Abstract
Patient: Female, 37-year-old
Final Diagnosis: Diabetic ketoacidosis • Fournier’s gangrene
Symptoms: Dysuria • pain
Medication: Canagliflozin
Clinical Procedure: Incision and drainage
Specialty: Endocrinology and Metabolic • General and Internal Medicine
Objective:
Adverse events of drug therapy
Background:
Sodium glucose co-transporter 2 (SGLT2) inhibitors have become an appealing treatment for diabetes due to their favorable cardiac and renal outcomes. However, reports continue to emerge describing potentially life-threatening adverse events such as Fournier’s gangrene and diabetic ketoacidosis associated with their use. Herein, we present a case of simultaneous Fournier’s gangrene and diabetic ketoacidosis after initiation of treatment with canagliflozin.
Case Report:
A 37-year-old female with diabetes presented to the hospital with a chief complaint of left gluteal pain associated with dysuria 1 month after canagliflozin was added to her regimen. On initial evaluation, the patient was afebrile and hemodynamically stable. Physical examination revealed suprapubic tenderness and induration in the left gluteal region extending to the perineum. Laboratory testing was significant for anion gap metabolic acidosis with the presence of serum ketones. Computed tomography of abdomen and pelvis revealed features suggestive of Fournier’s gangrene. The patient was treated for Fournier’s gangrene and diabetic ketoacidosis. Management included empirical antibiotic treatment, multiple surgical explorations with debridement as well as insulin infusion with aggressive fluid resuscitation. The patient was discharged with a urinary catheter, vacuum dressing, and colostomy with instructions to start a basal bolus insulin regimen and discontinue canagliflozin.
Conclusions:
This is the first case describing a simultaneous occurrence of Fournier’s gangrene and diabetic ketoacidosis with SGLT2 inhibitor therapy. Considering the growing popularity of these drugs, it is important to be aware of their more serious and potentially fatal complications. It is also important to promptly terminate SGLT2 inhibitors when harmful adverse effects are suspected.
MeSH Keywords: Diabetic Ketoacidosis, Fournier Gangrene, Sodium-Glucose Transporter 2
Background
Sodium glucose co-transporter 2 (SGLT2) inhibitors are a class of relatively new antihyperglycemic agents that have become an appealing treatment for diabetes due their favorable cardiac and renal outcomes [1–3]. These agents are recommended as 1 of 6 second-line therapy options after initial therapy with metformin [4]. SGLT2 inhibitors became available in the United States (US) in 2013. Currently the US Food and Drug Administration (FDA) has approved SGLT2 inhibitor use in patients with type 2 diabetes. Four SGLT2 inhibitors have been approved which include canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. These drugs act at the renal proximal tubule to inhibit the sodium glucose cotransporter-2, and to some extent the sodium glucose cotransporter-1. This results in decreased glucose reabsorption and the promotion of glucosuria which consequently reduces plasma glucose independently of insulin [5]. The most common adverse effects identified in clinical trials were genital mycotic and urinary tract infections (UTIs), but after FDA approval further adverse effects surfaced such as urosepsis, pyelonephritis, Fournier’s gangrene, ketoacidosis, and acute kidney injury [6].
Fournier’s gangrene (FG) and diabetic ketoacidosis (DKA) are 2 potentially life-threatening adverse effects of SGLT2 inhibitors. FG is a necrotizing soft tissue infection of the perineum, external genitalia, and perianal regions. It is a urological emergency requiring immediate surgical intervention and broad-spectrum antibiotics. DKA is a medical emergency, typically characterized by hyperglycemia, ketosis, and acidosis. However, what is unique with this class of drugs is that most cases of DKA are without profound hyperglycemia, which is one of the greatest fears with SGLT2 inhibitor use, that it may cause many DKA events to be missed. The association between DKA and SGLT2 inhibitors is presumably due to increased urinary excretion of glucose with diminished glycogen stores, compounded by increased ketone production and impaired excretion [4]. If not appropriately treated, DKA can lead to severe dehydration, diabetic coma and death.
The number of reported adverse effects associated with SGLT2 inhibitors is rising, but rarely are 2 potentially life-threatening adverse effects associated with SGLT2 inhibitors occurred in the same patient. Herein, we present a patient that developed FG and DKA after initiation of treatment with canagliflozin.
Case Report
A 37-year-old female with a past medical history significant for poorly controlled type 2 diabetes mellitus complicated by peripheral neuropathy, morbid obesity with a BMI of 45.8 kg/m2, obstructive sleep apnea, gastroesophageal reflux disease, depression and intellectual disability, was being treated with metformin 500 mg twice a day. Her hemoglobin A1c was 9.8%. Therefore, sitagliptin and canagliflozin were added to her regimen (Table 1). After 1 month she complained of pain in the left gluteal region associated with dysuria and treatment with trimethoprim/sulfamethoxazole for a presumed urinary tract infection was initiated. Urine culture was not collected at that time. Given no improvement on her symptoms, she decided to go to the hospital 5 days later.
Table 1.
Medications prior to admission.
| Canagliflozin | 100 mg daily |
| Cetirizine | 10 mg daily |
| Citalopram | 40 mg daily |
| Levothyroxine | 175 mcg daily |
| Lisinopril | 5 mg daily |
| Pantoprazole | 40 mg nightly |
| Pravastatin | 40 mg daily |
| Sitagliptin-metformin | 50–1000 mg BID |
| Trazodone | 100 mg nightly |
| Valacyclovir | 500 mg daily |
BID – twice a day.
On initial evaluation, the patient was afebrile (36.9°C), tachycardic (117 beats/minute), with blood pressure of 144/79 mmHg and respiratory rate of 19 breaths/minute. She appeared lethargic and in distress. Physical examination was significant for suprapubic tenderness and induration to the left gluteal region extending to the perineum. Laboratory workup revealed blood glucose of 402 mg/dL, a serum bicarbonate of 12 mmol/L (normal range, 20–30 mmol/L), an elevated anion gap of 24 mmol/L (normal range, 7–17 mmol/L) and a lactate of 1.8 mmol/L. Serum electrolytes, creatinine and blood urea nitrogen (BUN) were within normal range with serum osmolarity of 295 mOsm/kg (normal range, 285–295 mOsm/kg). Urinalysis was significant for glycosuria and ketonuria (glucose 4+, ketones 1+). Measurement of serum ketones revealed b-hydroxybutyrate of 2.49 mmol/L. Arterial blood gas analysis showed a pH of 7.23 with a PCO2 of 34 mmHg. A computed tomography of the abdomen and pelvis showed marked inflammatory changes with subcutaneous edema and air within the medial left gluteal soft tissues and locules of air extending into the presacral soft tissues suggestive of Fournier’s gangrene (FG) (Figure 1).
Figure 1.
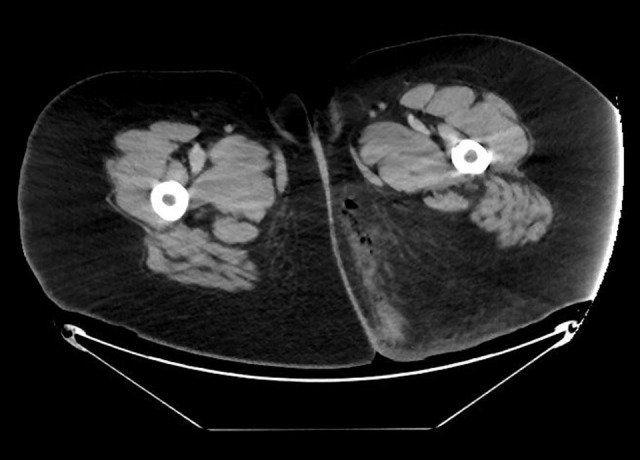
Abdominal computed tomography showing subcutaneous edema and air within the medial left gluteal soft tissues confirming the diagnosis of Fournier’s gangrene.
The patient received an initial bolus of intravenous (IV) normal saline 0.9%, was subsequently started empirically on IV ceftazidime 2 g, IV clindamycin 600 mg, and vancomycin 2 g and decision was made for debridement, incision and drainage (I&D) of the left gluteal region. Over the course of hospital day 2, the patient became hypotensive and tachycardic. Due to the concerns of ongoing infection and sepsis, she underwent second surgical exploration after the necessary fluid resuscitation and subsequently was admitted to surgical Intensive Care Unit (ICU). She was intubated, required vasopressors, and her antibiotics were switched to vancomycin and piper-acillin/tazobactam. During the next 3 to 6 days she required 4 additional surgical re-explorations with further debridement (6 in total since admission). On hospital day 7, a laparoscopic transverse loop colostomy for fecal diversion to prevent further infection of the perineum was performed.
Interestingly, initial DKA management included only subcutaneous insulin. On hospital day 3, when serum ketones were identified and anion gap persisted, insulin infusion with aggressive fluid resuscitation were started. The following day, anion gap closed, and acidosis resolved. On day 17, the patient was transferred to the general medical floor eventually was discharged on hospital day 28 in a medically stable condition with a urinary catheter, vacuum dressing, and colostomy in place to a short-term rehabilitation (STR) facility with instructions to start insulin glargine 18 U and discontinue canagliflozin and sitagliptin-metformin.
Discussion
We report the simultaneous occurrence of 2 potentially fatal complications of SGLT2 inhibitor use – Fournier’s Gangrene (FG) and diabetic ketoacidosis (DKA) – in a patient with type 2 diabetes. The incidence of FG is 1.6 out of 100 000 males annually in the US, with limited literature published on the occurrence in both males and females [7]. Risk factors for FG include hypertension, obesity, congestive heart failure, tobacco use, immunosuppression, and diabetes (although a major risk factor, yet the overall incidence remains low among diabetics [8]. A recently published data analysis identified 55 cases of FG among patients receiving SGLT2 inhibitor therapy in a nearly 6-year period since the drug’s approval by the FDA in March 2013 to January 2019. The database used was the FDA Adverse Events Reporting System (FAERS), which consists of voluntarily reported cases of adverse drug events [6]. In comparison, in the same analysis only 19 cases of FG were identified in patients receiving other classes of antihyperglycemic agents in a 35-year period [6]. This significantly higher number of FG cases reported with SGLT2 inhibitors raises the concern that these agents are an additional risk factor of FG.
Although the exact mechanism of SGLT2 inhibitor associated FG is unknown, it is believed that an inciting event allowing entry of the causative microorganism(s) into the host tissue is important in its development [6]. Since SGLT2 inhibitors promote glucosuria, they provide substrate for microorganisms to grow thereby increasing the risk of urogenital tract infections [9–11]. However, the association among SGLT2 inhibitors and UTIs has been inconclusive in several randomized control trials (RCTs) and meta-analyses [12]. More recently, a population-based cohort study comparing the use of SGLT2 inhibitors with the use of DPP4 inhibitors or GLP1 agonists showed that there is similar risk of UTIs among the different second-line antidiabetic medications [13] However, mycotic genital infections occurred more consistently in those receiving SGLT2 inhibitors in comparison to other antihyperglycemic agents, especially within a month after initiation of treatment [9]. Therefore, the increased risk of urinary and urogenital tract infections remains implicated in precipitating FG in those taking SGLT2 inhibitors. Our patient was being treated for a UTI prior to admission which may subsequently have predisposed her to develop FG. Additionally, she had other comorbidities associated with FG, including morbid obesity (BMI of 45.8 kg/m2) and a smoking history.
DKA is another complication of SGLT2 inhibitor use that our case identifies. Typical precipitants of DKA include infection or insufficient insulin therapy, yet the emerging reports of SGLT2 inhibitors associated DKA have led to a safety communication released by the FDA regarding the risk of DKA with the use of these agents with a majority of cases occurring in patients with type 2 diabetes. This has motivated clinicians and researchers to investigate further the incidence and the pathophysiology of DKA associated with these agents [4].
Although there is an alarming increase in reported cases over a short period of time, DKA associated with SGLT2 inhibitors is only partially understood. SGLT2 inhibitors are associated with decreased insulin levels, increased glucagon secretion, and impaired ketone excretion. Furthermore, the increased glucagon to insulin ratio favors ketogenesis leading to conditions favoring the development of DKA [5]. Therefore, in circumstances where ketogenesis may naturally be increased, such as the combination of diminished food intake, increased stress, and impaired insulin secretion, a substance that favors further ketone production and reduced clearance could directly promote ketoacidosis.
While there are no definitive diagnostic criteria for DKA, its presence is generally accepted by the simultaneous occurrence of hyperglycemia (blood glucose >250 mg/dL), anion-gap acidosis, and increased plasma ketones which is usually determined by measuring b-hydroxybutyrate levels [14]. Our patient was hyperglycemic with a blood glucose of 402 mg/dL, acidotic with an elevated anion gap of 24, CO2 of 12 mmol/L, pH of 7.3 and lactate of 1.2 mmol/L. Urine analysis also showed the presence of ketones. While sepsis alone can trigger DKA, it is more likely to occur in patients with type 1 diabetes or those with known insulin deficiency. Our patient was obese, formerly on metformin only, and had no prior history of DKA or features suggestive of insulin deficiency, and therefore a hyperosmolar hyperglycemic state would have been a more likely presentation, but in the presence of SGLT2 inhibition, increased ketone generation and impaired excretion could have easily fostered the development of ketoacidosis. A number of stressors such as sepsis from FG and surgery contributed to DKA development in the setting of SGLT2 inhibition.
To the best of our knowledge, this is the first case describing the simultaneous occurrence of 2 potentially fatal adverse effects of SGLT2 inhibitor therapy – DKA and FG. In light, of the FDA’s warnings and the growing popularity of SGLT2 inhibitor therapy, especially in patients with other comorbidities, such as cardiovascular disease and obesity, it is important to be mindful of this drug’s more serious and potentially fatal complications. It is also important to promptly terminate SGLT2 inhibitors when harmful adverse effects are suspected to prevent further progression.
Conclusions
SGLT2 inhibitors are a promising class of antihyperglycemic agents, due to their insulin independent glycemic and extra-glycemic effects. Although this drug is generally well tolerated by most patients, it has been associated with serious adverse effects. As this class of drug has only been on the market for the past 6 years, there is limited information of its true risk in clinical practice. Caution should be taken when clinicians prescribe this class of antihyperglycemic agents and the risks of developing adverse effects should also be considered. Additionally, we encourage clinicians to voluntarily report all adverse drug effects in order to adequately conduct post-marketing studies to determine the true risk of SGLT2 inhibitors in clinical practice.
References:
- 1.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 3.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 4.Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: A systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37:187–94. doi: 10.1002/phar.1881. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–52. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bersoff-Matcha SJ, Chamberlain C, Cao C, et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: A review of spontaneous postmarketing cases. Ann Intern Med. 2019;170:764–69. doi: 10.7326/M19-0085. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen MD, Krieger JN, Rivara FP, et al. Fournier’s gangrene: Population-based epidemiology and outcomes. J Urol. 2009;181:2120–26. doi: 10.1016/j.juro.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voelzke BB, Hagedorn JC. Presentation and diagnosis of Fournier gangrene. Urology. 2018;114:8–13. doi: 10.1016/j.urology.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019;21:434–38. doi: 10.1111/dom.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D, Wang T, Shen S, et al. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: A meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:348–55. doi: 10.1111/dom.12825. [DOI] [PubMed] [Google Scholar]
- 11.Geerlings S, Fonseca V, Castro-Diaz D, et al. Genital and urinary tract infections in diabetes: Impact of pharmacologically induced glucosuria. Diabetes Res Clin Pract. 2014;103:373–81. doi: 10.1016/j.diabres.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Puckrin R, Saltiel MP, Reynier P, et al. SGLT-2 inhibitors and the risk of infections: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55:503–14. doi: 10.1007/s00592-018-1116-0. [DOI] [PubMed] [Google Scholar]
- 13.Dave CV, Schneeweiss S, Kim D, et al. Sodium-glucose cotransporter-2 inhibitors and the risk for severe urinary tract infections: A population-based cohort study. Ann Intern Med. 2019 doi: 10.7326/M18-3136. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–43. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]


