Abstract
Neonatal lupus erythematosus (NLE) should be considered when a newborn develops atrioventricular heart block along with the presence of autoantibodies to Sjogren’s syndrome autoantigens in the maternal serum. NLE can also present with features such as cutaneous lesions, hepatic dysfunction or haematological abnormalities. Differential diagnosis usually includes congenital infections as there is a significant overlap of symptoms with NLE. We report a case of NLE who had multiorgan involvement with macular erythematous skin lesions present at birth, and on investigation was found to have cytomegalovirus (CMV) infection. The diagnostic dilemma was whether to consider this infection as symptomatic or just colonisation. In the infant described, the absence of end organ damage specific to CMV infection (hearing loss, intracranial calcifications, retinitis, brain involvement) made a diagnosis of symptomatic CMV unlikely.
Keywords: dermatology, materno-fetal medicine
Background
Neonatal lupus erythematosus (NLE) should be considered as a differential diagnosis in any neonate presenting with thrombocytopenia, neutropenia and/or anaemia, with concurrent rash and/or hepatitis. Differential diagnosis should include conditions with similar cutaneous manifestations such as congenital infections.
We report a case of NLE with concomitant cytomegalovirus (CMV) colonisation.
Case presentation
A 2560 g appropriate-for-age female infant was delivered at 37+6 weeks of gestation by caesarean section in view of meconium stained amniotic fluid and accompanying fetal distress in the form of fetal bradycardia. Apgar score was 8 and 9 at 1 and 5 min of life, respectively. There were no complications during pregnancy. The mother was primigravida and there was no history of known systemic diseases.
Immediately after birth, the child had confluent macular erythematous rash and petechiae over the face, extensor surfaces of extremities and trunk as well as soles (figure 1). On physical examination, no other systemic manifestations were detected. She was roomed in with her mother. Owing to new-onset tachypnoea 48 hours after delivery, she was admitted to the neonatal intensive care unit. Empirical antibiotics were started. She was administered oxygen (fraction of inspired oxygen 21%–30% via blender) by a low flow nasal cannula for 5 days, with a target preductal saturation of 94%–98% as per unit policy. A mild diffuse interstitial pattern was seen on chest X-ray. Haemoglobin was 157 g/L, platelet count was 30×109/L and C-reactive protein was positive (83.95 mg/L, >10 mg/L being positive). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 63 and 18 units/L, respectively, gamma glutamyl transferase was 123 units/L and serum creatinine was 0.6 mg/dL. Bacterial cultures of blood and cerebrospinal fluid were sterile. There was no evidence of any conduction abnormality or cardiac dysfunction on ECG and echocardiography. In view of rash with accompanying haematological involvement, new onset tachypnoea, diffuse interstitial pattern in chest X-ray and clinical findings not supported by cultures or cardiology workup, alternate diagnoses were considered. Paediatric dermatologist opinion was sought for the infant. A diagnosis of NLE was considered. In view of persistently low platelet counts, intravenous immunoglobulin (IvIg, Reliance Laboratories) 1 g/kg was administered. Oral steroid (prednisolone, 1 mg/kg/day in two divided doses) was administered for 2 weeks. Platelet count showed an improving trend (105×109/L) on day 11 of life. There was no clinical bleed throughout the hospital stay.
Figure 1.
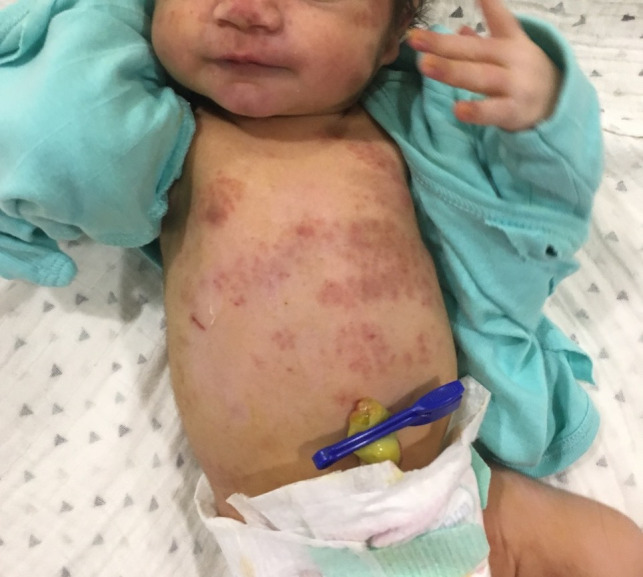
Macular erythematous rash and petechiae over the face and trunk.
The mother’s prenatal history was normal and laboratory tests revealed that HIV was negative, rapid plasma reagin was non-reactive and rubella titres showed immunity. Mother was not diagnosed with any autoimmune disorder previously. There was no history of photosensitivity, mucosal ulceration (either intranasal or palatal lesions), joint pains or cutaneous vasculitis. Her family history was unremarkable.
Investigations
In view of above-mentioned findings (rash and haematological findings), NLE and TORCH (Toxoplasmosis, Others, Rubella, Cytomegalovirus, Herpes) infections were considered. Autoantibodies for anti-Sjogren’s-syndrome-antigen A (anti-SSA/Ro) and anti-Sjogren’s-syndrome-antigen B (anti-SSB/La) were positive (both >200 RU/mL; BioPlex 2200; Bio-Rad Laboratories; for each test: positive >25 RU/mL; negative <15 RU/mL). Antinuclear antibody was also positive (ANA; 4.32 antibody index unit, negative result is <1). Her mother was also positive for ANA (4.32 antibody index unit, negative result is <1), anti-SSA/Ro and anti-SSB/La, thereby confirming the diagnosis of neonatal lupus.
Serology for rubella and herpes was negative. Chikungunya IgM was negative. Infant’s urine DNA PCR for CMV (Rotor Gene 3000, Corbett Research, Australia) was positive. To rule out false positivity, repeat testing for CMV DNA was done which was also positive. DNA PCR for CMV was not detectable in cerebrospinal fluid (CSF). Auditory brainstem responses of the infant were normal. Cranial and abdominal ultrasonography as well as ophthalmic evaluation was normal.
On the basis of the above-discussed findings, a diagnosis of NLE with concomitant CMV was considered.
Outcome and follow-up
The dermatologist advised emollient application and avoidance of direct sun exposure for the infant. There was gradual improvement and at 2 weeks, all the lesions had disappeared. The child presented for a follow-up after 1 month. There was no evidence of residual skin changes such as telangiectasia, hypopigmentation or hyperpigmentation, atrophy or scarring. Repeat laboratory tests revealed platelet counts of 242×109/L, haemoglobin 131 g/L, AST level 43 units/L and the ALT level 28 units/L. At birth and during follow-up (till 12 weeks of age), neurological examination was normal.
We considered treatment for CMV as there was an overlapping clinical picture. Thrombocytopenia, rashes, transaminitis and pneumonitis could be present in neonatal lupus as well as congenital CMV infection. End organ damage specific to CMV infection (hearing loss, intracranial calcifications, retinitis, brain involvement) was absent in the index case. Moreover, rashes started fading over 1–2 weeks. Thus, neonatal lupus was considered as the primary disease. So, oral steroids as well as IvIg were administered. On follow-up after 1 month, the platelet count and transaminase levels were normal. Thus, symptomatic CMV was ruled out as clinical signs as well as laboratory parameters improved with above management.
Discussion
NLE is caused by passage of maternal autoantibodies to Sjogren’s syndrome autoantigens (anti-SSA/Ro or anti-SSB/La) to the fetus transplacentally.1 2 It is an uncommon syndrome and was first described in 1954 by McCuistion et al and Bridge and Foley who reported similar cases in infants with ANA-positive mothers.3–5 The incidence of NLE is between 1 in 12 500 to 1 in 20 000 live births with a higher incidence in girls and premature infants.6 The diagnosis of NLE is considered when a newborn develops atrioventricular heart block along with the presence of autoantibodies to Sjogren’s syndrome autoantigens in the maternal serum.1 2
NLE can also present with features such as cutaneous lesions (~40%), hepatic dysfunction (~35%) or haematological (~35%) abnormalities. Non-cardiac manifestations are usually transient and not life threatening.1 7 8 Cutaneous lesions include annular erythematous lesions or plaques with or without scales and appear predominantly on the face, scalp or neck.5 6 9 Although the eruptions are usually characteristic, they are usually misinterpreted as birth trauma, fungal infection or allergic reactions.2 10 In typical cases with positive autoantibodies, skin biopsy is not mandatory to confirm the diagnosis.5 The lesions usually resolve spontaneously over weeks or months along with the disappearance of maternal antibodies from the infant’s serum.5 9 Avoidance of exposure to sun through protective clothing and use of sunscreen is the primary management.1 2 11 The index case had predominantly macular rash which disappeared in 2 weeks.
Hepatobiliary involvement manifests as elevated liver enzymes and/or conjugated hyperbilirubinaemia after birth which resolves gradually in weeks to months. Some infants may have mild organomegaly (hepatomegaly and/or splenomegaly).5 9 12 Both the manifestations are usually transient.
Haematological disturbances including haemolytic anaemia, thrombocytopenia and neutropenia usually occur in the first few weeks of life. Such patients are usually asymptomatic.5 12 Haematological manifestations usually disappear in weeks to months.5 9 10 Those with severe hepatic or haematological involvement may require treatment with systemic corticosteroids and/or IvIg.6 13 In this case, the infant had persistent thrombocytopenia which responded to systemic corticosteroids and IvIg.
NLE should be considered as a differential diagnosis in any neonate presenting with thrombocytopenia, neutropenia, with concurrent rash and/or hepatitis.13 Mothers may be asymptomatic and thus the diagnosis may not be straightforward. In comprehensive reviews of NLE by Vanoni et al and Chao et al, the authors have highlighted that 25%–40% mothers may be asymptomatic at the time of birth of such infants.2 13 Differential diagnosis should include conditions with similar cutaneous manifestations. A complete workup should be undertaken, including a complete metabolic panel, complete blood cell count, ECG and echocardiograph and workup for TORCH group of infections as the differential diagnosis should include congenital infections.14
There is significant overlap in clinical features of congenital CMV infection and NLE. While most infants affected with congenital CMV are asymptomatic at birth, nearly 10% exhibit some signs such as intrauterine growth restriction, thrombocytopenia, chorioretinitis, hepatitis and/or sensorineural hearing loss.15–18 The extent of acute involvement may vary from mild to severe and could be fatal. In the infant described, the absence of end organ damage specific to CMV infection (hearing loss, intracranial calcifications, retinitis, brain involvement) made a diagnosis of symptomatic CMV unlikely. Rather, this patient had asymptomatic CMV colonisation at birth. CMV PCR assay of the saliva or urine is more sensitive and specific compared with urine CMV culture.19 In most of the cases, CMV is an asymptomatic, self-limited illness, which usually results in the establishment of latent infection or colonisation.20
Kimberlin et al performed a randomised trial of 6 weeks of intravenous ganciclovir in ‘symptomatic’ congenital CMV infants compared with no treatment.19 21 The intervention group had better total-ear hearing compared with no treatment group (73% vs 57%, p=0.01).22 However, the intervention described in this trial cannot be extrapolated to infants with ‘asymptomatic’ congenital CMV infection, since there are no controlled studies showing a benefit in this population.22 Thus, we did not treat CMV infection in the index case.
Here, we report a case of NLE who presented at birth with multiorgan involvement with macular erythematous skin lesions. To our knowledge, this is the first report of NLE with asymptomatic CMV colonisation at birth.
Learning points.
Neonatal lupus erythematosus (NLE) should be considered as a differential diagnosis in any neonate presenting with thrombocytopenia, neutropenia and/or anaemia, with concurrent rash and/or hepatitis.
Differential diagnosis should include conditions with similar cutaneous manifestations. A complete workup for an infectious aetiology should be undertaken.
Differential diagnosis usually includes congenital infections as there is a significant overlap of symptoms between congenital cytomegalovirus infection and NLE.
Footnotes
Contributors: GA: conceived and wrote the manuscript. SW: critically reviewed and finalised the manuscript. All authors involved in clinical care of the infant, read and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brucato A, Cimaz R, Stramba-Badiale M. Neonatal lupus. Clin Rev Allergy Immunol 2002;23:279–300. 10.1385/CRIAI:23:3:279 [DOI] [PubMed] [Google Scholar]
- 2.Vanoni F, Lava SAG, Fossali EF, et al. Neonatal systemic lupus erythematosus syndrome: a comprehensive review. Clin Rev Allergy Immunol 2017;53:469–76. 10.1007/s12016-017-8653-0 [DOI] [PubMed] [Google Scholar]
- 3.McCUISTION CH, SCHOCH EP. Possible discoid lupus erythematosus in newborn infant; report of a case with subsequent development of acute systemic lupus erythematosus in mother. AMA Arch Derm Syphilol 1954;70:782–5. 10.1001/archderm.1954.01540240088009 [DOI] [PubMed] [Google Scholar]
- 4.Bridge RG, Foley FE. Placental transmission of the lupus erythematosus factor. Am J Med Sci 1954;227:1–8. 10.1097/00000441-195401000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Hon KL. Leung, AKC neonatal lupus erythematosus. Autoimmune Dis 2012;2012:301274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisuthsarewong W, Soongswang J, Chantorn R. Neonatal lupus erythematosus: clinical character, investigation, and outcome. Pediatr Dermatol 2011;28:115–21. 10.1111/j.1525-1470.2011.01300.x [DOI] [PubMed] [Google Scholar]
- 7.Cimaz R, Spence DL, Hornberger L, et al. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr 2003;142:678–83. 10.1067/mpd.2003.233 [DOI] [PubMed] [Google Scholar]
- 8.Zuppa AA, Riccardi R, Frezza S, et al. Neonatal lupus: follow-up in infants with anti-SSA/Ro antibodies and review of the literature. Autoimmun Rev 2017;16:427–32. 10.1016/j.autrev.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 9.Lee LA. Cutaneous lupus in infancy and childhood. Lupus 2010;19:1112–7. 10.1177/0961203310370347 [DOI] [PubMed] [Google Scholar]
- 10.Hulsmann AR, Oranje AP. Educational paper: neonatal skin lesions. Eur J Pediatr 2014;173:557–66. 10.1007/s00431-013-1956-0 [DOI] [PubMed] [Google Scholar]
- 11.Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol 2009;10:365–81. 10.2165/11310780-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12.Silverman E, Jaeggi E. Non-Cardiac manifestations of neonatal lupus erythematosus. Scand J Immunol 2010;72:223–5. 10.1111/j.1365-3083.2010.02443.x [DOI] [PubMed] [Google Scholar]
- 13.Chao MM, Luchtman-Jones L, Siverman RA. Hematological complications of neonatal lupus: case report and review of the literature. J Pediatr Hematol Oncol 2013;35:e344–6. [DOI] [PubMed] [Google Scholar]
- 14.Jaffery F, Khan F, Bustillo J. Visual diagnosis: a 3-week-old girl with an unusual rash. Pediatrics in Review 2017;38:e35–7. 10.1542/pir.2016-0035 [DOI] [PubMed] [Google Scholar]
- 15.Britt W. Infectious disease of the fetuses and newborn infant. Philadelphia: Elsevier Saunders; 2011. Cytomegalovirus; 706.. [Google Scholar]
- 16.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005;5:70–7. 10.1186/1471-2458-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bialas MK, Swamy GK, Permar SR. Perinatal cytomegalovirus and varicella zoster virus infections: epidemiology, prevention, and treatment. Clin Perinatol 2015;42:61–viii.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vimercati A, Chincoli A, De Gennaro A, et al. Clinical Management of Infections in Pregnancy: Update in Congenital Cytomegalovirus and Toxoplasmosis : Malvasi A, Tinelli A, Di Renzo G, et al., Management and therapy of late pregnancy complications. Springer, Cham, 2017. [Google Scholar]
- 19.Bialas KM, Swamy GK, Permar SR. Perinatal cytomegalovirus infections: epidemiology, prevention, and treatment. Neoreviews 2015;16:e231–5. 10.1542/neo.16-4-e231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozenblyum EV, Levy DM, Allen U, et al. Cytomegalovirus in pediatric systemic lupus erythematosus: prevalence and clinical manifestations. Lupus 2015;24:730–5. 10.1177/0961203314565443 [DOI] [PubMed] [Google Scholar]
- 21.Kimberlin DW, Lin C-Y, Sánchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003;143:16–25. 10.1016/S0022-3476(03)00192-6 [DOI] [PubMed] [Google Scholar]
- 22.Kimberlin DW, Jester PM, Sánchez PJ, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015;372:933–43. 10.1056/NEJMoa1404599 [DOI] [PMC free article] [PubMed] [Google Scholar]


