Abstract
Background:
The World Health Organization (WHO) HCV-elimination strategy advocates scaling-up screening and treatment to reduce HCV incidence by 80% by 2030, but little is known about how this could be achieved and the costs of doing so.
Methods:
A general population HCV transmission, screening, and treatment model was calibrated with available data from Pakistan, incorporating cost data on diagnostics and HCV treatment. We modelled the impact and costs of alternative strategies for scaling-up screening and HCV treatment to determine what is needed and the resulting costs of achieving the WHO HCV incidence target in Pakistan.
Findings:
One-time screening of 90% of the 2018 population by 2030, with 80% referral to treatment, leads to 14 million individuals being screened and 350,000 treated annually, decreasing incidence by 27% over 2018–2030. Prioritising screening to higher prevalence groups (people who inject drugs (PWID) and adults >30 years) and introducing re-screening (annually for PWID, otherwise 10-yearly) increases the number screened and treated by half and decreases incidence by 51%. Decreasing HCV incidence by 80% requires doubling the primary screening rate, increasing referral to 90%, re-screening the general population every 5-years, and re-engaging those lost-to-follow-up every 5-years. This could cost USD$8 billion, reducing to USD$4 billion with lowest costs for diagnostic tests and drugs, including healthcare savings, and implementing a simplified treatment algorithm.
Interpretation:
Pakistan will need to invest up to 9% of their yearly health expenditure to enable sufficient scale-up in screening and treatment to achieve the WHO HCV-elimination target for incidence.
Funding:
UNITAID
Keywords: mathematical model, hepatitis C virus, direct-acting antiviral treatment, prevention, HCV elimination targets, incidence, mortality, screening, case-finding, Ab, RNA, diagnosis, cascade of care, cost-effectiveness, budget impact analysis
Introduction
Following the development of highly effective direct-acting antiviral (DAA) treatments for hepatitis C virus (HCV), the World Health Organization (WHO) developed a Global Health Sector Strategy (GHSS) to eliminate HCV as a public health threat, setting targets to reduce the incidence of new HCV infections by 80% and HCV-related mortality by 65% by 2030 (compared with 2015 levels).1 In low to middle-income countries (LMIC), where 50–80% of the global HCV-burden resides2,3, diagnosis rates are low (13.8%), with 8.7% initiating treatment in 2015, most of which occurred in Egypt.3
Pakistan has the second-largest HCV-burden worldwide, with an estimated 7.0 million infections in 2013.4,5 Insufficient treatment occurs, leading to increasing HCV-related morbidity.5,6 Pakistan’s HCV epidemic is generalised, with most HCV transmission being attributable to routine community and medical-related practices.7,8
Since 2005, national and provincial hepatitis control programmes have been treating HCV-infected patients, with about 150,000–160,000 patients initiating treatment annually by 2015.9 From 2016, DAA therapies became available in Pakistan, and both the public and private sectors have been initiating programmes to scale-up HCV treatment.9 This scale-up depends on identifying infected cases; however, it is unknown what levels of screening, linkage-to-care, and resources are needed to scale-up treatment sufficiently for reaching the WHO HCV-elimination targets.
We evaluate the impact and cost of different strategies to scale-up screening and treatment in Pakistan, to determine what is required to reach the WHO HCV-elimination targets for incidence.
Methods
Model description
A previous HCV transmission, treatment, and disease progression model for Pakistan5 was adapted to incorporate a detailed cascade of care (CoC) for HCV (Figure 1, Supplementary Figure S1).
Figure 1.
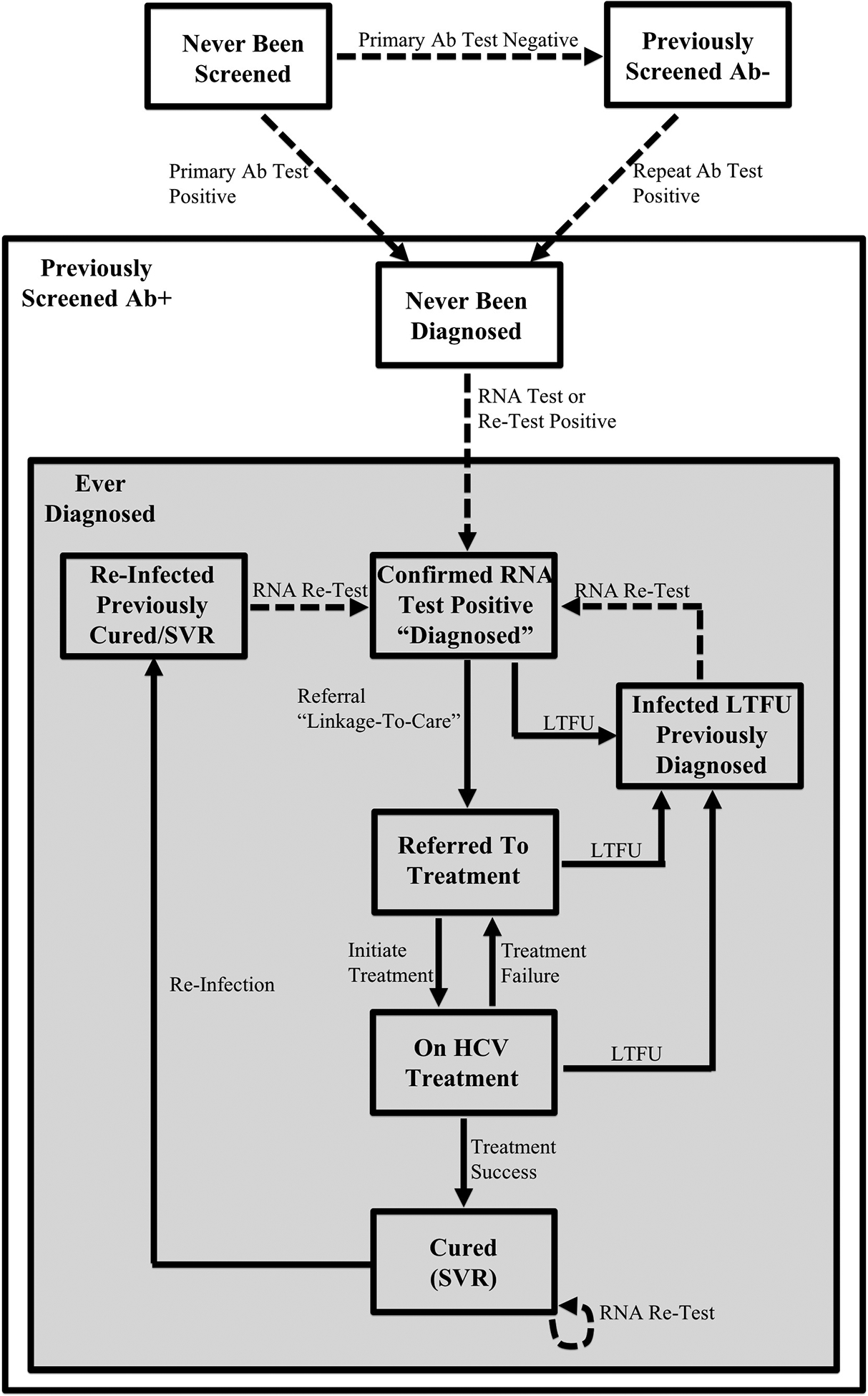
Simplified HCV screening model schematic illustrating the key aspects of the screening and treatment intervention pathway. Broadly, the population is split into three categories: (i) those who have never been screened (and eligible for primary Ab screening), (ii) those who have been previously screened Ab- (and eligible for Ab re-screening), and (iii) those who have been previously screened Ab+ (i.e. known Ab+ status). The lattermost category of individuals with known Ab+ status is divided into two sub-categories to indicate whether they have ever been diagnosed or not. The full HCV screening model schematic, including demographic and behavioural compartments, disease progression stages, HCV transmission dynamics, and the screening and treatment intervention cascade of care can be found in Supplementary Figure S1.
Common to the previous model, the new model incorporates population growth, age-structure and gender; HCV transmission in the general population and among PWID; and HCV-associated disease progression. Briefly, we divided the population into three age-categories: Young (0–19 years), Young Adult (20–29 years), and Adult (30+ years). Individuals enter the Young category at a birth-rate dependent on population growth and are initially susceptible. They transition through the age-categories, with some Young Adults becoming PWID. Mortality is age- and gender-specific, with PWID experiencing heightened drug-related mortality.10 Susceptible individuals become HCV-infected at a rate dependent on their gender, age, and HCV prevalence in the population. PWID have additional infection risk. Most newly infected individuals become chronically infected and progress to compensated cirrhosis if left untreated, and eventually end-stage liver disease (ESLD – decompensated cirrhosis and hepatocellular carcinoma (HCC)). ESLD is associated with increased mortality.11 Re-infection can occur after successful treatment or spontaneous clearance.12,13
The cascade of care (CoC) in the new model begins with diagnosis of HCV infection through antibody (Ab) screening and RNA confirmation. Diagnosed persons can then be referred to care and initiated on treatment, which can result in a sustained virologic response (SVR, effective cure).
We assume that each year, a proportion of the population are Ab screened for the first time, with previously tested seronegative individuals having specified re-testing rates; with these rates being variable across sub-groups. Those testing antibody-positive (Ab-positive) can receive RNA testing, whereupon they become diagnosed with active infection if they are RNA-positive. Any Ab-positive individuals who are not aware of their current infection status can also be RNA (re-)screened. Depending on possible eligibility criteria or the prioritisation of certain sub-groups, a proportion of newly diagnosed individuals are referred to treatment. If treated, patients either achieve SVR or fail treatment, following which they can be re-treated. Although loss-to-follow-up (LTFU) can occur along the CoC, re-engagement can result in them re-entering the diagnosed category.
Baseline model parameterisation and calibration
The model was parameterised using demographic, behavioural, and HCV epidemiological data from Pakistan (Table 1, Supplementary Tables S1–S2). The model was calibrated to country-level demographic data over 1960–2015, including population growth14,15, and to HCV seroprevalence data (by age group and overall) from a national survey7 in 2007–2008 (4.8% overall), surveys among PWID16,17 (56–69%), and HCV seroprevalence trends among blood-donors. These suggest HCV seroprevalence increased by 0.39% (95% confidence interval (CI) −0.17 to 0.94%) per decade over 1994–2014 (Supplementary Figure S2).
Table 1.
Summary of main baseline model parameters with associated uncertainty ranges. Rates are per year.
| Parameter | Baseline value or fitted range when stated [Uncertainty Distribution/Range] |
Source |
|---|---|---|
| Demographic Parameters | ||
| Average population growth rate per year | Pre-2000: Fitted 2.76% [2.53–2.99%] | Pakistan Economic Survey14; United Nations Department of Economic and Social Affairs, Population Division15 |
| Interim 2000–2015: Fitted 1.92% [1.54–2.31%] Post-2015: [Uniform 1.35–2.08%] | ||
| Proportion of Young Adults who initiate injecting drug use | Fitted values: Male: 0.032 [0.026–0.039], Female: 0.009 [0.0004–0.017] |
Calibrated to fit proportion of adults that are PWID (people aged 15 to 64; Male: 0.7%, Female: 0.01%)17 |
| Average mortality rate in each age group | Based on a life expectancy at birth of 66 years in 2015, and adjusted in model calibration to give proportion in each age group in 2015 (Young: 44%, Young Adult: 19%, Adult: 37%)15 | |
| Young Young Adult Adult |
1/56 | |
| 1/41 | ||
| Fitted values: Male: 0.023 [0.020–0.026] Female: 0.020 [0.017–0.024] | ||
| Additional drug-related mortality rate | 0.028 [Lognormal 0.017–0.039] | Based on estimates of drug-related mortality across Asia10 |
| Transmission Parameters | ||
| HCV transmission rate per susceptible in each age group |
β1 = 0.059 [0.052–0.066] β2 = 0.053 [0.023–0.085] β3 = 0.12 [0.10–0.14] |
Fit to chronic prevalence in each age category from 2007–2008 Pakistan national survey7 on viral hepatitis (Young: 1.5%, Young Adult: 3.2%, Adult: 6.9%) |
| Additional HCV transmission rate for injecting drug use | 0.61 [0.51–0.74] | Fit to chronic prevalence in PWID16 in 2012: 62.2% [55.5–68.8%] |
| Proportion of infections that spontaneously clear | 0.26 [Uniform 0.22–0.29] | Systematic review of spontaneous HCV clearance12 |
| Treatment Parameters | ||
| Treatment rate per capita before 2018 | Calibrated to historical treatment rates† | Pakistan Health Research Council (unpublished data) |
| Average duration on treatment | 24-weeks prior to 2016. Shortened to 12 weeks for pre-cirrhotic patients and 24 weeks for post-cirrhotic patients from 2016 onwards | World Health Organization HCV Treatment Guidelines13 |
| SVR rate with IFN and RBV treatment | 0.61 [Uniform 0.50–0.73] | Studies on SVR rates for conventional HCV treatment in Pakistan18 |
| SVR rate with new DAA treatments | 0.9 [Uniform 0.80–0.95] | World Health Organization HCV Treatment Guidelines13; Review of DAAs in Pakistan19 |
Total estimated historical HCV treatments prior to 2018, assuming a public/private sector split of 40%/60% (Supplementary Table S3). 2005–2010: 57,500; 2011: 137,970; 2012: 129,398; 2013: 137,910; 2014: 115,920; 2015–2017: 152,710 treatments. DAAs available from 2016 onwards.
Abbreviations. DAA: direct-acting antiviral; HCV: hepatitis C virus; IFN: interferon; PWID: people who inject drugs; RBV: ribavirin; SVR: sustained virologic response.
HCV disease progression and mortality rates came from the literature.4 Treatment before 2016 using interferon-based therapy (50% SVR)18 was modelled using in-country data (Supplementary Table S3); while DAA treatments (90–99% SVR) were used post-2016.13,19 No data on screening exists for Pakistan, so we calibrated the average screening rate (2.6–5.9%) to give the observed 150,000–160,000 treatments undertaken each year9, while assuming a treatment referral rate of between 35–70% based on Pakistan data.20
Uncertainty distributions were associated with most parameters and calibration data (Supplementary Tables S1–S2); the only exceptions being some unknown parameters estimated during the model calibration. To calibrate the model, these uncertainty distributions were randomly sampled to produce 4,000 paired sets of parameters and calibration data. For each set, the unknown model parameters were varied to fit the model to the sampled calibration data for chronic HCV prevalence by age and for PWID using a non-linear least squares optimisation algorithm (Matlab v2018b). Parameter sets that did not then produce model estimates within the 95%CI of the overall chronic HCV prevalence from the 2007–2008 national survey were discarded. This produced 1,151 model fits that were used for subsequent analyses. The results report the median and 95% uncertainty interval from these modelled runs. Further details are in the Supplementary Materials.
Model analyses
We used the calibrated model to evaluate various screening and treatment intervention scenarios from 2018. We assessed how each improved the CoC for HCV, and the percentage reduction in HCV incidence and mortality by 2030 compared to 2015. Scenarios were also compared to a counterfactual of no treatment from 2018 and a status-quo scenario of maintaining current levels of treatment (150,000–160,000 per year) until 2030.
In each scenario, we assume that all individuals with an Ab-positive test are tested for HCV RNA. We then assume different levels of referral to treatment, with the remainder LTFU. All referred patients initiate treatment, with those not achieving SVR being re-treated. For simplicity, we only incorporate one LTFU between diagnosis and referral, and assume that this captures the effect of other forms of LTFU between diagnosis and treatment, with data from Pakistan suggesting there is relatively little LTFU following treatment initiation (unpublished; Mafirakureva et al.), which can be accounted for in our intention to treat SVR rate. We assume a treatment duration of 12-weeks unless cirrhotic (24-weeks). The intervention scenarios are as follows (Supplementary Materials for more details):
-
Scenario S0:
No further treatment from 2018 onwards.
-
Scenario SQ:
While assuming a range of referral rates (35–70%), the average screening rate (2.6–5.9% of population per year) is calibrated to maintain current levels of treatment (‘Status Quo’).
-
Scenario S1:
One-time random screening of 90% of the 2018 Pakistan population by end of 2030, equating to ~6.2% screened annually, with 80% of diagnosed individuals referred to treatment. This is the screening target set by WHO.1,3
-
Scenario S2:
One-time screening (as Scenario S1) but prioritised first to individuals over 30 years of age and PWID, who have higher HCV prevalence, and then to the rest of the general population.
-
Scenario S3:
Prioritised one-time screening (as Scenario S2), but with periodic RNA re-screening of cured individuals and those previously screened RNA-negative, and Ab re-screening of individuals previously screened Ab-negative. Re-screening occurs every 10-years for non-PWID and annually for PWID.
-
Scenario S4:
Following these scenarios, we then determined what further improvements to the CoC are needed to reduce HCV incidence by 80% by 2030. This includes prioritised one-time screening as in Scenario S3, but with increased referral (90%), double the primary Ab screening rate (12.4% per year), re-screening every 5-years for non-PWID, and re-engaging those LTFU every 5-years for non-PWID and yearly for PWID (not included previously).
Estimation of screening and treatment costs
We estimated the total costs of each scenario to assess the affordability of widespread HCV screening and treatment scale-up. Cost data for undertaking HCV testing and treatment (including materials, equipment, and staff time) came from a Médecins San Frontières (MSF) HCV treatment clinic in Machar Colony,21,22 Karachi, from 2016–2017. A patient-level costing was undertaken using a provider’s perspective in 2018 US Dollars (USD$) (unpublished data; Mafirakureva et al.). Treatment costs were adapted for generic sofosbuvir ($15 for 28-day supply3) and daclatasvir ($21), giving a total treatment unit cost of $403 for a 12-week treatment course and $586 for 24-weeks, inclusive of drug, visit, and laboratory costs. All costs were valued at local rates. Screening unit costs for Ab and RNA testing were estimated as $10–17 and $34–41 per test, respectively, including staff costs. Actual Ab and RNA test kits cost $2.15 and $24.09, respectively. Healthcare costs for management of HCV disease (other than curative treatment) were not included in the baseline cost estimates due to a lack of data for Pakistan, but have been considered in the sensitivity analyses using adjusted costs from Cambodia (unpublished data; Mafirakureva et al.). We also estimated the cost per cure for each strategy. Costs and outcomes were discounted at 3.5% per year. More details in the Supplementary Materials.
Sensitivity analyses
We conducted sensitivity analyses to determine how the total costs of Scenario S4 would vary for the following changes in cost assumptions (more details in Supplementary Materials):
-
X1.
Assume lowest Pakistan DAA drug costs (from $109 to $18 for 12-weeks);
-
X2.
Assume lowest costs for diagnostic test kits (from $2.15 and $24 for Ab test and RNA confirmation to $0.40 and $15);
-
X3.
Include savings in healthcare costs for managing patients with HCV-related disease. Cost data from Cambodia adjusted to the Pakistan context (pre-cirrhosis: $15, compensated cirrhosis: $47, decompensated cirrhosis: $278, HCC: $339, compared to no healthcare costs in the base case);
-
X4.
Include costs for improving referral from 80% to 90% of diagnosed chronic infections and re-engaging people LTFU ($19 per patient referred or re-engaged, derived from assuming half a day of nurse time required to follow-up each patient);
-
X5.
Include savings from implementing a simplified treatment pathway (reduced visits and laboratory investigations);
-
X6.
Combine X1 to X5;
-
X7 and X8:
None or double the discount rate for costs and outcomes (3.5% in base analyses).
Role of the funding source
The funder of the study had no role in the study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Impact of baseline scenarios
Without treatment from 2018 (S0), our model projects chronic HCV prevalence will rise, from 3.7% [3.4–4.0%] to 4.5% [4.0–5.1%] (11.2 [10.1–12.4] million chronic infections) over 2018–2030, and incidence will increase by 19.5% [12.5–27.1%] relative to 2015 levels. If current levels of treatment are continued (SQ), then 25.0% [22.5–28.0%] of chronic infections in 2018 would be cured by 2030, chronic HCV prevalence and incidence would remain stable, but HCV-related mortality would increase by 32.3% [25.0–40.0%] relative to 2015.
Impact of improving cascade of care
Figure 2 shows the impact of each intervention scenario on mortality and incidence, with each CoC given in Figure 3.
-
Scenario S1:
One-time screening of General Population
One-time screening of 90% of the general population over 2018–2030 requires 13.8 [13.4–14.1] million individuals or 6.2% [6.1–6.3%] of the population being Ab and/or RNA-tested annually. On average, 350,000 [315,000–385,000] treatments are initiated annually and 56.4% [54.8–58.0%] of chronic infections in 2018 are cured by 2030. Compared to 2015 levels, incidence decreases by 26.5% [22.5–30.7%] by 2030 but mortality increases by 7.0% [1.1–13.5%]. Both are decreases compared to baseline scenarios S0 and SQ by 2030.
-
Scenario S2:
One-time screening with prioritisation for PWID and adults (30+ years)
Compared to Scenario S1, this results in the annual treatments increasing to 445,000 [400,000–490,000] and 72.9% [71.4–74.3%] of chronic infections in 2018 being cured by 2030. Incidence and mortality decrease by 40.8% [36.4–45.4%] and 14.8% [7.8–21.1%], respectively.
-
Scenario S3:
One-time prioritised screening with re-screening
Incorporating re-screening of previously treated individuals and individuals that previously tested Ab- negative increases the number of individuals screened by half (20.3 [19.7–20.8] million individuals annually) compared to Scenarios S1 and S2. This results in 490,000 [445,000–545,000] individuals being treated each year and 80.5% [78.2–82.8%] of chronic infections in 2018 being cured by 2030 (Figure 3). Incidence and mortality decrease by 50.8% [46.1–55.0%] and 17.9% [10.8–24.3%], respectively.
-
Scenario S4:
Improved scenario S3 with re-engagement of LTFU
Incremental to Scenario S3, doubling the primary screening rate (12.4% per year), improving treatment referral (90%), re-screening non-PWID every 5-years (instead of every 10-years), and re-engaging individuals LTFU to care (every 5-years) will substantially increase the number screened (Ab/RNA testing) annually to 36.4 [35.1–37.8] million. Overall, 139.7% [135.5–144.6%] of the population in 2018 are screened by 2030 and 109.0% [107.0–111.4%] of the chronic infections in 2018 are cured, with 660,000 [595,000–735,000] being treated annually. Incidence and mortality decrease by 84.8% [79.7–87.4%] and 52.1% [43.0–59.3%], respectively, reaching a 65% reduction in mortality by 2035 (Supplementary Table S6 and Supplementary Figure S7). Supplementary Figure S8 shows that the doubling in primary screening rate and the increase in referral have most benefit.
Figure 2.
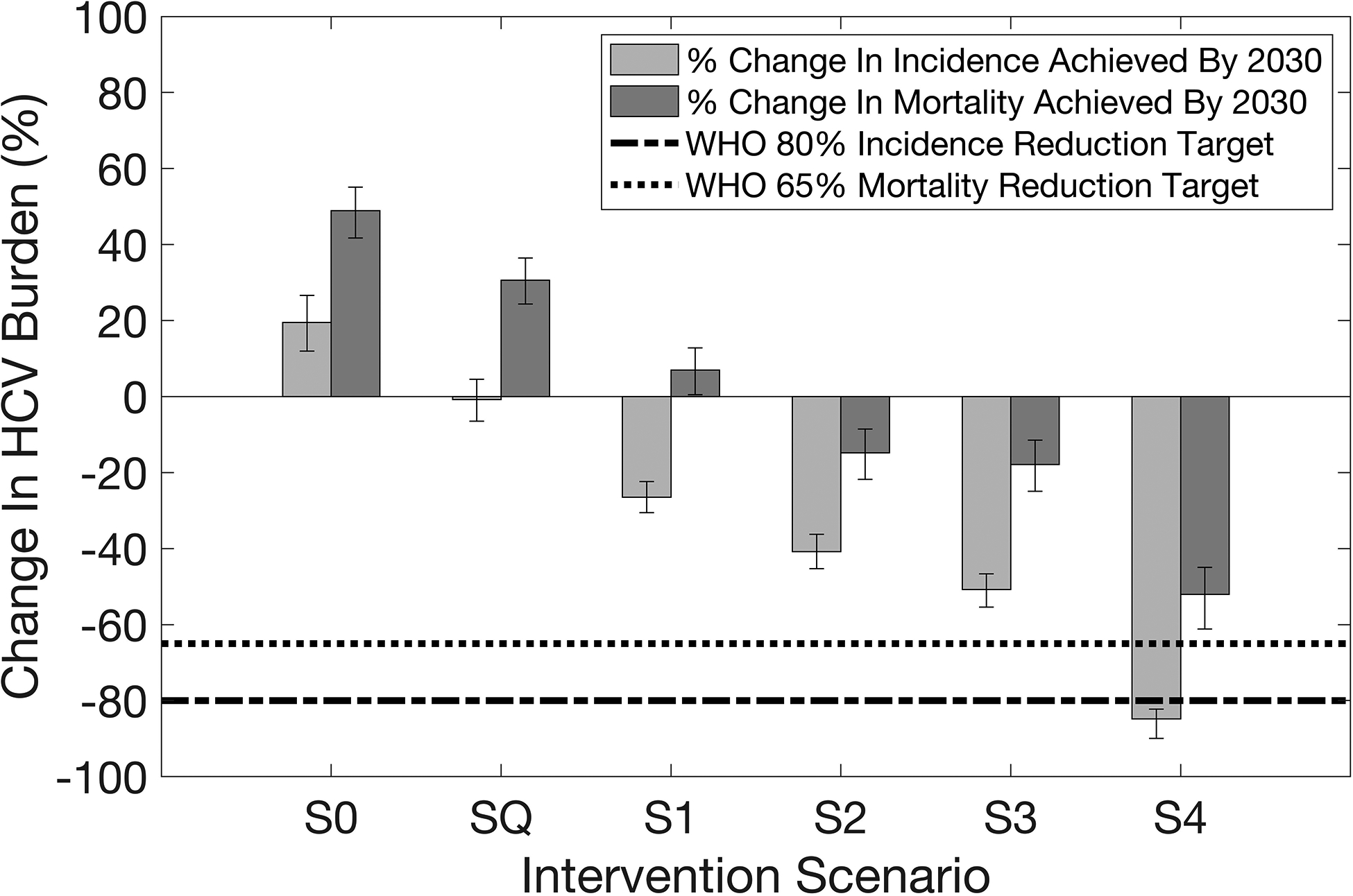
Relative change in incidence and mortality achieved by 2030 compared to 2015 levels for each screening intervention scenario. Intervention scenarios are as follows. Scenario S0: No screening or treatment from 2018 onwards. Scenario SQ: Maintaining status quo treatment of ~150,000 annual treatments from 2018. Scenario S1: One-time screening 90% of the general population by 2030 with 80% referral to care. Scenario S2: One-time screening as in Scenario S1, with prioritisation for PWID and adults (30+ years). Scenario S3: One-time prioritised screening as in Scenario S2, along with re-screening cured and previously Ab-negative/RNA-negative individuals from 2020 (every ten years for non-PWID and annually for PWID). Scenario S4: Scenario S3 with incremental improvements as described in the text. The height of each bar represents the median of 1,151 final model runs, with whiskers indicating 95% uncertainty intervals of runs.
Figure 3.
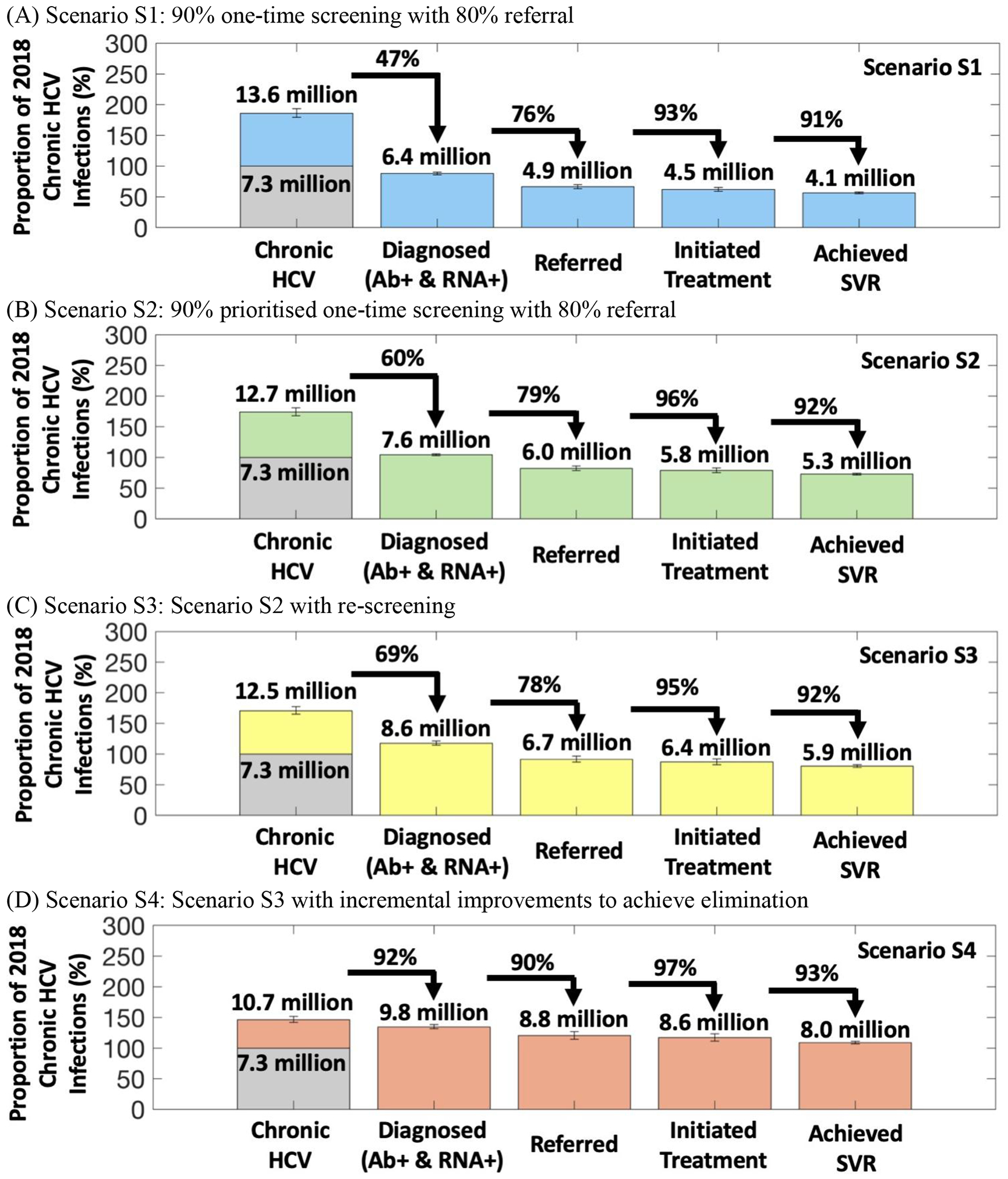
The cascade of care for Scenarios S1–S4. The heights of each bar show the proportions (numbers above each bar) that are diagnosed, referred, initiated treatment, and achieved SVR relative to the chronic HCV burden in 2018 in the first bar corresponding to 100% (shaded in grey). The full height of the first bar signifies the full burden of HCV infections over 2018–2030 for each scenario which is, specifically, the sum of the chronic HCV burden in 2018 with all new chronic infections that occur from 2018 until 2030 in that scenario. The transitions between each bar indicate the percentage of the previous step in the cascade of care that moves onto the following step. The height of each bar represents the median of 1,151 final model runs, with whiskers indicating 95% uncertainty intervals of runs.
For Scenarios S1–S4, the model suggests that one person will be treated for every 33–57 screening tests undertaken, with Scenario S2 achieving the lowest tests per treatment (Supplementary Figure S9).
Costs of improving cascade of care
For Scenarios S1–S3, the median estimated costs of the different intervention scenarios over 2018–2030 ranged from $3.4–5.1 billion (Figure 4a), with the cost increasing for each successive scenario and 50–60% of costs being due to screening. To reach the WHO elimination target for incidence, Scenario S4 increases the total screening and treatment costs by two-thirds (compared to Scenario S3) to $8.1 [7.7–8.5] billion over 2018–2030. The cost per cure ranges from $900–1,200 (Figure 4b) and is cheapest for Scenario S2.
Figure 4.
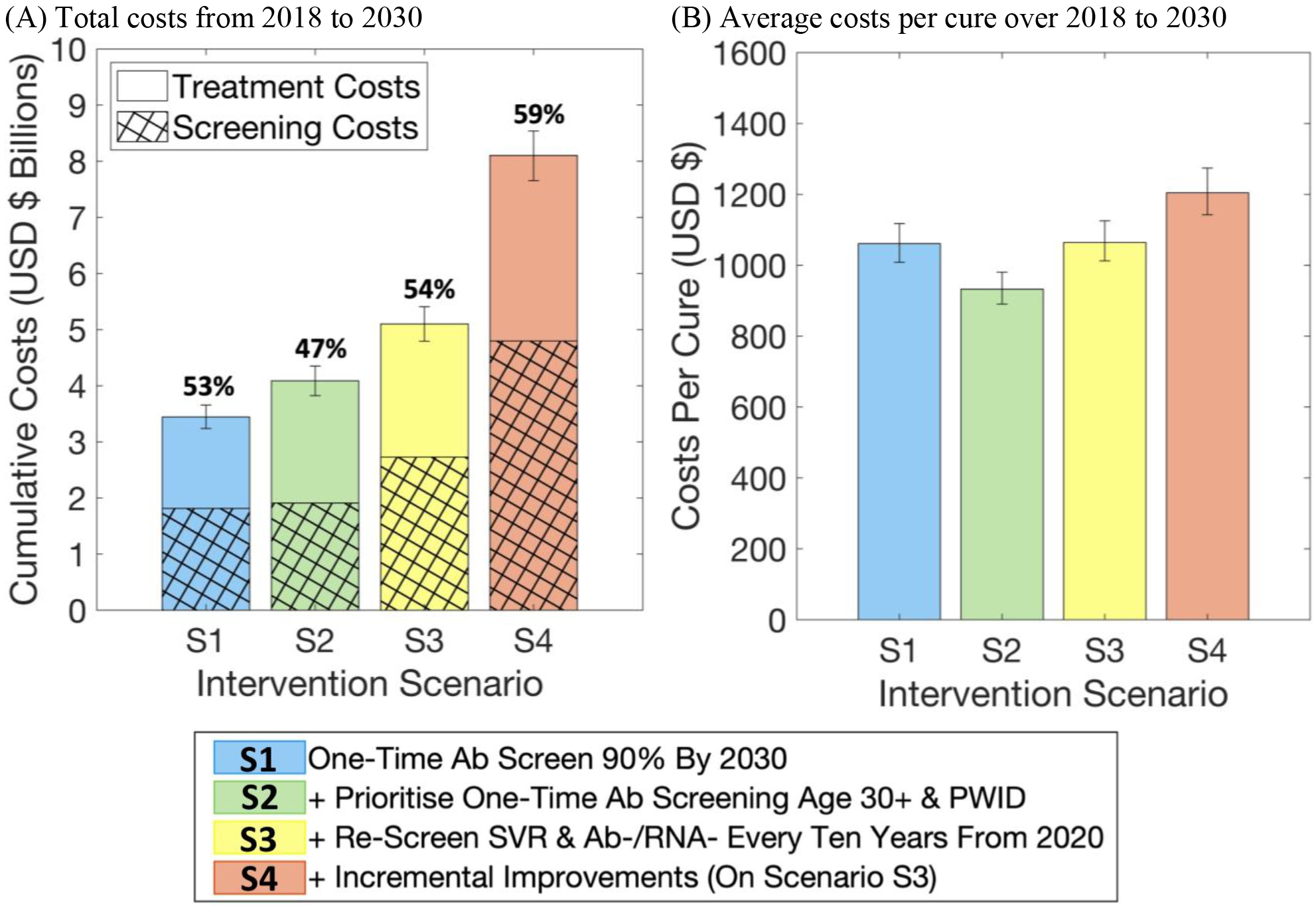
Estimated (A) total screening and treatment costs, and (B) average costs per cure, for each intervention scenario over 2018–2030. Costs and outcomes are discounted at a standard rate of 3.5% per year. The percentages above each bar in (A) indicate the proportion of total costs that are due to screening.
Sensitivity analysis
Our sensitivity analyses (Figure 5) show that reducing the costs of either diagnostics test kits or DAA regimens to the lowest available price could each reduce total costs by 10% ($7.2 billion) separately, while including savings in healthcare costs could reduce total costs by 13% ($7.0 billion). Including costs for improving referral and re-engaging patients LTFU is likely to have minimal effect on total costs (<1% change). Importantly, implementing a simplified screening and treatment pathway could have a considerable effect, reducing the overall budget for eliminating HCV by 16% ($6.7 billion), while combining these effects could reduce costs further to $3.9 billion by 2030. This results in $600 per cure. Changing the discount rate to either 0% or 7% varies the total costs by roughly +/−20%. Lastly, further reductions in costs of test kits and DAAs, possibly due to bulk purchasing, would only decrease costs marginally, with a 25% reduction in both only decreasing overall costs to $3.7 billion (Supplementary Figure S13).
Figure 5.
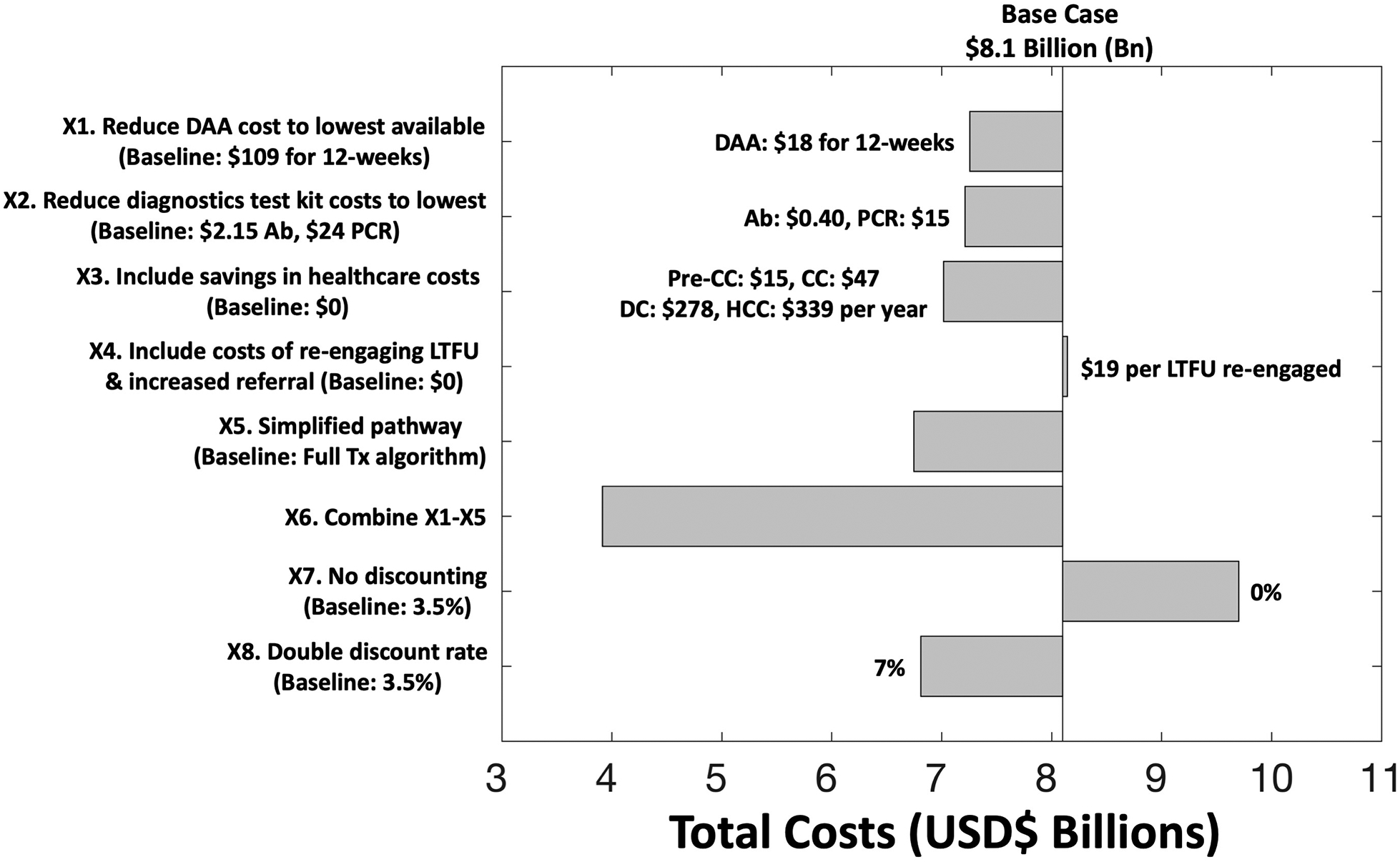
Univariate sensitivity analysis on total costs for Scenario S4. Implementing a simplified treatment pathway as in X5 includes fewer visits and laboratory investigations. Details are in Supplementary Materials. DAA: direct-acting antiviral; Ab: antibody; PCR: polymerase chain reaction; CC: compensated cirrhosis; DC: decompensated cirrhosis; HCC: hepatocellular carcinoma; LTFU: lost-to-follow-up; Tx: treatment.
Discussion
Considerable HCV screening and effective referral to treatment is needed to achieve the WHO elimination target for HCV incidence in Pakistan. Indeed, due to population growth and the expanding epidemic in Pakistan, our projections suggest that 140% of the current Pakistan population, or 278 million people, will need to be screened for HCV over 2018–2030, 90% of diagnosed individuals will need to be linked to treatment, interventions will need to re-engage individuals lost-to-follow-up, and regular re-screening will need to identify new (re-)infections. Although very cheap treatments and diagnostics are available in Pakistan (cheapest DAA price being $18 per 12-week treatment course), our estimates suggest that at its cheapest, this screening and treatment strategy will cost US$3.9 billion over 13 years, with the yearly costs making up 9.0% of the annual health budget of Pakistan.14 This translates to about $600 per cure.
Irrespective of the approach chosen, substantial improvements in the cascade of care for HCV are needed in Pakistan. Firstly, primary Ab screening rates need to be high to identify prevalent and incident infections. The yield of this screening can be improved through prioritising higher prevalence sub-groups or using other risk-based criteria for deciding whom to screen.8 However, it is important that such risk-based screening algorithms are evaluated before being deployed to ensure they capture most infections. Secondly, referral rates need to be high, as demonstrated by Egypt’s national testing and treatment programme, which has treated 88% of all diagnosed patients (https://www.who.int/hepatitis/news-events/egypt-hepatitis-c-testing/). Development of improved diagnostics, such as point-of-care (POC) tests for active HCV infection, could help with this through simplifying the pathway from HCV diagnosis to treatment23,24, as achieved with HIV POC testing in LMIC settings.25 Similarly, simplifying the treatment pathway in other ways and using incentives or nurse facilitators to reduce LTFU could also improve the referral to treatment.26,27 Lastly, because of continued exposure risk in the community, repeat screening is needed to identify (re-)infections. This is likely to incur substantial additional costs unless it can be targeted to those with identified risk.
Crucially, this work emphasises the immense effort and likely financial burden of a national HCV-elimination initiative in Pakistan. Our analyses show that a ‘screen-all’ approach will be needed, which will require improving access to screening for all patient subgroups, including marginalised subpopulations such as PWID or men who have sex with men (MSM), as well as patients with ESLD.28
Strengths and limitations
The main strength of our study is that, to our knowledge, it is the first to utilise detailed dynamic modelling to undertake a cost-impact analysis of what is needed to achieve the WHO HCV-elimination targets in a LMIC. In the Pakistan context, our cost estimates use local cost data based on real pathways of care to derive realistic cost projections for the actual implementation of screening and treatment in this setting. These budgetary estimates provide a basis to assess the economic feasibility of undertaking a large-scale HCV screening and treatment programme for achieving HCV elimination, which is crucial information for helping governments and other decision-makers. The modelling presented in this paper has fed into discussions with the Government of Pakistan who, on World Hepatitis Day 2019, announced a new Prime Minister’s Programme declaring their commitment to combating hepatitis C (https://www.who.int/hepatitis/news-events/pakistan-hepatitis-elimination-plan/en/).
Nevertheless, a number of limitations exist. First, we did not include the added costs of improving the infrastructure in Pakistan to enable country-wide HCV screening. Second, to estimate the cost savings related to prevented healthcare provision for HCV-associated morbidities, we adapted costs from Cambodia (unpublished data; Mafirakureva et al.) as data for Pakistan were not available. This was shown to offset 13% of the screening and treatment costs, emphasising the importance of obtaining local data to validate these estimates. Third, except for injecting drug use (IDU), we did not include any other risk-based stratifications in the model, and so it was not possible to properly evaluate risk-based screening or re-screening, which could be more efficient than the screening scenarios we modelled. Moreover, although the model likely captures the main characteristics of how IDU contributes to overall levels of HCV transmission in Pakistan, added detail could be included on variations in the age that people initiate IDU and their duration of injecting. Fourth, further modelling could also consider the impact and potential costs of scaling up prevention interventions, which preliminary projections (not shown) suggest could dramatically reduce the screening and treatment required for achieving HCV elimination, especially if the interventions are effective for the general population. However, evidence for the cost and effectiveness of suitable general population interventions do not exist, and so we did not include this scenario in our analyses. Fifth, we did not estimate the screening requirements and costs of achieving the WHO elimination target for mortality by 2030. In our analyses, even the most aggressive intervention (Scenario S4) only reached the 65% mortality reduction target by 2035, possibly suggesting that the mortality target may not feasible by 2030. Lastly, in Pakistan, healthcare decisions are made at the provincial, rather than national level following the devolution of the national programme in 2010.9 More detailed regional models are needed to determine geographical differences in the screening and treatment strategies needed in Pakistan’s highly variable epidemics.
Comparison with other studies
We have previously examined the required treatment scale-up needed to reach the WHO elimination targets in Pakistan.5 This analysis builds upon our previous work by determining the screening requirements and likely costs of achieving these targets.
Few studies have examined the impact and budgetary implications of HCV screening and treatment interventions in LMICs. A test and treat demonstration project evaluated the impact and cost-effectiveness of HCV micro-elimination in rural Egypt.29 An analysis for South Africa considered the budgetary requirements for scaling-up HCV screening and treatment,30, while analyses in Eastern Europe and Central Asia evaluated the cost-effectiveness of intervention packages for reducing HCV transmission among PWID.31 However, only the Egypt study used real context-specific cost data, while none incorporated the cascade of care into their models nor estimated the level of screening and treatment needed for HCV elimination. Moreover, the two analyses considering general population HCV epidemics did not account for population growth or the effect of interventions on ongoing HCV transmission, both being key factors characterising the HCV epidemic in Pakistan and other LMICs.
One other study considered the cost of achieving the HCV elimination target for mortality in Pakistan.32 However, their projections were based on conservative assumptions for the number of incident HCV infections (280,000 per year in 2018 instead of 650,000 in our model) occurring in Pakistan, likely due to them not modelling an increasing epidemic or growing population; both of which are important characteristics of the Pakistan epidemic.5,33 Also, their healthcare cost estimates were adapted from US data and staff costs were not included in their costs of treatment, meaning their cost estimates may not reflect the real costs in a Pakistan context. Our study improves on this analysis through using primary cost data from Pakistan and detailed epidemiological data to calibrate the increasing HCV epidemic in Pakistan.
Conclusions
Pakistan is a crucial target country for achieving global HCV-elimination because it harbours one-tenth of the global HCV-burden. Our findings suggest that considerable scale-up of screening and treatment interventions will be required at substantial cost to achieve the WHO HCV-elimination targets in Pakistan. Estimated annual screening and treatment costs could translate to around 9.0% of the current health expenditure of Pakistan, with this equating to 0.11% of their GDP in 2017–2018, or approximately $1.50 per person per year.14 The government of Pakistan has already made significant progress in initiating HCV prevention and control programmes9, and although political will exists9,33, greater health sector investment is required to effectively tackle the growing HCV-burden in Pakistan. Although specific to Pakistan, the insights from this study are applicable to other resource-limited settings with a high prevalence of HCV. Our analyses show that although the costs of achieving HCV elimination can be substantial, these can be reduced dramatically through improving accessibility to cheaper drugs and diagnostics tests, and developing simplified screening and treatment algorithms. This gives pointers for informing how other countries should work towards scaling-up HCV screening and treatment. To achieve global HCV-elimination, it is of paramount importance to tackle the HCV epidemics in settings such as Pakistan that have a high-burden of HCV – widespread intervention scale-up and resource investment cannot be delayed.
Supplementary Material
Table 2.
Model parameters for each screening and treatment intervention scenario for 2018 onwards.
| Ab screening & re-screening | RNA re-screening of known Ab+ status | Referral | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention scenario | Primary Ab screening rate‡ | Re-screening rate of SVR and previously screened uninfected‡ | Previously treated | Rate previously diagnosed LTFU linked back to care | % diagnosed HCV infections linked to treatment | |||
| All | Gen.b | PWID | Gen. | PWID | Gen. | PWID | All | |
| Scenario S0. No further treatment from 2018 | -- | -- | -- | -- | -- | -- | -- | -- |
| Scenario SQ. ~150,000–160,000 treatments/year | 2.6–5.9% | -- | -- | -- | -- | -- | -- | 35–70% |
| Scenario S1. One-time 90% screen by 2030 with 80% referreda | 6.2% [6.1–6.3%] |
-- | -- | -- | -- | -- | -- | 80% |
| Scenario S2. S1+ Target primary Ab screening Age 30+ & PWID | 6.2% [6.1–6.3%] |
-- | -- | -- | -- | -- | -- | 80% |
| Scenario S3. S2+ Re-screen SVR & Ab/RNA- from 2020 | 6.2% [6.1–6.3%] |
10% | 100% | 10% | 100% | -- | -- | 80% |
| Scenario S4. S3+ Incremental improvements* | 12.4% [12.1–12.6%] |
20% | 100% | 20% | 100% | 20% | 100% | 90% |
A 6.2% [6.1–6.3%] annual primary screening rate is equivalent to first-time Ab screening 180 [175–185] million individuals, or 90% of the 2018 population, by 2030. Doubling this to a 12.4% [12.1–12.6%] annual primary screening rate is equivalent to first-time Ab screen 280 [265–290] million individuals, or 140% of the 2018 population, by 2030.
Gen: General population rate for non-PWID groups.
Incremental improvements to Scenario S3, namely, increase referral to 90%, double primary Ab screening rate, re-screening every 5 years, and re-engage RNA+ LTFU.
We assume that all persons tested Ab-positive, either from primary Ab screening (ψ1) or Ab re-screening (ψ2), are subsequently tested for HCV RNA, i.e. there is no loss-to-follow-up at this stage
Research in context.
Evidence before this study
We searched PubMed for articles published on or before 31st August 2019, with ((“HCV” OR “hepatitis c virus” OR “hep c”) AND (“mathematical” OR “dynamic” OR “transmission”) AND (“model” OR “models” OR “modelling” OR “modeling”) AND (“case-finding” OR “screening” OR “treatment”) AND (“cost” OR “costs” OR “costing”)). Many HCV modelling studies have focussed on projecting the impact and economic implications of testing and treatment interventions in specific subgroups, including people who inject drugs (PWID), men who have sex with men (MSM), and prisoners. A dynamic modelling approach investigated the impact and cost-effectiveness of intervention packages to reduce HIV and HCV infections among PWID in Eastern Europe and Central Asia, but did not consider levels of interventions that would reach elimination. Other studies were conducted in high-income country settings, or utilised static models (e.g. Markov models) and so could not consider levels of treatment needed to reduce incidence. Only three studies presented cost-effectiveness or budget analyses of general population HCV screening and treatment interventions in low- or middle-income countries (LMIC), namely, Egypt, South Africa, and Pakistan. However, none of these studies used a dynamic modelling approach, nor did they consider the budgetary requirements for reducing country-level HCV incidence to WHO-advocated elimination levels. Moreover, the previous Pakistan analysis did not use locally collected cost data, which resulted in optimistic cost projections that are less representative of the Pakistan context.
Added value of this study
To our knowledge, this study is the first country-level estimation of the HCV screening and treatment requirements, and associated costs for achieving the WHO HCV elimination incidence targets in a generalised epidemic LMIC setting, specifically Pakistan. The realism of our projections is maximised through calibrating a detailed model to context specific data from Pakistan, and in using real-world costs of screening and treatment for Pakistan. Our results provide valuable and practical new information on how each stage of the cascade of care for HCV needs to be improved to achieve the WHO HCV-elimination target for incidence in Pakistan, and the costs of doing so. This includes ensuring effective referral to treatment following diagnosis, re-engaging individuals lost to follow up, and regular re-screening to identify new (re-)infections. Implementing effective HCV screening strategies is also paramount, with our findings suggesting that prioritised screening of population subgroups with higher prevalence of HCV, such as adults and PWID, can improve the efficiency and impact of screening. These considerations are likely to have substantial impact on optimising the costs of achieving the WHO HCV elimination targets, which our projections suggest will still be very large even with the cheapest available drug regimens and diagnostics. Importantly, though, our projections also show that substantial savings can be achieved through simplifying the screening and treatment pathway, a consideration that is relevant not only to Pakistan, but also to other LMIC settings.
Implications of all the available evidence
This study directly addresses the feasibility of eliminating HCV at a country level, a pre-requisite to achieving global elimination as set out by the WHO. To achieve this aim, it is crucial to achieve a high screening coverage of the whole population while strengthening all elements of the care continuum to ensure a high proportion are effectively cured. Meanwhile, to minimise costs, screening strategies should prioritise testing and re-testing to those with transmission potential and focus on maintaining high referral rates to ensure that diagnosed persons are adequately linked to care and initiated on treatment.
Acknowledgements:
PV, AGL, NKM, JGW and NM acknowledge funding from UNITAID for this work. NKM acknowledges funding from the National Institute for Drug Abuse (R01 DA03773), the National Institute for Allergy and Infectious Diseases (R01 AI147490), and the University of San Diego Center for AIDS Research (CFAR), a NIH funded program (P30 AI036214). PV and MH acknowledge support from NIHR Health Protection Research Unit in Evaluation of Interventions (HPRU EI). The authors would like to thank six anonymous reviewers, whose constructive feedback has improved the clarity and exposition of the manuscript. All modelling has been done in collaboration with Médecins San Frontières (MSF), the Pakistan HCV Task Force, and the US Centers for Disease Control and Prevention, Division of Viral Hepatitis (CDC-DVH).
Footnotes
Declaration of Interests: NKM has received unrestricted research grants and honoraria from Gilead and Merck unrelated to this work. PV has received unrestricted research grants and honoraria from Gilead, and honoraria from Abbvie.
References
- 1.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. (2016).
- 2.Graham CS & Swan T A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Res. 119, 89–96 (2015). [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Hepatitis Report, 2017. (2017).
- 4.Gower E, Estes C, Blach S, Razavi-Shearer K & Razavi H Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol 61, S45–57 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Lim AG et al. Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol 47, 550–560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibley A et al. The present and future disease burden of hepatitis C virus infections with today’s treatment paradigm - volume 3. J Viral Hepat 22 Suppl 4, 21–41 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Qureshi H, Bile KM, Jooma R, Alam SE & Afridi HU R. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East. Mediterr. Health J 16 Suppl, S15–23 (2010). [PubMed] [Google Scholar]
- 8.Trickey A et al. Importance and Contribution of Community, Social, and Healthcare Risk Factors for Hepatitis C Infection in Pakistan. The American Journal of Tropical Medicine and Hygiene (2017). doi: 10.4269/ajtmh.17-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of National Health Services, Regulations and Coordination (NHSRC), Pakistan. National Hepatitis Strategic Framework (NHSF) for Pakistan 2017–21; 1–63 (2018). [Google Scholar]
- 10.Mathers BM et al. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull. World Health Organ 91, 102–123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westbrook RH & Dusheiko G Natural history of hepatitis C. J. Hepatol 61, S58–68 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Micallef JM, Kaldor JM & Dore GJ Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 13, 34–41 (2006). [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. GUIDELINES FOR THE CARE AND TREATMENT OF PERSONS DIAGNOSED WITH CHRONIC HEPATITIS C VIRUS INFECTION. 1–108 (2019). [PubMed] [Google Scholar]
- 14.Finance Division, Government of Pakistan. Pakistan Economic Survey 2017–18. (2018).
- 15.United Nations, Department of Economic and Social Affairs, Population Division. United Nations World Population Prospects: The 2015 Revision. (2015). Available at: https://esa.un.org/unpd/wpp/. (Accessed: 30 June 2016)
- 16.Nelson PK et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 378, 571–583 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United Nations Office on Drugs and Crime. Drug Use in Pakistan 2013. (2013). Available at: http://www.unodc.org. (Accessed: 30 June 2016)
- 18.Umar M & Bilal M Hepatitis C, a mega menace: a Pakistani Perspective. J Pioneer Med Sci (2012). [Google Scholar]
- 19.Khaliq S & Raza SM Current Status of Direct Acting Antiviral Agents against Hepatitis C Virus Infection in Pakistan. Medicina (Kaunas) 54, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naveed A et al. Progress on scaling up testing and treatment for hepatitis C elimination in Punjab, Pakistan: Hepatitis prevention and treatment program. J Hepatol 70, e336 (2019). [Google Scholar]
- 21.Capileno YA et al. Management of chronic Hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan. PLoS ONE 12, e0175562 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalid GG et al. From risk to care: the hepatitis C screening and diagnostic cascade in a primary health care clinic in Karachi, Pakistan-a cohort study. Int Health (2018). doi: 10.1093/inthealth/ihy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freiman JM et al. Hepatitis C Core Antigen Testing for Diagnosis of Hepatitis C Virus Infection: A Systematic Review and Meta-analysis. Ann. Intern. Med 165, 345–355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heffernan A et al. Aiming at the Global Elimination of Viral Hepatitis: Challenges Along the Care Continuum. Open Forum Infect Dis 5, ofx252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govindasamy D et al. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings--a systematic review. J Int AIDS Soc 17, 19032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edlin BR et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin. Infect. Dis 40 Suppl 5, S276–85 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris M et al. Understanding hepatitis C intervention success-Qualitative findings from the HepCATT study. J Viral Hepat 25, 762–770 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Torres HA et al. The oncologic burden of hepatitis C virus infection: A clinical perspective. CA Cancer J Clin 67, 411–431 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiha G et al. An educate, test, and treat programme towards elimination of hepatitis C infection in Egypt: a community-based demonstration project. The Lancet Gastroenterology & Hepatology (2018). doi: 10.1016/S2468-1253(18)30139-0 [DOI] [PubMed] [Google Scholar]
- 30.Hecht R et al. The investment case for hepatitis B and C in South Africa: adaptation and innovation in policy analysis for disease program scale-up. Health Policy Plan 33, 528–538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabileau G et al. Intervention Packages to Reduce the Impact of HIV and HCV Infections Among People Who Inject Drugs in Eastern Europe and Central Asia: A Modeling and Cost-effectiveness Study. Open Forum Infect Dis 5, ofy040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chhatwal J et al. Assessment of the Feasibility and Cost of Hepatitis C Elimination in Pakistan. JAMA Netw Open 2, e193613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of National Health Services, Regulations and Coordination (NHSRC), Pakistan. National Health Vision Pakistan 2016–2025. 1–26 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


