Abstract
A 60-year-old woman presented with melena for 2 weeks. She had undergone hysterectomy and bilateral salpingo-oophorectomy to treat clear cell endometrial carcinoma 10 months before the presentation. She was anaemic and tachycardic; abdominal CT scan revealed a large duodenal mass. Her gastrointestinal bleed was not amenable to endoscopic intervention, so she had emergent laparotomy, pancreaticoduodenectomy with duodenal mass excision. Histopathology confirmed that the duodenal mass was a metastatic deposit from her clear cell endometrial cancer. Postoperatively, she was frail and chose hospice care and she died 90 days postoperatively. Clear cell endometrial cancer is a rare subtype of endometrial cancer, that has a worse prognosis compared with the more common endometrioid subtype. The duodenum is a rare site for metastatic endometrial cancer, and we report this case to alert clinicians to the possibility of metastases to the small intestine in patients with clear cell endometrial cancer.
Keywords: gynecological cancer, GI bleeding, stomach and duodenum, chemotherapy, end of life decisions (palliative care)
Background
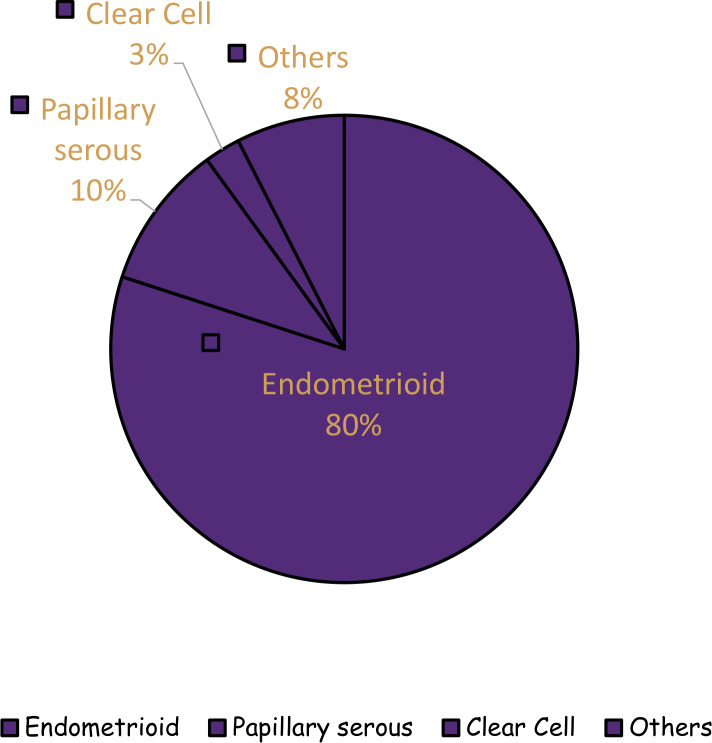
Endometrial cancer (EC) is the most common gynaecological malignancy with estimated 61 880 new cases and 12 160 deaths in the USA for 2019.1 There are various histological subtypes of EC including endometrioid, papillary serous carcinoma (PSC), mucinous, clear cell (CC) and squamous cell categories.2 The endometrioid subtype accounts for 80% of cases and is referred to as type 1 EC.3 4 The recognised environmental risk factors for endometrioid EC include obesity, nulliparity and diabetes mellitus.5 The non-endometrioid subtypes (collectively referred to as type 2), although less common, run a more aggressive clinical course and patients have worse outcomes compared with the endometrioid subtype.3 Although PSC and CC are only 10% and 3%, respectively, of all ECs, they account for 39% and 8%, respectively, of the total EC mortality (figure 1).6
Figure 1.
Histological subtypes of endometrial cancer.6
CC endometrial carcinoma is a rare subtype of EC which does not seem to be caused by the hyperestrogenemic states that predispose to endometrioid EC.2 6 It accounts for 3% of all ECs.6
In addition to having a rare subtype of endometrial carcinoma, our patient had an unusual pattern of metastasis which prompted this case report. We report a case of metastatic CC endometrial carcinoma cancer with a rare metastasis site manifesting as acute upper gastrointestinal bleeding.
Case presentation
A 60-year-old woman with a history of CC endometrial carcinoma who had undergone total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) 10 months prior presented with abdominal pain, melena and generalised weakness for 2 weeks. She did not receive adjuvant therapy following her TAH and BSO because she was lost to follow-up.
She had no family history of cancer, smoked marijuana but not tobacco and did not drink any alcohol. On examination, she was tachycardic with a regular heart rate of 124 beats/min and normotensive with a blood pressure of 117/82 mmHg. She had conjunctival pallor on general examination while her abdominal examination revealed a soft, non-tender abdomen with no palpable mass. Digital rectal examination revealed melena.
Investigations
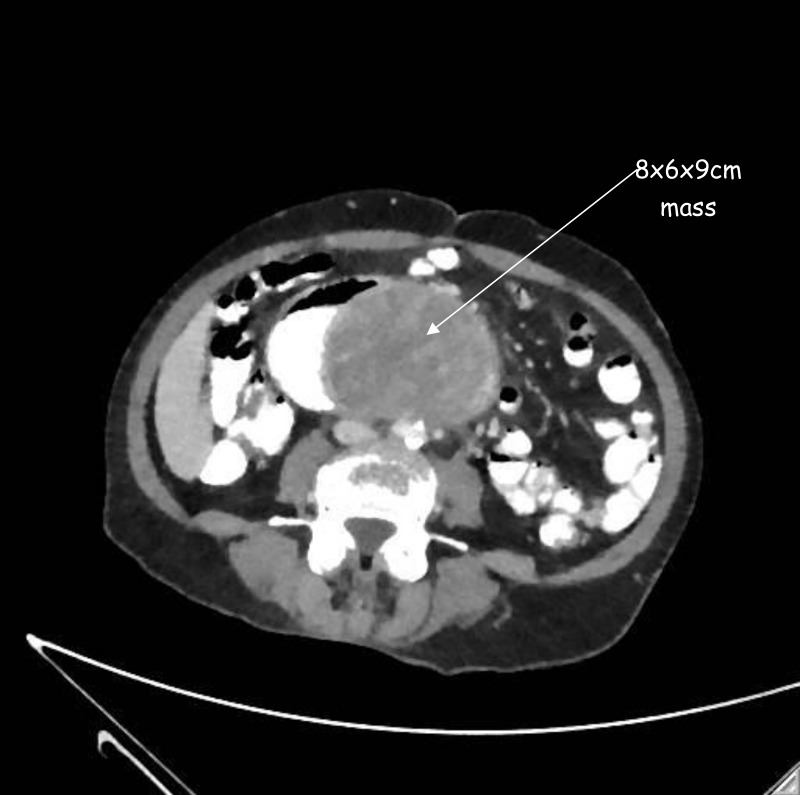
The haemoglobin level was 56g/L with mean corpuscular volume of 78.7 fL, blood urea nitrogen of 24 mg/dL and creatinine of 0.9 mg/dL. A rapid cross match of blood was done in preparation for transfusion because she was anaemic and tachycardic. Given her history of EC, a contrast-enhanced CT scan of her abdomen revealed a heterogeneously enhancing 8×6×9 cm mass arising from the duodenum, abutting the abdominal aorta but not eroding into the aorta (figure 2). For better evaluation of the gastrointestinal bleed a CT scan of the abdomen using the gastrointestinal bleed protocol was also obtained which showed evidence of active extravasation of contrast material in the duodenum. Invasive angiography of the abdomen was subsequently performed to confirm the finding on CT angiogram and potentially to perform embolisation of the culprit vessel if it was found. There was however no active extravasation on invasive angiography, so no embolisation was performed.
Figure 2.
Abdominal CT scan showing a duodenal mass (8×6×9 cm).
Esophagogastroduodenoscopy (EGD) was then performed to attempt to localise the source of the bleed and for potential therapeutic intervention. The EGD was however abandoned because the endoscope could not be advanced beyond the first part of the duodenum due to an extrinsic compression from the mass that was earlier noted on the CT scan.
Treatment
Transfusion of packed red blood cells was administered with appropriate rise in haemoglobin level. However, she had further episodes of melena with associated hypotension, so a massive blood transfusion protocol was activated, and she was admitted to the medical intensive care unit (ICU). Having failed to treat the persistent gastrointestinal bleed using endoscopic and interventional radiology means, she had emergent laparotomy and pancreaticoduodenectomy with excision of the duodenal mass. Intraoperatively, the mass was found to have invaded the duodenum with an overlying necrotic duodenal ulcer; however, there was no aortoenteric communication. The mass could not be excised intact, so it was dissected piece meal, but all the grossly visible tumour could not be excised. Postoperatively, she was transferred to the surgical ICU. She recovered well post operatively and was transferred out of the ICU 7 days postoperatively.
Histopathology and immunohistochemistry of the duodenal mass confirmed a similar histology with the primary EC that was found in the hysterectomy specimen 10 months prior to presentation.
Outcome and follow-up
Her postoperative course was complicated by wound dehiscence which necessitated abdominal closure 5 days postoperatively. After she was transferred out of the ICU, she also developed hyperactive delirium which further prolonged her hospital stay. She had repeat abdominal CT scan which showed no intraabdominal infection; blood and urine cultures also showed no infection. She was managed with supportive care and antipsychotic medication (quetiapine) in conjunction with consultant psychiatrist. On the Eastern Cooperative Oncology Group performance status scale, she had a score of 3; indicating a poor functional status. The medical oncologist, clinical oncologist and the palliative care physician discussed the options for adjuvant therapy with the patient and her family but the patient was not a candidate for any adjuvant therapy given her performance status of 3. After understanding the aggressive nature of the disease and the poor prognosis, the patient and her family opted for supportive care and she was discharged to inpatient hospice 40 days postoperatively. She subsequently died 90 days postoperatively.
Discussion
CC endometrial carcinoma is a high-risk histologic subtype of EC and carries a worse prognosis when compared with the endometrioid subtype.3 6 The National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology guidelines recommend primary surgical treatment for EC in order to allow for complete surgical staging.7 8 The recommended surgical treatment for EC is TAH, BSO, lymph node dissection, omentectomy and inspection of the peritoneal and diaphragmatic surfaces with or without peritoneal cytology.7 8 The NCCN guidelines recommend adjuvant systemic platinum-based chemotherapy with or without radiation for patients with Stages IB-IV EC with high-risk histology.7
Our patient had adequate surgical treatment as recommended by the guidelines. Pathologic staging after our patient’s TAH, BSO, omentectomy and lymph node dissection showed myometrial, serosal and bilateral fallopian tube involvement which was consistent with stage IIIA disease.9 10 Had she not been lost to follow-up, she would have received adjuvant therapy as recommended. Her lack of follow-up resulted in a missed opportunity for appropriate adjuvant chemotherapy and possible earlier detection of disease metastasis.
The lymph node dissection during her primary surgery was adequate and the pelvic and para aortic nodes had no tumour involvement on histopathology: She had no residual disease. The lack of lymph node involvement after primary surgery was a favourable prognostic factor for our patient although CC EC is a high-risk histologic subtype. The presence of lymphovascular space invasion (LVSI), especially if severe, is a marker that portends poor prognosis in EC.11 12 LVSI also puts patients at risk for both loco regional and distant spread and recurrence of EC.11 12 On the initial TAH, BSO histopathology, LVSI was present and we believe that this contributed to the development of duodenal metastasis via hematogenous spread in this case.
The common sites of recurrence for EC include the vagina, pelvic and para aortic lymph nodes, peritoneum and lungs.13 14 Other recognised, but less common sites of recurrence include the liver, spleen, adrenals, abdominal wall, muscle, brain and bones.13–15 To date, we know of three case of metastasis of endometrial carcinoma to the duodenum but none of them had CC histology.16–18 We believe that this is the first reported case of metastasis of CC endometrial carcinoma to the duodenum.
Both the primary TAH specimen and the duodenal metastasis specimen had DNA mismatch repair deficiency on immunohistochemistry (MSH6-deficiency). In patients with EC who experience recurrence after adjuvant first line platinum-based chemotherapy, the presence of microsatellite instability serves as a predictive biomarker for potential response to treatment with immune checkpoint inhibitors such as pembrolizumab.7 19 If her tumour demonstrated PD-L1 expression greater than 1%, she may have benefitted from pembrolizumab after failing first line chemotherapy.19
Learning points.
The finding of clear cell and other high-risk histologic subtypes in endometrial cancer (EC) should make the clinician consider metastatic disease to uncommon sites, including the small bowel as illustrated in this case. In patients with a history of high-risk endometrial cancer, even when evaluating common symptoms, metastatic disease should be considered as part of the differential diagnoses and appropriate work-up should be instituted. Efforts should also be made to provide additional educational materials to patients and their caregivers on the nature of these high-risk EC subtypes as education may help them maintain better follow up and potentially enable them to receive timely treatment. Patients with clear cell EC need strict follow-up to ensure adequate adjuvant therapy and monitoring for disease recurrence or progression.
We report this case to alert clinicians to the possibility of metastases to the small intestine in patients with clear cell EC.
Footnotes
Contributors: OE and CD identified the case for a case report. All authors developed the case report manuscript and reviewed the work prior to submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Mendivil A, Schuler KM, Gehrig PA. Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control 2009;16:46–52. 10.1177/107327480901600107 [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983;15:10–17. 10.1016/0090-8258(83)90111-7 [DOI] [PubMed] [Google Scholar]
- 4.Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol 2002;9:145–84. 10.1097/00125480-200205000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Raglan O, Kalliala I, Markozannes G, et al. . Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer 2019;145:1719–30. 10.1002/ijc.31961 [DOI] [PubMed] [Google Scholar]
- 6.McGunigal M, Liu J, Kalir T, et al. . Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: a national cancer database analysis. Int J Gynecol Cancer 2017;27:85–92. 10.1097/IGC.0000000000000844 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Uterine neoplasms (version 5.2019). Available: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf [Accessed 12 Feb 2020].
- 8.Colombo N, Preti E, Landoni F, et al. . Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi33–8. 10.1093/annonc/mdt353 [DOI] [PubMed] [Google Scholar]
- 9.Freeman SJ, Aly AM, Kataoka MY, et al. . The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics 2012;32:1805–27. 10.1148/rg.326125519 [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC. Ajcc cancer staging manual. 7th edition New York: Springer, 2010. [Google Scholar]
- 11.Bosse T, Peters EEM, Creutzberg CL, et al. . Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer--A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer 2015;51:1742–50. 10.1016/j.ejca.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Hirschowitz L, Zaino R, et al. . Pathologic prognostic factors in endometrial carcinoma (other than tumor type and grade). Int J Gynecol Pathol 2019;38 Suppl 1:S93–113. 10.1097/PGP.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurra V, Krajewski KM, Jagannathan J, et al. . Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging 2013;13:113–22. 10.1102/1470-7330.2013.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignatov T, Eggemann H, Costa SD, et al. . Endometrial cancer subtypes are associated with different patterns of recurrence. J Cancer Res Clin Oncol 2018;144:2011–7. 10.1007/s00432-018-2711-8 [DOI] [PubMed] [Google Scholar]
- 15.Creasman WT, Morrow CP, Bundy BN, et al. . Surgical pathologic spread patterns of endometrial cancer. A gynecologic Oncology Group study. Cancer 1987;60:2035–41. [DOI] [PubMed] [Google Scholar]
- 16.Singh T, Gandhi D, Arora T, et al. . Upper gastrointestinal bleeding due to metastatic endometrial adenocarcinoma. ACG Case Rep J 2019;6:e00138. 10.14309/crj.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider JJ, Shroff S, Moser AJ. Palliative segmental duodenectomy for bleeding, erosive endometrial cancer. Gynecol Oncol 2005;97:246–8. 10.1016/j.ygyno.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 18.Leitão C, Caldeira A, Banhudo A. A rare cause of intestinal bleeding: duodenal metastasis from endometrial cancer. Rev Esp Enferm Dig 2017;109:596. 10.17235/reed.2017.4822/2017 [DOI] [PubMed] [Google Scholar]
- 19.Ott PA, Bang Y-J, Berton-Rigaud D, et al. . Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1–Positive endometrial cancer: results from the KEYNOTE-028 study. JCO 2017;35:2535–41. 10.1200/JCO.2017.72.5952 [DOI] [PubMed] [Google Scholar]