Abstract
Bacterial virulence genes are often regulated at the transcriptional level by multiple factors that respond to different environmental signals. Some factors act directly on virulence genes; others control pathogenesis by adjusting the expression of downstream regulators or the accumulation of signals that affect regulator activity. While regulation has been studied extensively during in vitro growth, relatively little is known about how gene expression is adjusted during infection. Such information is important when a particular gene product is a candidate for therapeutic intervention. Transcriptional approaches like quantitative, real-time RT-PCR and RNA-Seq are powerful ways to examine gene expression on a global level, but suffer from many technical challenges including low abundance of bacterial RNA compared to host RNA, and sample degradation by RNases. Evaluating regulation using fluorescent reporters is relatively easy, and can be multiplexed with fluorescent proteins with unique spectral properties. The method allows for single-cell, spatiotemporal analysis of gene expression in tissues that exhibit complex three-dimensional architecture and physiochemical gradients that affect bacterial regulatory networks. Such information is lost when data are averaged over the bulk population. Herein, we describe a method for quantifying gene expression in bacterial pathogens in situ. The method is based on simple tissue processing and direct observation of fluorescence from reporter proteins. We demonstrate the utility of this system by examining the expression of Staphylococcus aureus thermonuclease (nuc), whose gene product is required for immune evasion and full virulence ex vivo and in vivo. We show that nuc-gfp is strongly expressed in renal abscesses, and reveal heterogeneous gene expression due in part to apparent spatial regulation of nuc promoter activity in abscesses fully engaged with the immune response. The method can be applied to any bacterium with a manipulatable genetic system and any infection model, providing valuable information for preclinical studies and drug development.
Keywords: Gene expression, virulence, fluorescence, confocal microscopy, histopathology, bacteria, pathogen, spatial regulation, Sae Two Component System, nuclease, kidney, abscess
SUMMARY
We describe here a method for analyzing bacterial gene expression in animal tissues at a cellular level. This method provides a resource for studying the phenotypic diversity occurring within a bacterial population in response to the tissue environment during an infection.
INTRODUCTION
Bacteria respond to changing physiological conditions and alterations in the nutritional state of their environment by differentially expressing genes required for adaptation and survival. For instance, opportunistic pathogens colonize body surfaces at relatively low densities, and are often harmless. However, once the bacterium has penetrated physical and chemical barriers, it must contend with host immune cell counter-defenses, and restricted nutrient availability 1. As an example, Staphylococcus aureus colonizes approximately one third of the population asymptomatically, but is also the cause of devastating skin and soft tissue infections, osteomyelitis, endocarditis, and bacteremia 2. The success of S. aureus as a pathogen is often attributed to its metabolic flexibility as well as an arsenal of surface-associated and secreted virulence factors that enable the bacterium to escape the bloodstream and replicate in peripheral tissues 3–5. Because host death due to staphylococcal disease is an evolutionary dead end and limits transmission to new hosts6, the commitment to virulence factor production must be carefully controlled.
A complex regulatory network of proteins and non-coding RNAs responds to a variety of environmental stimuli including cell density, growth phase, neutrophil-associated factors, and nutrient availability to ensure that virulence genes are expressed at the precise time and location within host tissues 7,8–13. For instance, the SaeR/S two component system (TCS) regulates expression of several virulence factors via the sensor kinase (SaeS) and the response regulator (SaeR)14. SaeS is autophosphorylated on a conserved histidine residue in response to host signals (e.g., human neutrophil peptides [HNPs], calprotectin)8,15,16. The phosphoryl group is then transferred to an aspartate residue on SaeR, activating it as a DNA-binding protein (SaeR~P) 17. The SaeR/S TCS regulates over 20 genes that contribute to pathogenesis including fibronectin binding proteins (FnBPs), leukocidins, and coagulase 14,18–20. Targets can be classified into high-affinity and low-affinity gene targets, which are likely induced as the level of SaeR~P rises when exposed to its cues 21. The SaeR/S activity is controlled by other regulators of gene expression such as the Agr quorum sensing system, repressor of toxins protein (Rot), and the alternative sigma factor B (SigB)22–24.
nuc is an Sae-dependent virulence gene in Staphylococcus aureus and encodes thermonuclease (Nuc), which is essential for escaping from neutrophilic extracellular traps (NETs) and for dissemination during the course of infection25,26. The expression of nuc is also strongly indirectly repressed by CodY in the presence of branched-chain amino acids and GTP 27, and directly repressed by the staphylococcal accessory regulator protein SarA28,29, whose activity is influenced by oxygen (redox state) and pH 30. Given that sae and nuc mutants are attenuated in mouse models of infection, there is interest in developing chemical interventions that inhibit their corresponding activities26,31. Despite this, there is no information regarding their regulation during infection.
Fluorescent reporters have been used to monitor and quantify gene expression on the single cell level. Herein, we present a method for quantifying S. aureus gene expression during infection that, when paired with in vitro transcriptome analysis and powerful imaging techniques like magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS), can reveal how bacterial physiology is regulated in vivo and the relative abundances of nutrients in certain niches. The method can be applied to any bacterial pathogen with a tractable genetic system.
Overview of the genome integrative vector
The genome integrative vector pRB4 contains 500 base pairs each from the upstream and downstream regions of the S. aureus USA300 SAUSA300_0087 pseudogene to facilitate homologous recombination. pRB4 is derived from the temperature-sensitive pMAD vector backbone containing the erythromycin resistance cassette (ermC) and thermostable beta-galactosidase gene bgaB for blue/white screening of recombinants 32. The engineered reporter construct also contains a chloramphenicol resistance marker (cat) for selection after genome integration and plasmid elimination, as well as EcoRI and SmaI sites to fuse the regulatory region of interest to superfolder green fluorescent protein (sGFP) (Figure 1). It is known that the choice of ribosome binding site (RBS) influences the activity of the reporter, and often requires empirical optimization 33. Thus, a RBS is not supplied. Here, the native ribosome binding site is used to provide for a more natural pattern of gene expression, but other sites may be used.
Figure 1: Schematic representation of the genome integrative reporter plasmid pRB4.
Plasmid pRB4 is a derivative of pMAD with a temperature sensitive origin of replication in S. aureus (Ori pE194ts). There are three drug resistance markers: (i) bla, conferring resistance to ampicillin in Escherichia coli; (ii) ermC, conferring erythromycin resistance in S. aureus; and (iii) cat, conferring resistance to chloramphenicol in S. aureus. The reporter construct is flanked by ~500 bp each from the upstream and downstream sequences of the pseudogene SAUSA300_0087 (reference genome: FPR3757) for double crossover homologous recombination and genome integration. sGFP, green fluorescent protein gene; CS, cloning site; TT, strong bidirectional transcriptional terminator. Figure is not to scale.
PROTOCOL
1. Generation of the fluorescent reporter strain
1.1).
Digest the genome integrative pRB4 vector sequentially with EcoRI and SmaI restriction enzymes. Following the manufacturer’s protocol, set up an overnight digestion with SmaI using 1 μg of pRB4 at 25°C, and then proceed with the second digestion for 1h at 37°C by adding EcoRI to the reaction mixture. Inactivate the enzymes by incubating at 65°C for 20 min.
1.2).
PCR amplify the regulatory region of interest from genomic DNA (in this case, a ~350 base pair DNA fragment containing the thermonuclease [nuc] promoter region), incorporating a 5’ EcoRI restriction site and a 3’ SmaI restriction site. The SmaI recognition sequence must be in-frame with the translational start codon. In this study, the PCR was performed using a high-fidelity DNA Polymerase according to the manufacturer’s recommendations with forward primer oRB015 (5’—ATCATTGAATTCTCCAAAGTAAATTATAAGTTATAC—3’) and reverse primer oRB016 (5’—GGGCATAACTAACACCTCTTTCTTTTTAG—3’). The annealing temperature was 53°C. Purify the resulting fragment using a PCR clean-up kit following the manufacturer’s instructions.
1.3).
Digest the resulting PCR product with EcoRI and SmaI as performed in step 1.1 and ligate the digested DNA fragment to the same sites of pRB4 using T4 DNA ligase as recommended by the manufacturer to generate the integrative construct. Introduce the ligation mixture into E. coli and confirm construct sequence.
1.4).
Electroporate the construct into S. aureus strain RN4220 as previously described 34. In brief, use 1 μg of plasmid with 70 μl of electro-competent cells (100 Ω, 25 μF, and 2.3 kV) in B2 broth (1% [w/v] Casein hydrolysate, 2.5% [w/v] Yeast extract, 2.5% [w/v] NaCl, 0.1% [w/v] K2PO4, 0.5% [w/v] Glucose). Select for erythromycin resistance (Emr) at the permissive temperature (30°C) on tryptic soy agar (TSA) supplemented with erythromycin (5 μg ml−1) and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal; 80 μg ml−1). The resulting transformants are blue due to plasmid-encoded β-galactosidase activity. Note: Transfer of DNA into clinical isolates is extremely difficult due to a strong restriction-modification barrier35. Thus, S. aureus RN4220 was used as an intermediate recipient of the fusion construct because it is restriction deficient and highly transformable.
1.5).
Transfer plasmid to S. aureus USA300 strain of interest using electroporation or phage-mediated transduction36 at the permissive temperature. In brief, Φ11 bacteriophage particles were propagated on the donor strain using TSA plates containing 5 mM CaCl2 overnight at 30oC, and were then sterilized by filtration through a 0.45 μM cellulose acetate syringe filter. The lysates were used to transduce S. aureus strain LAC to Emr. Note: LAC is a widely used clinical isolate that was previously made Em sensitive by serial passage in TSB medium to cure the strain of the native plasmid pUSA03 that confers Emr 37,38.
1.6).
Integrate the construct by two, successive homologous recombination events into the chromosome as described previously 32. The recombinants are chloramphenicol-resistant (Cmr) on TSA plates containing 5 μg ml−1 of chloramphenicol, erythromycin-susceptible (Erms), and no longer blue. Note: When possible, re-introduce the marked fusion into USA300 via phage-mediated transduction to avoid the accumulation of mutations associated with in vitro strain passage 39 and temperature shifts.
2. Animal Infection: Preparation of the inoculum, systemic infection, and tissue processing
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of Georgetown University.
2.1. Preparation of bacterial inoculum
2.1.1).
Streak S. aureus strains of interest for isolation on blood agar plates from a frozen glycerol stock. Incubate at 37°C for 16–24 h to confirm hemolytic phenotypes based on their ability to lyse red blood cells (RBCs) and form clear transparent zones.
2.1.2).
Initiate overnight cultures by inoculating single colonies of each strain to 4 ml of tryptic soy broth (TSB) in sterile glass tubes. Rotate the tubes at 37°C for 16–24 h in a tube roller set at approximately a 70-degree angle and 70 rotations per minute [RPM].
2.1.3).
Use a spectrophotometer to measure the optical density at 600 nm (OD600) of the cultures from step 2.1.2. Sterile medium is used as an optical reference (blank). Dilute these cells to an OD600 of 0.05 in 25 ml of sterile Tryptic Soy Broth (TSB) in separate, 125 ml DeLong flasks (5:1 flask to volume ratio) and incubate cultures in a water bath (for proper heat transfer to the cultures), shaking at 280 RPM and set to 37°C. Note: growth of the inoculum can be performed in various ways; one method for growing cells to exponential phase is presented. Regardless, it is important to consistently use the same method for preparing the cells for inoculation.
2.1.4).
At an OD600 of ~1, re-dilute the cells into fresh medium to a starting OD600 of 0.05 to ensure the cells achieve exponential phase and that factors that accumulate during overnight incubation have been reduced to exponential phase levels.
2.1.5).
Harvest the cells during exponential phase (OD600 ~0.6–0.8) by centrifuging at 3000 x g for 10 min at room temperature.
2.1.6).
Wash the pellets twice in equivalent volume of sterile 1X phosphate-buffered saline (PBS; pH 7.4).
2.1.7).
Suspend the cells in 1X PBS (pH 7.4) to a concentration of 1 × 108 colony forming units (CFUs) ml−1 or as appropriate for the desired experiment.
Note: it is important to determine the relationship between OD600 and CFU ml−1 for each strain of interest because strain-dependent alterations of cell size and shape can affect light scattering properties and ultimately, the infection dose.
2.2. Preparation of the animals and bacterial infection
2.2.1).
Acclimate mice for 7 days on purified diet AIN-93. The diet is formulated to provide adequate nutrition while reducing tissue auto-fluorescence associated with the consumption of plant-based ingredients used in standard mouse chow 40. Female C57/BL6 mice (6–8 weeks old) are used here, but the mouse strain and sex will depend on the study.
2.2.2).
Before infection, dilate mouse tail veins with lukewarm water.
2.2.3).
Infect animals by injecting 100 μl of the bacterial inoculum via tail-veins to produce a systemic infection. Save an aliquot of the initial inoculum if performing flow cytometry (see Section 4).
2.2.4).
Monitor animals daily and evaluate their health status using a monitoring system reviewed and approved by one’s Institutional Animal Care and Use Committee.
2.2.5).
Allow the infection to progress for the desired duration. Here, the experiments are terminated 3 days post-infection.
Note: Under these conditions, abscesses consist of a staphylococcal abscess community of bacteria, enclosed by fibrin deposits, and surrounded by concentric layers of immune cells5,41.
2.3. Harvesting the organs and tissue processing
2.3.1).
Euthanize the animals by CO2 inhalation and cervical dislocation as a secondary method and perform necropsy.
2.3.2).
Harvest kidney (right) and other vital organs (heart, liver, lungs, spleen) and transfer into 50 ml polypropylene tubes containing 10% [v/v] buffered formalin. Skip to section 2.3.4 below.
2.3.3).
Transfer the left kidney to a sterile 2 ml impact-resistant tube containing ~500 μl of 2 mm silica beads and 1 ml sterile 1x PBS (pH 7.4). Proceed to Section 4.
2.3.4).
Allow organs to fix in the dark at room temperature with gentle shaking or rotation for at least 24 hours but no more than 48 hours.
2.3.5).
Embed organs in clear tissue freezing medium and store the tissues at −80°C.
2.3.6).
Using a cryostat, section tissue into slices of 10 μm thickness.
2.3.7).
Dry the sections on a pre-cleaned, charged glass slide for 20 min in darkness, apply hard mounting medium with 4’,6-diamidino-2-phenylindole (DAPI) stain, and apply coverslip.
2.3.8).
Cure mounted slides at room temperature overnight, and transfer to 4°C for long-term storage.
3. Laser Scanning Confocal Microscopy and Image Processing
3.1).
Examine mounted slides to locate lesions using lasers appropriate for the fluorescent reporter being used. In this case, the green (GFP), red (tdTomato), and blue (DAPI) fluorescence signals are used.
3.2).
Acquire the image using the appropriate objective for visualizing individual cells (e.g., typically 20x or 40x objectives are used).
3.3).
Measure the fluorescence intensities in the confocal images.
3.3.1).
Open the confocal image in Image J and adjust brightness/contrast to properly visualize the fluorescence signal of the lesion.
3.3.2).
Define the region of interest, and by using the ‘thresholding’ option, set the lower and upper fluorescence limits as necessary.
3.3.3).
Define the centroid by selecting “Area” → “Mean gray value” → “Centroid” → “Limit to threshold” under the “Analyze” tab in Image J.
3.3.4).
Extract centroid fluorescence intensity value, or measure the mean fluorescence intensity in a given lesion per unit area (mean fluorescence intensity, MFI) for GFP and tdTomato. Here, areas of one μm2 were used. Note: a blinded second analysis minimizes bias when the identity of the sample is known.
3.3.5).
Plot the data and perform the appropriate statistical analyses for each comparison.
4. Flow cytometry analysis
4.1).
(Optional) Determine fusion activity of the inoculum on the day of the infection (from section 2.2.3)
4.1.1).
To 899 μl of 1X sterile PBS (pH 7.4) add 100 μl of each inoculum and 1 μl of the membrane permeant nucleic acid stain Syto 40. As a control, be sure to include a sample lacking Syto 40.
4.1.2).
Perform flow cytometry on all samples. Note: For the very first experiment, it is useful to include a vector only control for the fusion of interest to determine the proper voltage to use. A clear separation between positive and negative samples is essential for data analysis, indicating the reporter is well above background signal.
4.1.3).
To identify the bacterial population, plot events using Syto 40 (y-axis) and forward scatter (x-axis).
4.1.4).
Draw an inclusion gate around events that are both Syto 40 positive and the correct size of the bacterium based on the forward scatter (between 0.5 to 2.0 μm for S. aureus). Note: Be rigorous when identifying this population as it is critical to include events that are clearly within these parameters. Be sure this gate excludes all events for the negative controls under other conditions. In this study, roughly 70% the cell population met these requirements in the analysis.
4.2).
Analysis of tissue samples after sacrifice:
4.2.1).
Euthanize the animals, harvest the left kidneys, and transfer into bead-beating tubes containing 1 ml of 1x sterile PBS (pH 7.4) and 250 μl of 2 mm borosilicate beads.
4.2.2).
Disrupt tissues to release bacterial cells. For kidneys, use a homogenizer to disrupt cells. Here, we used three, 30 s bursts at 6800 rpm with 1 min cooling periods on wet ice between cycles to minimize heating samples.
4.2.3).
Pellet the larger tissue debris by centrifuging at 250 × g for 3 min in a tabletop microcentrifuge cooled to 4°C. Note: Typically, there is a cell pellet at the bottom of the tube and a layer of floating debris on the top. The middle aqueous layer contains the bacterial cells.
4.2.4).
Transfer 10 μl from the aqueous layer into a clean 1.5 ml microcentrifuge tube containing 1 μl Syto 40 stained in 989 μl of PBS (total volume 1 ml).
4.2.5).
Perform flow cytometry using the same data acquisition parameters and gating as for the initial inocula.
Note: Bacterial cell counts will be very low because the sample mainly contains tissue debris. The “Live gate” option can also be used if the cells are Syto 40 positive and size-gated when data are loaded into the application. This will also help to analyze more of the target events and reduce file size. Nearly one million events per sample are counted, but more event counts may be necessary depending on the severity of the infection and size of tissue analyzed.
4.2.6).
Data Analysis: Determine mean fluorescent intensity (MFI) for each fluorophore in a given sample using the inclusion gates determined in section 4.1.4. Normalize samples in flow analysis software to event counts. 10,000 counts are usually sufficient in the analysis. Note: It is not uncommon to find samples that contain less than this number of events since this is dependent on abscess formation and infection severity. Using this approach, 500–40,000 events are typically found in infected tissue.
REPRESENTATIVE RESULTS
We developed a plasmid derived from pMAD32 that can deliver any reporter fusion construct into the chromosome by double crossover homologous recombination (Figure 1). This construct allows for quantitative analysis of any regulatory region that supports the production of GFP protein and fluorescent signal above background. The plasmid confers ampicillin resistance (Apr) for maintenance and propagation in E. coli, and confers erythromycin resistance (Emr) in S. aureus. The construct also confers chloramphenicol resistance (Cmr), which allows for easy transfer of the integrated reporter fusion between various mutant strains for sophisticated genetic analysis in S. aureus.
As proof of principle, we fused the nuc regulatory region to gfp. We chose nuc because its gene product is required for full virulence and is required for restricting macrophages from S. aureus-induced abscesses 42,43. The construct was integrated into the chromosome as described in steps 1.4–1.6 of the protocol. The integrated fusion was then transferred to AH3926 that contains a PsarAP1-tdTomato fusion. The sarA P1 promoter has been shown previously to be constitutively active 44 and thus, labels all cells.
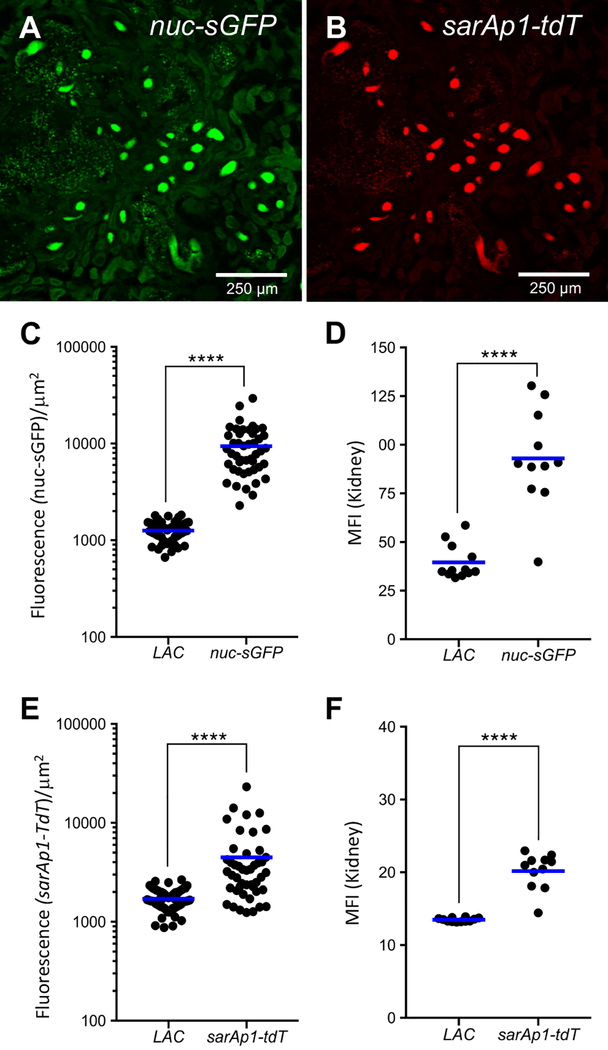
We first verified that the reporter fusions were active during in vitro shake flask growth in Tryptic Soy Broth (TSB; a rich, complex medium) and that the levels of fluorescence were above the cellular auto-fluorescent background (Figure 2). To determine how the fluorescent reporters behave in vivo, we used a modified renal abscess model5. We challenged groups of female C57BL/6 mice intravenously with 1×107 CFU each of S. aureus LAC cells lacking reporter fusions or LAC cells carrying both the nuc-sGFP and sarAp1-tdTomato fusions. Animals were sacrificed three days post infection. Then, harvested organs were fixed in 10% [v/v] buffered formalin, cryo-sectioned, and imaged by confocal microscopy after DAPI staining as described in steps 2 and 3 (Figure 3A, B). We analyzed the images using Image J and measured fluorescence per unit area in the renal lesions. As shown in Figure 3C, nuc-gfp fusion fluorescence was on average nearly 9-fold higher in cells carrying the fusion than in cells not carrying the reporter fusion; the latter signal constitutes the limit of detection (auto-fluorescence) (compare nuc-sGFP to LAC). Similarly, PsarAP1-tdtomato fusion fluorescence was ~6-fold higher than the no reporter control (Figure 3E, compare sarAP1-tdT vs LAC). Using flow cytometry, we confirmed the pattern of reporter activities using kidney homogenates and flow cytometry as described in step 4, though the fold differences in fluorescence were lower (Figures 3D, F).
Figure 2: Integrated nuc-sGFP and sarAp1-tdTomato reporters are expressed during in vitro growth.
S. aureus LAC cells (wild-type [WT]), with and without the A) nuc-sGFP or B) sarAp1-tdTomato reporters) were grown to exponential phase in TSB and re-diluted into the same medium. Optical density (OD) and fluorescence were measured at the indicated optical density values. Background-subtracted fluorescence intensity values (excitation/emission: sGFP, 485/535 nm; tdTomato [tdT], 535/590 nm) were divided by the optical density values to generate Relative Fluorescence Units. The data are the means +/− SEM from two independent experiments.
Figure 3: Fluorescent reporters are visible in renal tissue.
Groups of 13 C57BL/6 mice were challenged intravenously with LAC cells or LAC cells carrying both nuc-sGFP and sarAp1-tdTomato (tdT), and the infection was allowed to proceed for three days. Mice were euthanized, and organs were processed as described in steps 2 and 3 of the protocol. Representative kidney section showing multiple lesions and the associated fluorescence for A) nuc-sGFP and B) sarAp1-tdTomato. Scale bars = 250 μm (10x objective). The Relative Fluorescence Units per unit area (RFU [μm2]−1) associated with renal lesions were measured using image analysis as described in section 3.3 and were plotted for C) nuc-sGFP and E) sarAp1-tdTomato; each dot represents one lesion and bars indicate the median; 3–5 lesions analyzed per mouse. Flow cytometry analysis of D) nuc-sGFP and F) sarAp1-tdTomato fusion fluorescence in bacterial populations isolated from infected kidney homogenates three days post infection. The Mean Fluorescence Intensity (MFI) is indicated by the solid bar, and each dot is one kidney. LAC, reporterless wild-type strain. Statistics: Mann-Whitney Test; ****p < 0.05. The data are representative of two independent experiments. The excitation wavelengths for the fluorescence channels are as follows: DAPI, 405 nm; GFP, 488 nm; tdTomato, 561 nm. Emitted fluorescence data were collected over a range of wavelengths: DAPI, 419–481 nm; sGFP, 505–551 nm; tdTomato, 575–630 nm.
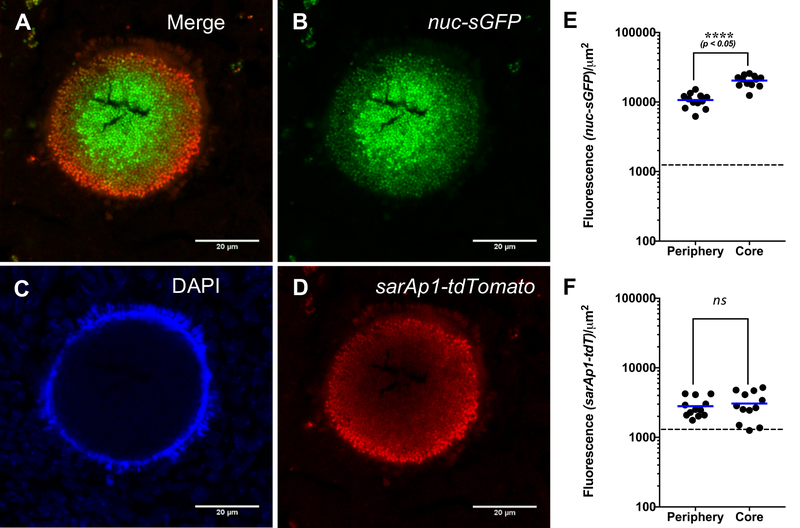
Interestingly, the fluorescence data from lesions formed by cells carrying fluorescent reporter fusions appeared to show higher variability than those lacking the reporters. We wondered whether the variation in the fluorescence measurements we observed was due to heterogeneous expression of the reporters (that is, of biological origins). Indeed, within ~100 μm distance we found that some lesions expressed either one or both reporters (Figure 4). Importantly, examining reporter activity with single cell resolution in staphylococcal abscess communities (SACs) revealed spatial regulation of nuc expression in abscesses circumscribed with strong DAPI staining, likely associated with the formation and release of neutrophil extracellular traps (Figure 5A–C) 45. For instance, we measured significantly higher nuc-sGFP fusion fluorescence in the interior core of the SAC compared with that on the periphery (Figure 5B, 5E). In the same abscess, the pattern for sarAp1-tdTomato fusion fluorescence appeared to be inverted (Figure 5D). However, the pattern was not statistically significant using the same number of animals (Figure 5F).
Figure 4: Abscesses exhibit variable expression of reporters in renal tissues.
Groups of 13 C57BL/6 mice were challenged with 1 × 107 CFU of S. aureus cells via the tail-vein. Animals were euthanized three days post infection. Kidneys were harvested, fixed, sectioned for fluorescence microscopy (40x objective) as described in step 2 of the protocol. Shown are three lesions in close proximity with variable levels of (A) nuc-sGFP and (B) sarAp1-tdTomato fluorescence. (C) Nucleic acid from staphylococci and host cells is indicated by the DAPI staining. The green and red channels are merged in (D). Similar results were seen in other sections. The images were acquired using a 40x objective and the scale bar indicates 25 μm. The excitation wavelengths for the fluorescence channels are as follows: DAPI, 405 nm; GFP, 488 nm; tdTomato, 561 nm. Emitted fluorescence data were collected over a range of wavelengths: DAPI, 419–481 nm; sGFP, 505–551 nm; tdTomato, 575–630 nm.
Figure 5: nuc-sGFP is spatially regulated in the staphylococcal abscess micro-environment.
Representative confocal laser scanning microscopy (CLSM) images of a staphylococcal abscess community (SAC) lesion produced by S. aureus strain LAC carrying nuc-sGFP and sarAp1-tdTomato reporter fusions. Shown are the (A) merged channels for nuc-sGFP and sarAp1-tdtomato, (B) nuc-sGFP fluorescence (green), (C) DAPI staining of nucleic acids (blue), and (D) sarAp1-tdTomato fluorescence (red). Asterisks indicate cells in the core (centroid) and arrows indicate cells on the periphery. The fluorescence intensities for (E) nuc-sGFP and (F) sarAp1-tdTomato are shown for cells in the core and periphery of the SAC. The excitation wavelengths for the fluorescence channels are as follows: DAPI, 405 nm; GFP, 488 nm; tdTomato, 561 nm. Emitted fluorescence data were collected over a range of wavelengths: DAPI, 419–481 nm; sGFP, 505–551 nm; tdTomato, 575–630 nm. The data are derived from 8 kidneys (one per mouse), and 1–2 lesions were imaged from each kidney. Bars indicate median. Dashed line, limit of detection. Statistics: Normal distribution of data, Student’s t-test (unpaired), ****p < 0.05. (Scale bar: 20 μm; applies to all images.)
DISCUSSION
Bacterial infectious diseases are an increasing health problem worldwide due to the acquisition of antibiotic resistance determinants 46. Because adaptation to host environments is essential for growth and survival during infection, strategies targeting gene expression programs that increase pathogen fitness may prove useful therapeutically. One such program is the set of genes controlled by the SaeR/S two component system (TCS), shown previously to play an essential role in immune evasion 47. SaeR/S is induced by a variety of factors, most notably those associated with neutrophils 8,20. During infection, S. aureus elicits a strong inflammatory response in which neutrophils and other phagocytes are recruited to the site of infection 2. Liquefaction necrosis and fibrin deposition follow, forming an abscess to prevent further tissue damage 48. Within these immune privileged sites, S. aureus cells use a number of Sae-dependent gene products to reprogram the abscess to facilitate bacterial multiplication 5,48. One Sae-dependent gene, nuc, codes for nuclease and metabolizes NETs to produce 2’-deoxyadenosine to kill macrophages 43. Thus, Nuc is an important secreted enzyme that is essential for full virulence 42 and is expressed in vivo (Figures 3–5).
Although the virulence factors of S. aureus have been studied extensively, how the bacterium grows in the host is an understudied area, as is understanding how its physiology is regulated during infection. Here we describe a method for probing bacterial gene expression and behavior on the single cell level using a modified integrative vector that mitigates concern for plasmid loss in the absence of selection. Our methodology allows for direct visualization of gene expression in abscesses without having to permeabilize cell walls, and without the need for antibodies and labeling. We detected strong expression of the nuc-sGFP fusion as well as the sarAp1-tdTomato fusion in renal abscess SACs formed by wild-type cells three days post infection in an acute systemic infection model. However, the experimental design can be modified to answer questions regarding gene expression in other sites. Indeed, because C57BL/6 mice are unable to clear S. aureus from their tissues, the bacteria invade diverse anatomical sites including the skeletal system (bones and joints) and the brain, heart, spleen, and liver, all of which have different physiology and nutritive properties 5,49. Thus, much can be learned by using S. aureus to probe the nature of host tissues. It is important to note that it is known that certain host niches are hypoxic in nature or otherwise exhibit a strong oxygen gradient 50. Fluorescent proteins require molecular oxygen for activity, and some are more sensitive to oxygen partial pressures than others 51. While we see fluorescence signal deep within the abscess, the magnitudes may be an underestimate. Using codon-optimized fluorescent proteins developed for use in Clostridium difficile could be used to mitigate this concern 52. A second point to note is that the fluorescent proteins (sGFP and tdTomato) produced by the reporter strains used in this study are stable. Therefore, the fluorescent data reflect accumulation over the course of experiment rather than a recent response. Generating a construct containing an unstable sGFP or tdTomato gene would greatly increase the utility of the system for dynamic experiments.
The reporter system described here provides a powerful tool to quantitatively study gene regulation in vitro and in vivo. Because the reporter is maintained in single copy on the chromosome, the system is well-suited for strongly expressed genes (that is, having high promoter activity). Biosynthetic genes or other lowly expressed genes may not be visible because the level of fluorescence expression could fall below the limit of detection or background auto-fluorescence. It is known that the ribosome binding site (RBS) influences the activity of reporter fusions 33. A potential solution to this problem is to use a stronger RBS such as the translation initiation region (TIR) 53, or to integrate tandem copies of promoters of interest into the chromosome. Alternatively, stable multi-copy plasmids could be used 54.
A limitation of the method described is that, unlike a cDNA preparation from RNA extracted from the tissues, a relatively small number of genes can be interrogated simultaneously (limited by available fluorescent channels without spectral overlap). However, what is gained can be potentially more informative. qRT-PCR and other readouts that average the bulk population are unable to capture population heterogeneity at the site of infection. Indeed, we observed heterogeneity in the level of nuc-sGFP and sarAp1-tdTomato expression between abscess lesions in close proximity (Figure 4). This is similar to what was recently reported by Cassat et al.55 At this time, the origin of this heterogeneity is not known. However, nutrient availability, variable induction of nutrient acquisition systems, or other host factors could possibly explain the phenomenon. Moreover, the host-pathogen interaction occurs within microenvironments of organs or abscesses that have complex three-dimensional structure and various cell types. Within these structures, tissues can vary considerably with respect to pH, osmolarity, oxygenation, and nutrient availability, a phenomenon known as metabolic zonation 56,57. We serendipitously discovered that a pattern of spatial regulation arises in wild-type cells residing in the abscess (Figure 5). This is to our knowledge the first observation of its kind in a Gram-positive pathogen during infection. The spatial regulation we report is similar to that obtained by Davis et al. in splenic tissue containing microcolonies of the Gram-negative pathogen Yersinia pseudotuberculosis 58. In that instance, host produced nitric oxide (NO*) stimulated the production of nitric oxide dioxygenase (Hmp) in cells on the periphery of the microcolony closest to the diffusing NO*, sparing interior cells the need to induce expression of hmp. In staphylococcal abscesses, nuc expression is strongest at the core of the SAC, and weakest along the periphery. Because abscesses are surrounded by a cuff of neutrophils, it is tempting to speculate that neutrophil-derived cues are guiding the behavior of the staphylococci, polarizing the cells into two phenotypically distinct populations reminiscent of morphogenic regulation of differentiation during development in higher life 59. The mechanistic underpinning of this pattern is unknown and is a subject of intense study in our laboratory.
We believe our method provides an effective tool for studying the fine detail of gene expression in individual cells during infection. The method provides a unique opportunity to observe and to eventually understand the physiological origins of heterogeneity and spatial patterning of gene expression in tissues. This heterogeneous behavior must be taken into account when developing new treatment modalities, as only a subpopulation of cells (i.e., those expressing the genes) may be targeted by the therapeutic.
ACKNOWLEDGMENTS
We thank Alexander Horswill for the gift of the PsarAP1-tdTomato fusion, and Karen Creswell for help with flow cytometry analysis. We also thank Alyssa King for advice on statistical analysis. This work was funded in part by an NIH Exploratory/Developmental Research Award (grant AI123708) and faculty startup funds to SRB. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
DISCLOSURES:
The authors declare that they have no competing financial interests.
Contributor Information
Ranjan K. Behera, Department of Biology, Georgetown University, Washington DC
Kevin D. Mlynek, Department of Biology, Georgetown University, Washington DC
Matthew S. Linz, Department of Biology, Georgetown University, Washington DC
Shaun R. Brinsmade, Department of Biology, Georgetown University, Washington DC
REFERENCES
- 1.Hood MI & Skaar EP Nutritional immunity: transition metals at the pathogen-host interface. Nature Reviews Microbiology. 10 (8), 525–537, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy F Staphylococcus aureus infections. The New England Journal of Medicine. 339 (8), 520–532, (1998). [DOI] [PubMed] [Google Scholar]
- 3.Grosser MR et al. Genetic requirements for Staphylococcus aureus nitric oxide resistance and virulence. PLoS Pathogen. 14 (3), e1006907, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentino M et al. Genes contributing to Staphylococcus aureus fitness in abscess- and infection-related ecologies. MBio. 5 (5), e01729–01714, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng A et al. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB Journal. 23 (10), 3393–3404, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers LA, Levin BR, Richardson AR & Stojiljkovic I Epidemiology, hypermutation, within-host evolution and the virulence of Neisseria meningitidis. Proceedings of the Royal Society B: Biological Sciences. 270 (1525), 1667–1677, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramanian D et al. Staphylococcus aureus Coordinates Leukocidin Expression and Pathogenesis by Sensing Metabolic Fluxes via RpiRc. MBio. 7 (3), e00818–00816, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiger T, Goerke C, Mainiero M, Kraus D & Wolz C The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. Journal of Bacteriology. 190 (10), 3419–3428, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss A, Broach WH & Shaw LN Characterizing the transcriptional adaptation of Staphylococcus aureus to stationary phase growth. Pathogens and Disease. 74 (5), pii:ftw046, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaban N & Novick RP Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proceedings of the National Academy of Sciences of the United States of America. 92 (5), 1619–1623, (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohl K et al. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. Journal of Bacteriology. 191 (9), 2953–2963, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majerczyk CD et al. Staphylococcus aureus CodY negatively regulates virulence gene expression. Journal of Bacteriology. 190 (7), 2257–2265, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara PJ, Milligan-Monroe KC, Khalili S & Proctor RA Identification, cloning, and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus. Journal of Bacteriology. 182 (11), 3197–3203, (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Yeo WS & Bae T The SaeRS Two-Component System of Staphylococcus aureus. Genes (Basel). 7 (10), E81, (2016).27706107 [Google Scholar]
- 15.Giraudo A, Calzolari A, Cataldi A, Bogni C & Nagel R The sae locus of Staphylococcus aureus encodes a two-component regulatory system. FEMS Microbiology Letters. 177 (1), 15–22, (1999). [DOI] [PubMed] [Google Scholar]
- 16.Cho H et al. Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Staphylococcus aureus Infections. PLoS Pathogen 11 (7), e1005026, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun F et al. In the Staphylococcus aureus two-component system sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. Journal of Bacteriology. 192 (8), 2111–2127, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang X et al. Inactivation of a two-component signal transduction system, SaeRS, eliminates adherence and attenuates virulence of Staphylococcus aureus. Infection and Immunity. 74 (8), 4655–4665, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraudo AT, Cheung AL & Nagel R The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Archives of Microbiology. 168 (1), 53–58, (1997). [DOI] [PubMed] [Google Scholar]
- 20.Flack CE et al. Differential regulation of staphylococcal virulence by the sensor kinase SaeS in response to neutrophil-derived stimuli. Proceedings of the National Academy of Sciences of the United States of America. 111 (19), E2037–2045, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mainiero M et al. Differential target gene activation by the Staphylococcus aureus two-component system saeRS. Journal of Bacteriology. 192 (3), 613–623, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D & Cheung A Repression of hla by rot is dependent on sae in Staphylococcus aureus. Infection and Immunity. 76 (3), 1068–1075, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischoff M et al. Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. Journal of Bacteriology. 186 (13), 4085–4099, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novick R & Jiang D The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology. 149 (Pt 10), 2709–2717, (2003). [DOI] [PubMed] [Google Scholar]
- 25.Olson ME et al. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infection and Immunity. 81 (4), 1316–1324, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berends E et al. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. Journal of Innate Immunity. 2 (6), 576–587, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters NR et al. A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Molecular Microbiology. 101 (3), 495–514, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassat J et al. Transcriptional profiling of a Staphylococcus aureus clinical isolate and its isogenic agr and sarA mutants reveals global differences in comparison to the laboratory strain RN6390. Microbiology. 152 (Pt 10), 3075–3090, (2006). [DOI] [PubMed] [Google Scholar]
- 29.Kiedrowski M et al. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One. 6 (11), e26714, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto DF et al. Staphylococcus aureus SarA is a regulatory protein responsive to redox and pH that can support bacteriophage lambda integrase-mediated excision/recombination. Molecular Microbiology. 74 (6), 1445–1458, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo WS et al. The FDA-approved anti-cancer drugs, streptozotocin and floxuridine, reduce the virulence of Staphylococcus aureus. Scientific Reports. 8 (1), 2521, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaud M, Chastanet A & Débarbouillé M New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, Gram-positive bacteria. Applied and Environmental Microbiology. 70 (11), 6887–6891, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malone CL et al. Fluorescent reporters for Staphylococcus aureus. Journal of Microbiological Methods. 77 (3), 251–260, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenk S & Laddaga RA Improved method for electroporation of Staphylococcus aureus. FEMS Microbiology Letters. 73 (1–2), 133–138, (1992). [DOI] [PubMed] [Google Scholar]
- 35.Monk IR & Foster TJ Genetic manipulation of Staphylococci—breaking through the barrier. Frontiers in Cellular and Infection Microbiology. 2 (49), (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novick RP Genetic systems in staphylococci. Methods Enzymol. 204 (587–636), (1991). [DOI] [PubMed] [Google Scholar]
- 37.Diep BA et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 367 (9512), 731–739, (2006). [DOI] [PubMed] [Google Scholar]
- 38.Boles BR, Thoendel M, Roth AJ & Horswill AR Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One. 5 (4), e10146, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villaruz AE et al. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. Journal of Infect Diseases. 200 (5), 724–734, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves PG, Nielsen FH & Fahey GCJ AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Journal of Nutrition. 123 (11), 1939–1951, (1993). [DOI] [PubMed] [Google Scholar]
- 41.Thomer L, Schneewind O & Missiakas D Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annual Review of Pathology. 11 343–364, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson M et al. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infection and Immunity. 81 (4), 1316–1324, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thammavongsa V, Missiakas D & Schneewind O Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 342 (6160), 863–866, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung AL, Nast CC & Bayer AS Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infection and Immunity. 66 (12), 5988–5993, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pilsczek FH et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. Journal of Immunology. 185 (12), 7413–7425, (2010). [DOI] [PubMed] [Google Scholar]
- 46.Uhlemann AC, Otto M, Lowy FD & DeLeo FR Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infection, Genetics and Evolution. 21 563–574, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voyich JM et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. Journal of Infectious Diseases. 199 (11), 1698–1706, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng A, DeDent A, Schneewind O & Missiakas D A play in four acts: Staphylococcus aureus abscess formation. Trends in Microbiology. 19 (5), 225–232, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balasubramanian D, Harper L, Shopsin B & Torres VJ Staphylococcus aureus pathogenesis in diverse host environments. Pathogens and Disease. 75 (1), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vitko NP, Grosser MR, Khatri D, Thurlow LR & Richardson AR Expanded Glucose Import Capability Affords Staphylococcus aureus Optimized Glycolytic Flux during Infection. MBio. 7 (3), e00296–00216, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landete JM et al. Use of anaerobic green fluorescent protein versus green fluorescent protein as reporter in lactic acid bacteria. Applied Microbiology and Biotechnol. 99 (16), 6865–6877, (2015). [DOI] [PubMed] [Google Scholar]
- 52.Buckley AM et al. Lighting Up Clostridium Difficile: Reporting Gene Expression Using Fluorescent Lov Domains. Scientific Reports. 6 23463, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bose JL Genetic manipulation of staphylococci. Methods in Molecular Biology. 1106 101–111, (2014). [DOI] [PubMed] [Google Scholar]
- 54.Krute CN et al. Generation of a stable plasmid for in vitro and in vivo studies of Staphylococcus. Applied and Environmental Microbiology. 10.1128/AEM.0237016, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cassat JE et al. Integrated molecular imaging reveals tissue heterogeneity driving host-pathogen interactions. Science Translational Medicine. 10 (432), pii: eaan6361, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handlogten ME, Hong SP, Westhoff CM & Weiner ID Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). American Journal of Physiology-Renal Physiology. 289 (2), F347–358, (2005). [DOI] [PubMed] [Google Scholar]
- 57.Hijmans BS, Grefhorst A, Oosterveer MH & Groen AK Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences . Biochimie Journal. 96 121–129, (2014). [DOI] [PubMed] [Google Scholar]
- 58.Davis KM, Mohammadi S &.Isberg, R. R. Community behavior and spatial regulation within a bacterial microcolony in deep tissue sites serves to protect against host attack. Cell Host & Microbe. 17 (1), 21–31, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stenman JM et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 322 (5905), 1247–1250, (2008). [DOI] [PubMed] [Google Scholar]