Abstract
Background:
Twin pregnancy is an iatrogenic complication following in vitro fertilization (IVF) that can be decreased using elective single-embryo transfer. However, the risks associated with twin pregnancy following IVF as compared to singleton IVF pregnancy need to be further evaluated.
Aim:
This study aims to compare the maternal, perinatal, and neonatal complications in singleton and twin pregnancies following IVF-intracytoplasmic sperm injection (ICSI). Settings and Design: Retrospective observational cohort study using previously collected routine patient data.
Materials and Methods:
Singleton and twin deliveries following IVF/ICSI from January 2014 to August 2015 were included. Data were collected from patient records and the obstetricians of the patients.
Statistical Analysis Used:
SPSS was used for analysis. Student's t-test and Fisher's exact test were used for continuous and categorical data, respectively. Significance was kept at 0.05.
Results:
There were 897 singleton and 382 twin deliveries (total of 1661 babies). The mean gestational age at delivery was lower in twin deliveries (34.9 ± 3.1 weeks) as compared to singleton deliveries (36.8 ± 3.2 weeks, P < 0.001). The overall incidence of maternal complications was higher in twin pregnancies (29.3% vs. 21.3%, odds ratio = 1.53, 95% confidence interval = 1.17–2.01; P = 0.003). The mean birth weight of babies was significantly lower (2.02 ± 0.58 kg vs. 2.71 ± 0.68 kg; P < 0.001) and the incidence of stillbirth plus neonatal death was higher (7.5% vs. 4.6%, P = 0.01) in the twin group as compared to the singleton group.
Conclusion:
Twin deliveries, following IVF/ICSI deliver at lower gestational age, have lower birth weight and have higher odds of stillbirth plus neonatal death as compared to singleton deliveries following IVF/ICSI.
KEYWORDS: In vitro fertilization, maternal complications, neonatal complications, singleton delivery, twin delivery
INTRODUCTION
During the past four decades, the incidence of twin pregnancies has increased worldwide.[1] This increase is mainly attributable to increased use of fertility drugs and assisted reproductive technology (ART).[2,3] European registries have reported the incidence of multiple deliveries after ART to be 18%.[4] In the US, this incidence was even higher at over 39.4% of all infants delivered with ART, compared to only 3.5% among the total birth population.[5] This increased prevalence of twin (or multiple) gestation in in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles is a result of multiple embryo transfer to improve the chances of a pregnancy.
Twin pregnancies are known to be at a significantly higher risk of maternal as well as perinatal complications after natural conception,[6,7,8,9] and it is expected that these would be associated with increased risk in ART-associated pregnancies as well. Twin pregnancy following ART is considered an iatrogenic complication that can be prevented by limiting the number of embryo transfer. Many countries now promote elective single-embryo transfer (eSET) since the odds of a multiple live birth with eSET are 2% as compared to 29% with double embryo transfer (DET).[10]
Although singleton IVF pregnancy has been proven to carry more risk than a singleton naturally conceived pregnancy, the evidence is limited to derive the same conclusion in the case of twin pregnancies; in fact, the risks from IVF twin pregnancies have been shown by at least some of the studies to be comparable to (or not significantly different from) that of a naturally conceived twin pregnancy.[11,12,13,14],15,16 Another study comparing the ultrasonographic findings in twin and singleton pregnancies following assisted reproduction found larger placental volume in twins, suggesting a higher production of placental hormones that would better support an early twin pregnancy, compared with a singleton pregnancy.[17]
Keeping these studies into consideration, it is difficult to infer indirectly how much higher the risk in twin IVF pregnancies is, compared to singleton IVF pregnancies. Further, it is also important to note that eSET is shown to have a lower pregnancy rate (odds ratio [OR] = 0.5) than DET, particularly in women older than 33 years.[10] Since a large proportion of women that seek IVF treatment are older, this assumes significance. Thus, it becomes important to have data on the extent by which the twin IVF gestation increases the maternal and perinatal risk as compared to a singleton IVF delivery. This information is required for prognostication of patients since the patient would want to know the reduction in maternal and perinatal risks expected from a singleton IVF pregnancy in place of a twin IVF pregnancy when she chooses the less effective eSET over DET.
Globally, there are very few studies that have directly compared the outcomes from singleton and twin pregnancies following ART,[5,18] and none on Indian population. Most authors reporting on IVF deliveries have stopped just short of directly comparing these two groups.[15,19]
The objective of this study was to compare the maternal, perinatal, and neonatal complications in singleton versus twin pregnancies conceived following IVF/ICSI. The hypothesis that was tested was that these complications are higher in IVF twin pregnancies as compared to IVF singleton pregnancies.
MATERIALS AND METHODS
Study design and setting
This was a retrospective observational cohort study conducted at an infertility center located in Ahmedabad, India. It was conducted on previously collected (archival) routine patient data, i.e. the data had been collected prior to the conception of the study.
Inclusion criteria
All patients who underwent IVF/ICSI at our center between January 1, 2014, and August 31, 2015 (the study period), who subsequently had a singleton or a twin pregnancy (more than 20 weeks of gestation).
Exclusion criteria
Exclusion criteria were nonavailability of follow-up data till 1 month age of the newborn, or till the end result of pregnancy in case of stillbirth, and patients with abortion before 20 weeks of gestation.
Data collection
Archival patient data, including follow-up data that had already been collected routinely in the center, was used for performing this study. These data had been collected previously using two approaches: (a) Post-ART treatment and detection of clinical pregnancy, at the time of referral of the patient to the obstetrician, the latter was provided with a pregnancy monitoring pro forma which included maternal, perinatal, and neonatal outcomes, and this pro forma was to be sent to the center within a month of delivery and (b) the patients were also directly contacted for the follow-up information. This dual data collection strategy is followed in our center to ensure the availability of complete data as well as reduction of related biases for any future studies.
Assisted reproductive technology protocol and ovarian stimulation used in the patients
All the patients underwent controlled ovarian hyperstimulation with the flexible antagonist protocol. Ovarian stimulation was started from day 2 or 3 of the menstrual cycle, after confirming the baseline scan, i.e., when there is no follicular cyst >10 mm and endometrial thickness <6 mm. Gonadotropins were started in a dose between 150 IU and 450 IU according to age, antral follicle count, anti-Mullerian hormone, body mass index, and previous cycle response. Antagonist 0.25 mg cetrorelix was added once the leading follicle attained a size of 14 mm as per flexible antagonist protocol. When two or more follicles reached 17 mm, the final oocyte maturation was triggered with 250 mcg of recombinant human chorionic gonadotropin (hCG). If there was a risk of ovarian hyperstimulation syndrome, suggested by the presence of more than 15 follicles of size larger than 15 mm, or when the serum progesterone was more than 1.5 ng/ml, then 0.2 mg of triptorelin was used as a trigger instead of hCG. Ovum pick up was planned 35 h after the trigger. A maximum of two embryos were transferred either on day 3 or day 5. Luteal support was given vaginally with 400 mg vaginal micronized progesterone twice daily for 14 days after embryo transfer. Surplus good quality embryos were vitrified.
For fresh oocyte/embryo recipient cycles and vitrified and warmed embryo transfer cycles, hormone replacement therapy was started with estradiol valerate from day 2 of the menstrual cycle in escalating dose of 4 mg to 8 mg after a normal baseline sonography. Once the endometrium reached 7 mm or more with triple-layer echogenicity, serum progesterone was measured. Transvaginal micronized progesterone 400 mg was administered twice daily if the serum progesterone was < 0.5 ng/ml. A maximum of two embryos were transferred either on days 3 or 5 post progesterone treatment.
Serum beta-hCG was measured 14 days after embryo transfer and if it was found to be >20 mIU/ml, transvaginal sonography was carried out 1 week later to confirm the pregnancy and the number of gestational sacs. Once the gestational sac was visible on sonography, all the patients were referred to an obstetrician of their own choice for further antenatal care and delivery, as obstetric care is not provided at the center. Patients were provided with a format of standard antenatal care (frequency of monitoring, medications, ultrasound monitoring, etc.).
Outcomes assessed
Pregnancy outcomes and frequency of maternal, perinatal, and neonatal complications were compared between singleton and twin groups (the two study arms). The maternal complications that were specifically assessed included preeclampsia, premature rupture of membranes (PROM), preterm labor, gestational diabetes mellitus (GDM), antepartum hemorrhage, postpartum hemorrhage, and liquor abnormalities including oligohydramnios and polyhydramnios. The perinatal outcomes that were recorded were prematurity, birth weight, stillbirths, neonatal deaths, and congenital anomalies. For maternal outcomes, the denominator was the number of pregnancies, and for perinatal outcomes, the denominator was the number of babies.
Subgroup analysis
Patients were divided into four subgroups based on the treatment: fresh embryo transfer using self-oocytes, fresh embryo transfer using donor oocytes, vitrified warmed cycles using self-oocytes, and vitrified warmed cycles using donor oocytes. The outcomes following singleton deliveries were then compared with twin deliveries in each of these four subgroups.
Statistical analysis
Continuous variables were expressed as means ± standard deviation, and Student's t-test was used for between-group comparisons. The incidences and categorical data were analyzed using the Fisher's exact test and ORs along with 95% confidence intervals (CIs). Two-tailed P < 0.05 was considered to be statistically significant. SPSS (IBM, New York, United States) was used for all analysis.
STROBE statement was followed for reporting of this manuscript.
RESULTS
Baseline characteristics
Out of a total of 3637 embryo transfers conducted during the study, there were 1279 pregnancies that extended beyond 20 weeks' gestation (35.2%). These 1279 pregnancies formed the sample size for this study. The follow-up period was as chosen in the study design and was available for all these patients in the datasheet. Of these, there were 897 singleton deliveries and 382 multiple-birth deliveries. None of the patients had a transfer of more than two embryos. There were 11 patients with dichorionic triamniotic pregnancies, out of which three had missed abortion and rest eight underwent fetal reduction. As a result, all multiple-birth deliveries were twin deliveries (764 babies) only. Thus, in total, there were 1661 babies.
There were 1760 embryo transfers with fresh self-cycles, of which a total of 394 pregnancies extended beyond 20 weeks. Of these, 28.9% were twin pregnancies. There were 1033 embryo transfers with fresh recipient cycle, and 37.5% of these had pregnancy extending beyond 20 weeks. There were 272 singletons and 103 twin deliveries with thaw self-cycle, and 91 singleton and 32 twin deliveries with thaw recipient cycles.
The distribution of maternal age, gestational age, lower segment cesarean section (LSCS), and maternal complications are represented in Table 1. Patients were also stratified with respect to the treatment groups. The mean maternal age was comparable between the singleton and twin groups, overall as well as in each of the four treatment subgroups. The vast majority of deliveries were through LSCS.
Table 1.
Comparison of maternal age, gestational age at delivery, rate of lower segment cesarean section, and incidence of maternal complications between singleton and twin pregnancies
| Statistics | Fresh self |
Fresh recipient |
Thaw self |
Thaw recipient |
All treatment groups combined |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single (n=280) | Twins (n=114) | Single (n=254) | Twins (n=133) | Single (n=272) | Twins (n=103) | Single (n=91) | Twins (n=32) | Single (n=897) | Twins (n=382) | ||
| Maternal age (years) | Mean±SD | 30.2±3.3 | 29.7±3.2 | 36.7±5.4 | 35.6±5.4 | 30.0±3.5 | 29.8±3.3 | 36.1±4.9 | 34.1±5.5 | 32.6±5.3 | 32.1±5.1 |
| P | 0.26 | 0.04* | 0.65 | 0.05 | 0.17 | ||||||
| Gestational age at delivery (weeks) | Mean±SD | 37.2±2.5 | 35.0±3.3 | 36.5±3.0 | 35.0±2.9 | 36.7±3.7 | 34.8±3.2 | 36.4±3.6 | 34.3±3.0 | 36.8±3.2 | 34.9±3.1 |
| P | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | ||||||
| LSCS | n (%) | 253 (90.4) | 107 (93.9) | 240 (94.5) | 132 (99.3) | 256 (94.1) | 97 (94.2) | 85 (93.4) | 32 (100) | 834 (93.0) | 368 (96.3) |
| P | 0.26 | 0.02* | 0.98 | 0.34 | 0.02* | ||||||
| Maternal complications | n (%) | 53 (18.9) | 26 (22.8) | 65 (25.6) | 41 (30.8) | 57 (20.9) | 32 (31.0) | 16 (17.6) | 13 (40.6) | 191 (21.3) | 112 (29.3) |
| OR (95% CI) | 1.27 (0.75-2.17) | 1.30 (0.82-2.08) | 1.72 (1.03-2.86) | 3.23 (1.33-8.33) | 1.53 (1.17-2.01) | ||||||
| P | 0.41 | 0.28 | 0.04* | 0.01* | 0.003* | ||||||
*P<0.05; Statistically significant. n=Number of pregnancies, SD=Standard deviation, CI=Confidence interval, OR=Odds ratio, LSCS=Lower segment caesarean section
Gestational age
The mean gestational age at delivery was significantly lower by 2 weeks in twin deliveries (34.9 ± 3.1 weeks) as compared to singleton deliveries [36.8 ± 3.2 weeks, P < 0.001; Table 1]. The majority of deliveries (64.1%) in the singleton group were term deliveries (37–42 weeks). On the other hand, the majority of deliveries in the twin group (64.7%) were between 32 and 37 weeks [Figure 1]. The percentages of deliveries with gestational age of 28–32 weeks (9.2% vs. 3.3%) and 20–28 weeks (5.2% vs. 3.6%) were also higher in the twin group as compared to the singleton group (P < 0.001). This pattern was the same across all treatment groups.
Figure 1.
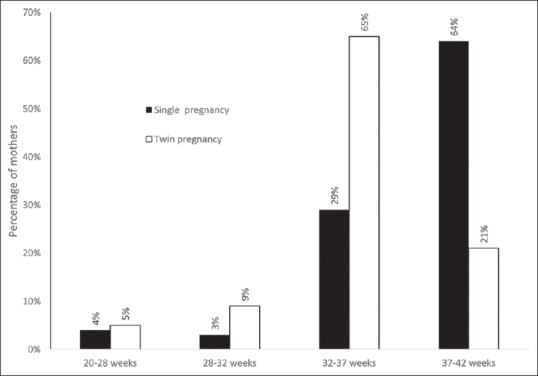
The distribution of singleton and twin pregnancies with respect to the gestational age groups
Maternal complications
The most common maternal complications were preeclampsia (12.8% in twins vs. 9.8% in singletons), PROM (10.5% in twins vs. 4.8% in singleton), and oligohydramnios (3.7% in twin vs. 3.9% in singleton). Other complications were less common. PROM had significantly higher incidence in twin deliveries as compared to singleton deliveries (OR = 2.32, 95% CI = 1.48–3.64; P < 0.001). This also resulted in an overall higher incidence of maternal complications in twin pregnancies compared with singleton deliveries [29.3% vs. 21.3%, OR = 1.53, 95% CI = 1.17–2.01, P = 0.003; Table 2].
Table 2.
Comparison of maternal complications between singleton and twin deliveries
| Maternal complication | Singleton delivery (n=897), n (%) | Twin delivery (n=382), n (%) | OR (95% CI) | P |
|---|---|---|---|---|
| Preeclampsia | 88 (9.8) | 49 (12.8) | 1.35 (0.93-1.96) | 0.12 |
| PROM | 43 (4.8) | 40 (10.5) | 2.32 (1.48-3.64) | <0.001* |
| Oligohydraminos | 35 (3.9) | 14 (3.7) | 0.94 (0.50-1.76) | 1.00 |
| APH | 11 (1.2) | 9 (2.4) | 1.94 (0.80-4.73) | 0.14 |
| GDM | 16 (1.8) | 6 (1.6) | 0.88 (0.34-2.26) | 1.00 |
| PPH | 5 (0.6) | 1 (0.3) | 0.47 (0.05-4.02) | 0.68 |
| Polyhydraminos | 2 (0.2) | 1 (0.3) | 1.17 (0.11-12.99) | 1.00 |
| All complications combined | 191 (21.3) | 112 (29.3) | 1.53 (1.17-2.01) | 0.003* |
*Significant P value in Fisher’s exact test. PROM=Premature rupture of membranes, APH=Antepartum hemorrhage, GDM=Gestational diabetes mellitus, PPH=Postpartum hemorrhage, n=Total number of subjects, CI=Confidence interval, OR=Odds ratio
The same pattern was also seen in each of the four subgroups, with PROM being the only maternal complication that was significantly higher in twin deliveries in fresh self, fresh recipient, and thaw self-cycles. The incidence of preeclampsia was higher in twin deliveries in the thaw recipient subgroup (OR = 3.45, P = 0.04), but was similar in all other subgroups as well as overall.
Perinatal complications
The distribution of singleton and twin deliveries by weight grade of the baby is represented in Figure 2. The majority of singleton babies (69.1%) were in weight Grade D (2.51–4 kg) whereas a higher percentage of twins was in the lower grades, i.e., C (1.51–2.50 kg), B (1.1–1.5 kg) and A (<1 kg). The mean birth weight of babies was significantly lower in the twin group (2.02 ± 0.58 kg) as compared to the singleton group (2.71 ± 0.68 kg; P < 0.001), and this was seen consistently in all the four subgroups also [Table 3].
Figure 2.
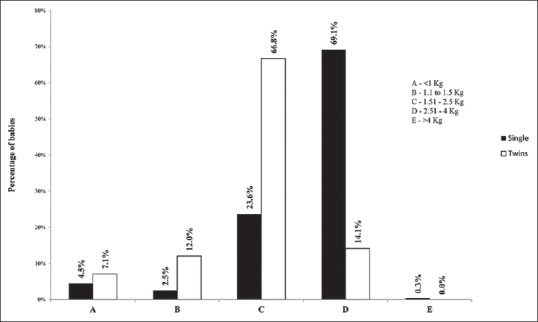
The distribution of singleton and twin babies with respect to the weighted grade at birth
Table 3.
Comparison of perinatal complications in babies between singleton and twin deliveries
| Perinatal indicators | Statistics | Fresh self |
Fresh recipient |
Thaw self |
Thaw recipient |
Combined |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single (n=280) | Twins (n=228) | Single (n=254) | Twins (n=266) | Single (n=272) | Twins (n=206) | Single (n=91) | Twins (n=64) | Single (n=897) | Twins (n=764) | ||
| Weight of baby (kg) | Mean±SD | 2.76±0.54 | 2.03±0.55 | 2.61±0.68 | 2.01±0.56 | 2.78±0.75 | 2.06±0.63 | 2.63±0.79 | 1.84±0.57 | 2.71±0.68 | 2.02±0.58 |
| P | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | ||||||
| Death of child (still birth + early + late neonatal death) | n (%) | 8 (2.9) | 16 (7.0) | 14 (5.5) | 11 (4.1) | 16 (5.9) | 21 (10.2) | 3 (3.3) | 9 (14.1) | 41 (4.6) | 57 (7.5) |
| OR (95% CI) | 2.57 (1.08-6.11) | 0.74 (0.33-1.66) | 1.82 (0.92-3.58) | 4.80 (1.25-18.5) | 1.68 (1.11-2.55) | ||||||
| P | 0.03* | 0.46 | 0.08 | 0.03* | 0.01* | ||||||
| Still birth | n (%) | 6 (2.1) | 8 (3.5) | 12 (4.7) | 5 (1.9) | 7 (2.6) | 14 (6.8) | 2 (2.2) | 3 (4.7) | 27 (3.0) | 30 (3.9) |
| OR (95% CI) | 1.66 (0.57-4.86) | 0.39 (0.13-1.11) | 2.76 (1.09-6.97) | 2.19 (0.36-13.49) | 1.32 (0.78-2.24) | ||||||
| P | 0.35 | 0.07 | 0.03* | 0.41 | 0.31 | ||||||
| Early neonatal death | n (%) | 2 (0.7) | 7 (3.1) | 2 (0.8) | 3 (1.1) | 8 (2.9) | 7 (3.4) | 1 (1.1) | 4 (6.3) | 13 (1.5) | 21 (2.8) |
| OR (95% CI) | 4.40 (0.91-21.4) | 1.44 (0.24-8.67) | 1.16 (0.41-3.25) | 6.00 (0.65-55.0) | 1.92 (0.96-3.86) | ||||||
| P | 0.09 | 1 | 0.78 | 0.16 | 0.06 | ||||||
| Late neonatal death | n (%) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 3 (1.1) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 2 (3.1) | 1 (0.1) | 6 (0.8) |
| OR (95% CI) | NC | NC | NC | NC | 7.09 (0.85-59.04) | ||||||
| P | 0.45 | 0.25 | 1 | 0.17 | 0.05 | ||||||
| Congenital anomalies | n (%) | 4 (1.4) | 3 (1.3) | 8 (3.2) | 4 (1.5) | 6 (2.2) | 8 (3.9) | 2 (2.2) | 0 (0.0) | 20 (2.2) | 15 (2.0) |
| OR (95% CI) | 0.92 (0.2-4.15) | 0.47 (0.14-1.58) | 1.79 (0.61-5.25) | NC | 0.88 (0.45-1.73) | ||||||
| P | 1 | 0.25 | 0.28 | 0.51 | 0.71 | ||||||
*P<0.05; Statistically significant. n=Number of babies, SD=Standard deviation, OR=Odds ratio, CI=Confidence interval, NC=Not calculable due to presence of zero in outcome
Overall, the child mortality characterized by either stillbirth, early or late neonatal death was 5.9% (98 deaths out of 1661 births). A significant difference was observed in this between singleton babies and twin babies (4.6% vs. 7.5%, P = 0.01). This significant difference was also observed in fresh self (7% vs. 2.9% P = 0.03) and thaw recipient (14.1% vs. 3.3%, P = 0.03) groups. However, other indicators of perinatal complications were comparable between the groups [Table 3]. The incidence of congenital anomalies was also comparable (2% in twin vs. 2.2% in singleton; P = 0.71).
Post hoc power analysis
Post hoc power analysis was performed with alpha = 0.05 for “all maternal complications combined” (dichotomous variable), “gestational age at delivery” (continuous variable), and “weight of the baby” (continuous variable). The power for these three outcome variables was 85.9%, 100%, and 100%, respectively.
DISCUSSION
In the present study, twin deliveries following ART had significantly lower mean gestational age at delivery, lower mean birth weight, and higher incidences of “stillbirth plus neonatal death,” as compared to singleton deliveries following ART. The overall maternal complication rates were also significantly higher, primarily because of higher incidence of PROM in twin pregnancies.
Studies directly comparing singleton IVF pregnancies with multiple birth (or twin) IVF pregnancies are limited. In the ART surveillance report from the United States for the year 2014, the authors compared the percentage of low birth weight babies and preterm babies among ART-singleton and ART-twin pregnancies.[5] They reported the percentages to be much higher in twin pregnancies (55.2% incidence of low birth weight in twins vs. 8.9% among singletons; 62.2% preterm babies among ART-twins vs. 13.2% among singletons). These percentages were found to be equally higher in twin infants born with spontaneous pregnancy also (55.2% low birth weight babies and 56.6% preterm babies).[5] In other studies, which included both singleton IVF and twin IVF pregnancies, the authors stopped short of comparing these two groups directly.[15,19,20]
In the present study, the findings of lower gestational age at delivery (by about 2 weeks), lower birth weights (by 25.5%), and higher odds of stillbirth plus neonatal death (1.68 times), stand on par with the existing literature. Individually, the differences in risks of stillbirth, early neonatal death, and late neonatal death were not significantly different. It could be because of the beta error. The risk of congenital anomalies was also similar.
The most surprising finding in our study was that, apart from PROM, no other maternal complication had significantly increased risk in twins. It was because of the increased risk of PROM that the overall odds of maternal complications in twins were higher by 1.53 times.
In addition, the complication rates for single and twin pregnancies found in the present study were also compared with historical population-based data for single and twin pregnancies obtained from literature.[21] The incidences of maternal complications, low birth weight (<2500 g), and preterm delivery were available for comparison and were found to be significantly higher for IVF singleton pregnancies from the current data as compared to population-based historical data for singleton pregnancies from the literature, with OR of 3.69, 3.72, and 7.16, respectively (P < 0.001 for all; data not included). Similarly, the ORs for these three complications were 2.31, 4.63, and 6.40, respectively, for IVF twin pregnancies from the current data versus population-based historical data for twin pregnancies. While the information on the proportion of IVF deliveries in this population-based data was not available, this proportion is generally only about 2%,[22] and hence, these population-based data can be taken as control data.
Naturally conceived twin pregnancies are known to be at a significantly higher risk of both maternal and neonatal complications as compared to singleton pregnancies.[6,7,8,9] Singleton pregnancies following ART have also been shown to be associated with increased complications as compared to naturally conceived singleton pregnancies.[18,19,23,24] Subfertility along with preexisting metabolic or vascular abnormalities, advanced maternal age, and hormonal therapy used in women undergoing ART are considered to be major factors for lower birth weight and adverse outcomes in ART pregnancies.[16,23,25] However, studies comparing naturally conceived versus IVF/ICSI conceived twins have shown conflicting results. There are many individual studies that suggested that twin ICSI pregnancies may carry similar or even less risk of complications as compared to naturally conceived twins.[11,16] Various meta-analyses comparing ART twins with naturally conceived twins have reported similar perinatal mortality.[26,27] Some studies have reported a lower risk of perinatal death for twins among ART births than naturally conceived twins.[28,29] One of the hypotheses put forward to explain this phenomenon is that the number of monochorionic (MC) twins may be less in IVF than in spontaneous twin pregnancies.[25] It is a known fact that MC monozygotic twins have a lesser mean birth weight and higher perinatal mortality compared to dizygotic (DZ) twins.[30,31,32] The vast majority of assisted-reproduction twins are DZ following DET, and the rate of monozygotic twinning has been reported to be lower in ART pregnancies than in spontaneous pregnancies in various studies.[33]
Studies also exist that report higher obstetric and perinatal complications in twins conceived through ART as compared to spontaneous twins.[16,20,34,35,36] In a systematic review, Palomba et al.[19] found that in multiple-birth pregnancies in ART, compared to spontaneously conceived multiple-birth deliveries; there was an increased risk of PROM (Relative Risk, RR 1.20), preeclampsia (RR 1.11), GDM (RR 1.78), preterm birth (RR 1.08), and low birth weight (RR 1.04). They did not find any difference in perinatal mortality. Another review in 2013 concluded that while some antenatal complications were more frequent in ART twin pregnancies than in spontaneous twin pregnancies, their prevalence was low, and thus, their impact on the morbidity and mortality of ART-twin pregnancies was considered by the authors to be limited.[16]
The patient profile seen in our center is like any other infertility center, and the outcomes analyzed do not depend on the IVF protocols used. Hence, the results are generalizable. The findings of our study will help in prognosticating the patients who are carrying a twin pregnancy after IVF. This will also help the medical practitioners in counseling the patients to take an informed decision on whether to transfer one or more embryos at a time. In some countries, a large proportion of patients undergoing IVF have two or more embryos transferred in one transfer. For example, as per the data from a National ART Registry, 43.4% of patients undergoing self-cycles had two embryos transferred and 44.8% had three or more embryos transferred, resulting in 25.1% twin and high order pregnancies.[37] In egg donation cycles, 38% had two and 54.6% had three or more embryos transferred at one time, resulting in 38.4% multiple pregnancies. On the other hand, many centers across the world are now increasing their proportion of eSET. For example, the Australian and New Zealand Assisted Reproduction Database has shown a considerable reduction in the proportion of DET, from 29.6% in 2010 to only 14% in 2015.[38,39] The benefit of higher pregnancy rate with DET or multiple embryo transfer needs to be weighed against the higher risks associated with a subsequent twin or higher-order pregnancy, as found in the present study as well as other existing literature. The results of the present study suggest that for the majority of patients, twin pregnancies should be avoided if possible. Twin pregnancy following IVF/ICSI is an iatrogenic complication and can be easily prevented by limiting the number of embryos to be transferred. Thus, this study will help in encouraging eSET and changing the practice of transferring two or more embryos at a time.
A single eSET cycle may have a lower success rate as compared to a single DET cycle. However, patients failing to achieve a live birth after first elective SET undergo a second embryo transfer with frozen embryo and hence do not require repeat stimulation. A recently published study comparing the cumulative outcomes after two eSET cycles versus a single DET cycle reported similar or better live birth rates with two eSET cycles, with significantly lower incidence of neonatal complications.[40] As expected, the incidence of multiple gestations was drastically lower in two eSET cycles as compared to one DET cycle: 4.2% versus 45% in self-oocyte group and 4% versus 51.2% in donor oocyte group.[40] Another study comparing the cost-effectiveness of SET and DET reported that eSET followed by an additional frozen-thawed single-embryo transfer if available, was less costly as compared to DET.[41] Further, in women under 32 years, eSET followed by an additional frozen–thawed SET if available was more effective than DET, although DET was more effective in women aged 32 or older.[41] Thus, there might be a subset of patients in whom DET to increase pregnancy rates may be justifiable in spite of increased risks, and more studies need to be performed to define this subset.
Adequate sample size with required follow-up data is one of the strengths of this study. The power of the study was found to be more than 80%, and even close to 100% for some variables in post hoc analysis. Further, all data is from a single institution, and all patients were managed with the same IVF protocol, thus reducing confounding due to variations in the treatment protocol. However, the study has all the limitations that exist in any study based on the precollected archival data. Further, since our center does not provide obstetric care beyond 12 weeks of pregnancy, hence much of these data were based on patient reporting or reporting by their concerned obstetricians, and this may be a source of bias or error.
CONCLUSION
Multiple-birth deliveries following IVF, as compared to singleton deliveries following IVF, have lower gestational age, lower birth weight, higher odds of stillbirth plus neonatal death, and slightly higher incidence of LSCS. The overall maternal complication rate is higher due to a higher incidence of PROM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the help of Dr. Mira Dave in data collection, and Dr. Lalit and Miss Nisha, for their suggestions in improving the final manuscript.
REFERENCES
- 1.American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. ACOG Practice Bulletin No. 144: Multifetal gestations: Twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol. 2014;123:1118–32. doi: 10.1097/01.AOG.0000446856.51061.3e. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni AD, Jamieson DJ, Jones HW, Jr, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–25. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 3.Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: An American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril. 2012;97:825–34. doi: 10.1016/j.fertnstert.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 4.European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Calhaz-Jorge C, de Geyter C, Kupka MS, de Mouzon J, Erb K, et al. Assisted reproductive technology in Europe, 2012: Results generated from European registers by ESHRE. Hum Reprod. 2016;31:1638–52. doi: 10.1093/humrep/dev319. [DOI] [PubMed] [Google Scholar]
- 5.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology Surveillance – United States, 2014. MMWR Surveill Summ. 2017;66:1–24. doi: 10.15585/mmwr.ss6606a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjerregaard-Andersen M, Lund N, Jepsen FS, Camala L, Gomes MA, Christensen K, et al. A prospective study of twinning and perinatal mortality in urban Guinea-Bissau. BMC Pregnancy Childbirth. 2012;12:140. doi: 10.1186/1471-2393-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rezavand N, Veisi F, Malek-Khosravi Sh, Zangeneh M, Kohzadi M. Assessment of frequency of twin pregnancy and neonatal outcome in deliveries of Mo'tazedi hospital, Kermanshah in 2004-2007. J Obstet Gynaecol India. 2014;64:19–22. doi: 10.1007/s13224-013-0449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obiechina Nj, Okolie V, Eleje G, Okechukwu Z, Anemeje O. Twin versus singleton pregnancies: The incidence, pregnancy complications, and obstetric outcomes in a Nigerian tertiary hospital. Int J Womens Health. 2011;3:227–30. doi: 10.2147/IJWH.S22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kullima AA, Audu BM, Geidam AD. Outcome of twin deliveries at the University of Maiduguri teaching hospital: A 5-year review. Niger J Clin Pract. 2011;14:345–8. doi: 10.4103/1119-3077.86781. [DOI] [PubMed] [Google Scholar]
- 10.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: Meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945. doi: 10.1136/bmj.c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulet SL, Schieve LA, Nannini A, Ferre C, Devine O, Cohen B, et al. Perinatal outcomes of twin births conceived using assisted reproduction technology: A population-based study. Hum Reprod. 2008;23:1941–8. doi: 10.1093/humrep/den169. [DOI] [PubMed] [Google Scholar]
- 12.Minakami H, Sayama M, Honma Y, Matsubara S, Koike T, Sato I, et al. Lower risks of adverse outcome in twins conceived by artificial reproductive techniques compared with spontaneously conceived twins. Hum Reprod. 1998;13:2005–8. doi: 10.1093/humrep/13.7.2005. [DOI] [PubMed] [Google Scholar]
- 13.Baxi A, Kaushal M. Outcome of twin pregnancies conceived after assisted reproductive techniques. J Hum Reprod Sci. 2008;1:25–8. doi: 10.4103/0974-1208.39593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agustsson T, Geirsson RT, Mires G. Obstetric outcome of natural and assisted conception twin pregnancies is similar. Acta Obstet Gynecol Scand. 1997;76:45–9. doi: 10.3109/00016349709047783. [DOI] [PubMed] [Google Scholar]
- 15.Dhont M, De Sutter P, Ruyssinck G, Martens G, Bekaert A. Perinatal outcome of pregnancies after assisted reproduction: A case-control study. Am J Obstet Gynecol. 1999;181:688–95. doi: 10.1016/s0002-9378(99)70514-4. [DOI] [PubMed] [Google Scholar]
- 16.Jauniaux E, Ben-Ami I, Maymon R. Do assisted-reproduction twin pregnancies require additional antenatal care? Reprod Biomed Online. 2013;26:107–19. doi: 10.1016/j.rbmo.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Póvoa A, Matias A, Xavier P, Blickstein I. Can early ultrasonography explain the lower miscarriage rates in twin as compared to singleton pregnancies following assisted reproduction? J Perinat Med. 2018;46:760–3. doi: 10.1515/jpm-2017-0087. [DOI] [PubMed] [Google Scholar]
- 18.Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertil Steril. 2013;99:731–7. doi: 10.1016/j.fertnstert.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Palomba S, Homburg R, Santagni S, La Sala GB, Orvieto R. Risk of adverse pregnancy and perinatal outcomes after high technology infertility treatment: A comprehensive systematic review. Reprod Biol Endocrinol. 2016;14:76. doi: 10.1186/s12958-016-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin J, Sheng X, Wang H, Liang D, Tan H, Xia J. Assisted reproductive technology and risk of congenital malformations: A meta-analysis based on cohort studies. Arch Gynecol Obstet. 2015;292:777–98. doi: 10.1007/s00404-015-3707-0. [DOI] [PubMed] [Google Scholar]
- 21.Santana DS, Silveira C, Costa ML, Souza RT, Surita FG, Souza JP, et al. Perinatal outcomes in twin pregnancies complicated by maternal morbidity: Evidence from the WHO Multicountry Survey on Maternal and Newborn Health. BMC Pregnancy Childbirth. 2018;18:449. doi: 10.1186/s12884-018-2082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: Results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Hum Reprod. 2018;33:1586–601. doi: 10.1093/humrep/dey242. [DOI] [PubMed] [Google Scholar]
- 23.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 24.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: A systematic review and meta-analysis. Hum Reprod Update. 2012;18:485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 25.Ochsenkühn R, Strowitzki T, Gurtner M, Strauss A, Schulze A, Hepp H, et al. Pregnancy complications, obstetric risks, and neonatal outcome in singleton and twin pregnancies after GIFT and IVF. Arch Gynecol Obstet. 2003;268:256–61. doi: 10.1007/s00404-003-0518-5. [DOI] [PubMed] [Google Scholar]
- 26.Qin J, Wang H, Sheng X, Liang D, Tan H, Xia J. Pregnancy-related complications and adverse pregnancy outcomes in multiple pregnancies resulting from assisted reproductive technology: A meta-analysis of cohort studies. Fertil Steril. 2015;103:1492–5080. doi: 10.1016/j.fertnstert.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: A meta-analysis of cohort studies. Fertil Steril. 2016;105:73–850. doi: 10.1016/j.fertnstert.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Declercq E, Luke B, Belanoff C, Cabral H, Diop H, Gopal D, et al. Perinatal outcomes associated with assisted reproductive technology: The Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART) Fertil Steril. 2015;103:888–95. doi: 10.1016/j.fertnstert.2014.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzsimmons BP, Bebbington MW, Fluker MR. Perinatal and neonatal outcomes in multiple gestations: Assisted reproduction versus spontaneous conception. Am J Obstet Gynecol. 1998;179:1162–7. doi: 10.1016/s0002-9378(98)70125-5. [DOI] [PubMed] [Google Scholar]
- 30.Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: A systematic review and meta-analysis. Obstet Gynecol. 2011;118:928–40. doi: 10.1097/AOG.0b013e31822f129d. [DOI] [PubMed] [Google Scholar]
- 31.Corney G, Thompson B, Campbell DM, MacGillivray I, Seedburgh D, Timlin D. The effect of zygosity on the birth weight of twins in Aberdeen and northeast Scotland. Acta Genet Med Gemellol (Roma) 1979;28:353–60. doi: 10.1017/s0001566000008916. [DOI] [PubMed] [Google Scholar]
- 32.Dubé J, Dodds L, Armson BA. Does chorionicity or zygosity predict adverse perinatal outcomes in twins? Am J Obstet Gynecol. 2002;186:579–83. doi: 10.1067/mob.2002.121721. [DOI] [PubMed] [Google Scholar]
- 33.Källén B, Olausson PO, Nygren KG. Neonatal outcome in pregnancies from ovarian stimulation. Obstet Gynecol. 2002;100:414–9. doi: 10.1016/s0029-7844(02)02069-0. [DOI] [PubMed] [Google Scholar]
- 34.Qin JB, Wang H, Sheng X, Xie Q, Gao S. Assisted reproductive technology and risk of adverse obstetric outcomes in dichorionic twin pregnancies: A systematic review and meta-analysis. Fertil Steril. 2016;105:1180–92. doi: 10.1016/j.fertnstert.2015.12.131. [DOI] [PubMed] [Google Scholar]
- 35.Pourali L, Ayati S, Jelodar S, Zarifian A, Sheikh Andalibi MS. Obstetrics and perinatal outcomes of dichorionic twin pregnancy following ART compared with spontaneous pregnancy. Int J Reprod Biomed (Yazd) 2016;14:317–22. [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Li Y, Li C, Zhang W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril. 2014;101:385–91. doi: 10.1016/j.fertnstert.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Indian Society for Assisted Reproduction. NARI 2014 National ART Registry of India (2010-2011-2012) Mumbai: Indian Society for Assisted Reproduction; 2014. [Google Scholar]
- 38.Fitzgerald O, Harris K, Paul RC, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2015. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales Sydney; 2017. [Google Scholar]
- 39.Macaldowie A, Wang YA, Chambers GM, Sullivan EA. Assisted Reproductive Technology in Australia and New Zealand 2010. Canberra: Australian Institute of Health and Welfare; 2012. [Google Scholar]
- 40.Mehta VP, Patel JA, Gupta RH, Shah SI, Banker MR. One plus one is better than two: Cumulative reproductive outcomes are better after two elective single blastocyst embryo transfers compared to one double blastocyst embryo transfer. J Hum Reprod Sci. 2018;11:161–8. doi: 10.4103/jhrs.JHRS_117_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Loendersloot LL, Moolenaar LM, van Wely M, Repping S, Bossuyt PM, Hompes PG, et al. Cost-effectiveness of single versus double embryo transfer in IVF in relation to female age. Eur J Obstet Gynecol Reprod Biol. 2017;214:25–30. doi: 10.1016/j.ejogrb.2017.04.031. [DOI] [PubMed] [Google Scholar]


