Abstract
Segmental arterial mediolysis (SAM) is an uncommon condition and commonly missed diagnostic aetiology of acute abdominal pain, initially described in 1976. SAM is a non-inflammatory, non-atherosclerotic vasculopathy mostly involving the abdominal arteries with notable asymmetric involvement of the walls of the mesenteric arteries and their branches. Clinical presentation ranges from postprandial abdominal discomfort suggestive of mesenteric ischaemia to intra-abdominal bleeding. Pathophysiological explanation and prognosis of these cases are not well understood and therefore no clear guidelines for management exist. In this case report, we emphasise the imaging modalities used to reach the diagnosis and the management options available.
Keywords: radiology, general practice / family medicine
Background
This case highlights the imaging characteristics and clinical presentations of segmental arterial mediolysis (SAM). Despite being a relatively uncommon diagnosis with yet unknown incidence, SAM is an important diagnosis for the general medicine practitioners to be aware of given the risks of life-threatening spontaneous intraperitoneal haemorrhage and acute organ ischaemia. An increasing number of cases have been reported over the past few years mostly based on single centre retrospective studies and largely due to more well-defined radiological imaging characteristics. This is a commonly overlooked cause of abdominal pain where an early diagnosis and lifestyle modifications may prevent disease progression and catastrophic sequelae.
Case presentation
A 71-year-old healthy woman presented to an outlying hospital with the primary symptoms of 1 week of acute abdominal pain associated with nausea and vomiting. Pain was worse after eating, intermittent, non-radiating and predominantly located in epigastrium and left lower quadrant. A computed tomography (CT) abdomen and pelvis was done that demonstrated abnormal perivascular fat stranding around mesenteric vessels as shown in figure 1. The patient was subsequently discharged home on a proton pump inhibitor. Patient returned with persistent pain to another outlying hospital within a few hours, and a repeat CT abdomen and pelvis with contrast was done. This showed non-specific stranding around the coeliac trunk with irregularity of vessels raising concern for mesenteric vasculitis. Patient was transferred to our hospital for further evaluation by rheumatology. On presentation to our hospital, she was afebrile and blood pressure was elevated at 134/88 mm Hg without tachycardia. On initial examination, she was pain free, abdomen was non-tender, bowel sounds were normal and no masses or organomegaly were appreciated.
Figure 1.
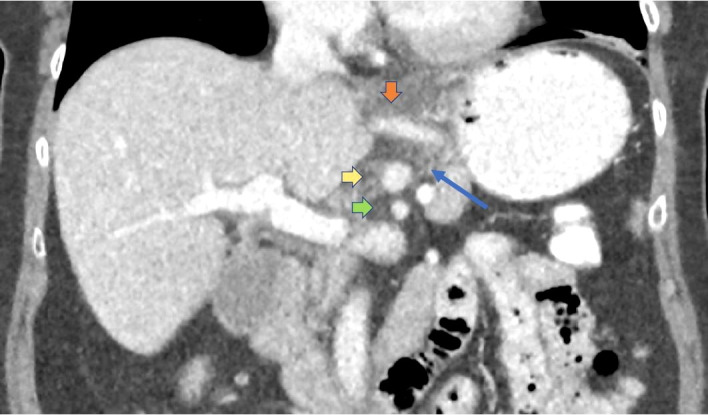
Initial CT abdomen and pelvis with intravenous contrast performed in standard portal venous phase (70–80 s postcontrast injection); coronal two-dimensional reformatted image through upper abdomen. Abnormal perivascular hazy fat stranding (long blue arrow) seen as increased soft tissue attenuation around the mesenteric arteries. This is a non-specific finding that can reflect oedema, inflammation or cellular infiltration. Limited detailed assessment of the arterial walls due to venous timing of imaging. Orange vertical arrow: left gastric artery and accessory left hepatic artery. Yellow horizontal arrow: coeliac artery. Green horizontal arrow: superior mesenteric artery.
Investigations
Normal erythrocyte sedimentation rate (ESR) of 5 mm/hour. Complete blood count and metabolic profile were normal. Lactic acid level was normal at 0.7 mmol/L. Inflammatory and autoimmune workup was done with results noted in table 1.
Table 1.
Inflammatory and autoimmune workup results
| Laboratory tests | Result (ref range) |
| Hepatitis-B surface antigen | Non-detected |
| Hepatitis-C antibody | <1 signal/cut-off ratio |
| HIV-1 and HIV-2 antibody antigen screen | Non-detected |
| ANA screen | Negative |
| Double-stranded DNA antibody | <4 IU/mL |
| Sjogren’s antibody (SS-A and SS-B) | <1 IU/mL |
| Smooth muscle antibody | <1 IU/mL |
| RNP antibody | <1 IU/mL |
| Chromatin antibody | <1 IU/mL |
| Complement C3 | 155 (83–193 mg/dL) |
| Complement C4 | 24 (15–57 mg/dL) |
| Complement total CH50 | 60 (31–60 U/mL) |
ANA, Antinuclear Antibody; CRP, C-Reactive Protein; ESR, Erythrocyte sedimentation rate; RNP antibody, Ribonucleoprotein antibody; SS-A, Ro Antibody; SS-B, La Antibody.
On resuming clear liquid diet, she reported acute recurrence of pain. Due to concern for mesenteric ischaemia, CT angiogram (CTA) was done this time with standard angiographic phase (15–20 s after contrast administration) as shown in figure 2. Patient had positive angiographic evidence of mesenteric artery arteriopathy diffusely involving coeliac artery and extending to diffusely involve hepatic arteries and left gastric artery; similarly, characterised and diffusely involved inferior mesenteric artery was noted. Superior mesenteric artery and remaining intra-abdominal and pelvic arteries appeared spared. CTA appearance and distribution strongly favoured SAM as shown in figure 2.
Figure 2.
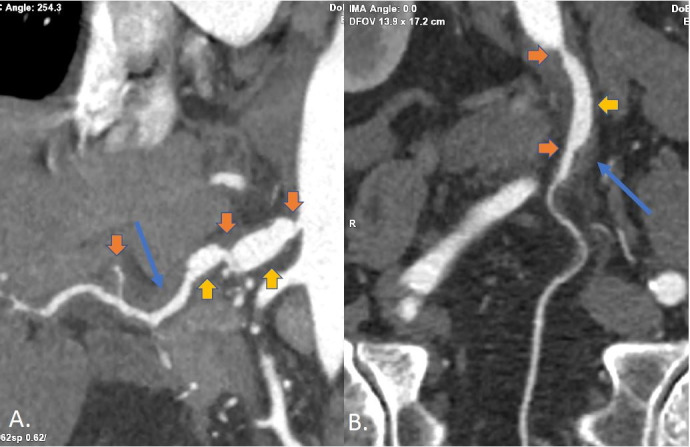
Subsequent dedicated computed tomography angiogram (CTA) abdomen and pelvis with intravenous contrast performed in standard angiographic phase (15–20 s postcontrast injection); representative three-dimensional reformatted images through the coeliac and common hepatic arteries (A) as well as the inferior mesenteric artery (B). Orange arrows: multifocal skip pattern of irregular non-atherosclerotic luminal strictures from arterial mural wall thickening/thromboses. Yellow arrows: associated poststenotic fusiform aneurysmal arterial dilations. Long blue arrows: persistent abnormal hazy fat stranding seen as increased soft tissue attenuation around the involved arteries. The angiographic phase timing and thin slices obtained with dedicated CTA allow excellent reformats to be generated along vessel lengths for improved visualisation and characterisation of findings of segmental arterial mediolysis of mesenteric arteries. The aorta, splenic artery, renal arteries and iliac arteries were spared.
Treatment
Vascular surgery was consulted and conservative management was recommended. A lipid profile showed elevated total cholesterol and mildly elevated triglyceride and she was started on atorvastatin 40 mg per day. Amlodipine was started for elevated blood pressure.
Outcome and follow-up
Our patient was subsequently encouraged to advance diet as tolerated, starting with small and frequent meals. She was followed up by the primary care provider and reported no recurrence of pain, though she had continued to avoid large meals.
Discussion
SAM is a non-inflammatory, non-atherosclerotic condition most commonly involving the abdominal arterial lining but carotid and retroperitoneal involvement have been reported as well.1 2 Clinical presentation ranges from postprandial abdominal discomfort suggestive of mesenteric ischaemia to intra-abdominal bleeding. Imaging findings can mimic inflammatory vasculitis, such that the diagnosis of SAM is one of exclusion. Vasospasm is thought to be a precursor of SAM, though exact pathogenesis of disease remains unclear.3 In one single centre study, the renal artery was found to be most frequently involved, followed by the superior mesenteric and coeliac arteries, although it can involve any arterial branch of the intra-abdominal vasculature. In another study, the middle colic artery was reportedly involved in up to 50% of cases. The published data are based on single centre studies such that the criteria for case inclusions and sample bias have influenced the results reported.4–6 In a single centre study, approximately 70% of cases were men and the median age of presentation was 51 years.5 Some authors have raised questions regarding relationship of fibromuscular dysplasia (FMD) and SAM. Although in contrast to SAM, FMD has been shown to effect mostly women, usually earlier in life and involving mainly renal and carotid arteries.7–10
With more advanced CT angiography techniques, more cases are being diagnosed based on a combination of imaging and clinical criteria. CT angiogram in these cases will typically show dissecting aneurysm, multifocal non-atherosclerotic skip pattern of irregular luminal strictures, due to arterial mural wall thickening and poststenotic aneurysmal dilatation of arteries. Kalva et al in their study of 14 patients, reported dissection in 10 patients. Naidu et al in their larger sample of 111 patients reported that dissection was seen in 86% of cases.5 11 The angiographic phase timing and thin slices obtained with dedicated CT angiography allow excellent reformats to be generated along vessel lengths for improved visualisation and characterisation of findings of SAM. The most common imaging findings that lead to secondary organ ischaemia or acute intraperitoneal haemorrhage are arterial dissection and pseudoaneurysm formation, respectively.5 11 Kalva et al formulated criteria for non-invasive diagnosis of SAM, which included clinical and imaging characteristics. The clinical criteria for diagnosis included absence of connective tissue disease, FMD, arteritis in the presence of acute or chronic abdominal/flank/back pain. The imaging characteristics for diagnosis were presence of dissection, beading, wall thickening, aneurysm in the absence of continuous aortic dissection or atherosclerosis. The absence of serological markers such as antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) was included in diagnostic criteria, as the imaging findings commonly mimic inflammatory vasculitis.11
SAM should be considered in the differential diagnosis for patients presenting with unexplained abdominal pain in middle-aged and elderly patients. In addition, patients with known history of SAM presenting with acute abdominal pain should undergo CTA abdomen and pelvis early in their clinical course in order to avoid delay in diagnosis of acute intraperitoneal haemorrhage or organ ischaemia.
Several authors have described SAM patients presenting with intra-abdominal haemorrhage secondary to rupture of aneurysms or pseudoaneurysms of involved arteries, subsequently treated with endovascular coil embolisation and arterial ligation.12–15 Fortunately our patient had no intra-abdominal haemorrhage and she had an excellent response to medical management with significant improvement in her pain. Mortality rate of 50% is reported in patients initially presenting with haemorrhage and several case reports have been focused on this critical presentation.15–17 Prognosis of SAM is otherwise unknown in patients presenting with less severe symptoms. Naidu et al recently performed a single centre study in which they reported haematoma in only 9% of cases. They also reported common imaging characteristics and progression of disease, based on imaging follow-up. Ninety-seven out of 111 patients were followed up by imaging in 12 months, with reported progression in approximately 20% of cases. Interestingly most of these cases showing imaging progression did not have concomitant clinical symptomatology. Even with progression noted on follow-up imaging, no deaths were reported and only one patient required coil embolisation.5 In this case series and other case reports, regression or stability of imaging findings was seen in 80% cases.18–21 Contrary to this, another pooled case study reported mortality rate of 40% with SAM.22 Again, physicians should keep in mind that the results on reported outcomes have been skewed due to sampling bias and the high mortality rate is an overestimate due to a large number of cases being less symptomatic and undiagnosed. The case studies reporting more favourable outcomes had patient populations in which haemorrhage was not the initial presentation.
No standardised guidelines exist for the management of these cases. For patients not presenting with acute haemorrhage, antiplatelet or anticoagulation have been initiated when there is evidence of organ ischaemia or infarction based on discretion of the vascular surgeon. Lifestyle modifications including dietary modification and strict control of dyslipidaemia and hypertension may be crucial to prevent further shear pressure on arteries. This is usually a self-limiting disease treated conservatively, though no established guidelines exist for optimal medical therapy. Our patient was treated with an antihypertensive agent in addition to both low-dose aspirin and statin. This case report highlights an uncommon diagnosis where early detection may prevent potential life-threatening complications.
Learning points.
Segmental arterial mediolysis (SAM) most frequently presents in the middle-aged and elderly population with acute abdominal pain.
Prognosis of disease is not well-studied and most results are based on single centre retrospective studies. Many SAM cases show a non-progressive course.
Patients may benefit from computed tomography angiogram imaging follow-up to document stability, resolution or worsening of arterial irregularities in order to stratify those who may be at risk for acute intraperitoneal haemorrhage or organ ischaemia.
Early lifestyle modifications with strict control of blood pressure may prevent progression of the disease.
Footnotes
Contributors: MR wrote the initial manuscript, performed study design. AKR and MEM reviewed the initial manuscript and edited it. MR and AKR performed data collection. MEM performed image collection and editing. All authors revised and edited the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Phillips CK, Lepor H. Spontaneous retroperitoneal hemorrhage caused by segmental arterial mediolysis. Rev Urol 2006;8:36–40. [PMC free article] [PubMed] [Google Scholar]
- 2. Ro A, Kageyama N, Takatsu A, et al. Segmental arterial mediolysis of varying phases affecting both the intra-abdominal and intracranial vertebral arteries: an autopsy case report. Cardiovasc Pathol 2010;19:248–51. 10.1016/j.carpath.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 3. Slavin RE, Gonzalez-Vitale JC. Segmental mediolytic arteritis: a clinical pathologic study. Lab Invest 1976;35:23–9. [PubMed] [Google Scholar]
- 4. Shenouda M, Riga C, Naji Y, et al. Segmental arterial mediolysis: a systematic review of 85 cases. Ann Vasc Surg 2014;28:269–77. 10.1016/j.avsg.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 5. Naidu SG, Menias CO, Oklu R, et al. Segmental arterial Mediolysis: abdominal imaging of and disease course in 111 patients. AJR Am J Roentgenol 2018;210:899–905. 10.2214/AJR.17.18309 [DOI] [PubMed] [Google Scholar]
- 6. Inada K, Maeda M, Ikeda T. Segmental arterial mediolysis: unrecognized cases culled from cases of ruptured aneurysm of abdominal visceral arteries reported in the Japanese literature. Pathol Res Pract 2007;203:771–8. 10.1016/j.prp.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 7. Slavin RE, Saeki K, Bhagavan B, et al. Segmental arterial mediolysis: a precursor to fibromuscular dysplasia? Mod Pathol 1995;8:287–94. [PubMed] [Google Scholar]
- 8. Hall ET, Gibson BA, Hennemeyer CT, et al. Segmental arterial mediolysis and fibromuscular dysplasia: what comes first, the chicken or the egg? Cardiovasc Pathol 2016;25:113–5. 10.1016/j.carpath.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 9. Lie JT. Segmental mediolytic arteritis. not an arteritis but a variant of arterial fibromuscular dysplasia. Arch Pathol Lab Med 1992;116:238–41. [PubMed] [Google Scholar]
- 10. Slavin RE. Segmental arterial mediolysis: course, sequelae, prognosis, and pathologic-radiologic correlation. Cardiovasc Pathol 2009;18:352–60. 10.1016/j.carpath.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 11. Kalva SP, Somarouthu B, Jaff MR, et al. Segmental arterial mediolysis: clinical and imaging features at presentation and during follow-up. J Vasc Interv Radiol 2011;22:1380–7. 10.1016/j.jvir.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 12. Pillai AK, Iqbal SI, Liu RW, et al. Segmental arterial mediolysis. Cardiovasc Intervent Radiol 2014;37:604–12. 10.1007/s00270-014-0859-4 [DOI] [PubMed] [Google Scholar]
- 13. Britto MM, Lukies M, Milne C, et al. Case of segmental arterial Mediolysis. BMJ Case Rep 2018;35:pii: bcr-2017-223731 10.1136/bcr-2017-223731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beerle C, Soll C, Breitenstein S, et al. Spontaneous rupture of an intrahepatic aneurysm of the right hepatic artery caused by segmental arterial mediolysis. BMJ Case Rep 2016;2016:pii: bcr2015214109. 10.1136/bcr-2015-214109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hashimoto T, Deguchi J, Endo H, et al. Successful treatment tailored to each splanchnic arterial lesion due to segmental arterial mediolysis (SAM): report of a case. J Vasc Surg 2008;48:1338–41. 10.1016/j.jvs.2008.05.056 [DOI] [PubMed] [Google Scholar]
- 16. Rott G, Boecker F. Segmental arterial Mediolysis of omental arteries with haemoperitoneum: case report with embolization of the left omental artery and brief review of literature. Case Rep Radiol 2018;2018:1–5. 10.1155/2018/4749356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gulati G, Ware A. Segmental arterial mediolysis: a rare non-inflammatory cause of mesenteric bleeding. BMJ Case Rep 2015;2015:pii: bcr2015210344. 10.1136/bcr-2015-210344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michael M, Widmer U, Wildermuth S, et al. Segmental arterial mediolysis: cta findings at presentation and follow-up. AJR Am J Roentgenol 2006;187:1463–9. 10.2214/AJR.05.0281 [DOI] [PubMed] [Google Scholar]
- 19. Ryan JM, Suhocki PV, Smith TP. Coil embolization of segmental arterial mediolysis of the hepatic artery. J Vasc Interv Radiol 2000;11:865–8. 10.1016/S1051-0443(07)61802-8 [DOI] [PubMed] [Google Scholar]
- 20. Gahide G, Servant S, Giroux MF. Vanishing renal artery disease: a segmental arterial mediolysis story. Kidney Int 2011;80:1002. 10.1038/ki.2011.279 [DOI] [PubMed] [Google Scholar]
- 21. Sakano T, Morita K, Imaki M, et al. Segmental arterial mediolysis studied by repeated angiography. Br J Radiol 1997;70:656–8. 10.1259/bjr.70.834.9227264 [DOI] [PubMed] [Google Scholar]
- 22. Tameo MN, Dougherty MJ, Calligaro KD. Spontaneous dissection with rupture of the superior mesenteric artery from segmental arterial mediolysis. J Vasc Surg 2011;53:1107–12. 10.1016/j.jvs.2010.11.034 [DOI] [PubMed] [Google Scholar]


