Abstract
Background
The Centers for Disease Control and Prevention advises that patients with moderate to severe asthma belong to a high-risk group that is susceptible to severe coronavirus disease 2019 (COVID-19). However, the association between asthma and COVID-19 has not been well-established.
Objective
The primary objective was to determine the prevalence of asthma among patients with COVID-19 in a major US health system. We assessed the clinical characteristics and comorbidities in asthmatic and nonasthmatic patients with COVID-19. We also determined the risk of hospitalization associated with asthma and/or inhaled corticosteroid use.
Methods
Medical records of patients with COVID-19 were searched by a computer algorithm (March 1 to April 15, 2020), and chart review was used to validate the diagnosis of asthma and medications prescribed for asthma. All patients had PCR-confirmed COVID-19. Demographic and clinical features were characterized. Regression models were used to assess the associations between asthma and corticosteroid use and the risk of COVID-19–related hospitalization.
Results
Of 1526 patients identified with COVID-19, 220 (14%) were classified as having asthma. Asthma was not associated with an increased risk of hospitalization (relative risk, 0.96; 95% CI, 0.77-1.19) after adjusting for age, sex, and comorbidities. The ongoing use of inhaled corticosteroids did not increase the risk of hospitalization in a similar adjusted model (relative risk, 1.39; 95% CI, 0.90-2.15).
Conclusions
Despite a substantial prevalence of asthma in our COVID-19 cohort, asthma was not associated with an increased risk of hospitalization. Similarly, the use of inhaled corticosteroids with or without systemic corticosteroids was not associated with COVID-19–related hospitalization.
Key words: COVID-19, SARS-CoV-2, asthma, risk factors, morbidity, severity, corticosteroid, long-acting β-agonist, allergic rhinitis, rhinosinusitis
Abbreviations used: BMI, Body mass index; CAD, Coronary artery disease; CDC, Centers for Disease Control and Prevention; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; DM, Diabetes mellitus; HTN, Hypertension; ICD-10, International Classification of Diseases, Tenth Revision; ICS, Inhaled corticosteroid; ICU, Intensive care unit; LABA, Long-acting β-agonist; OSA, Obstructive sleep apnea; RR, Relative risk; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel betacoronavirus that was first detected in December 2019. Coronavirus disease 2019 (COVID-19) has rapidly spread globally, causing severe pneumonia along with additional complications including death in the most severely affected individuals. Community spread likely has occurred rapidly because the virus transmits easily, even in asymptomatic patients, and remains viable in respiratory droplets and fomites.1 Three months after first emerging, fueled by community transmission, there were approximately 2.6 million cases reported globally—including 900,000 cases in the United States and 40,000 cases in Illinois according to the Centers for Disease Control and Prevention (CDC). The outcomes of COVID-19 are worsened by several comorbidities, including hypertension (HTN), chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), cardiovascular disease, and obesity.2 , 3 Whether asthma stands among these exacerbating factors requires further study.
Asthma is one of the most common chronic diseases in the United States (∼8%-9% of the population), with acute exacerbations being a frequent cause of hospitalizations and/or emergency room visits.4 , 5 Respiratory viruses are well-known triggers of asthma exacerbations.6, 7, 8 Coronaviruses are respiratory viruses and have been implicated in both upper respiratory tract infections and asthma exacerbations.9 What is currently unclear is how SARS-CoV-2 impacts patients with asthma. Data from published studies suggest that the prevalence of asthma in the COVID-19 population in China was ≤1%.10 , 11 The reported prevalence of asthma in patients with COVID-19 in the United States varies from 7.4% to 17%.2 , 12, 13, 14
Currently, the CDC classifies patients with underlying moderate to severe asthma as a high-risk group that is susceptible to severe COVID-19. For patients with asthma, the symptoms of COVID-19, including cough, shortness of breath, and chest tightness, are difficult to distinguish from those of a severe asthma exacerbation. This symptom pattern overlap may make it more difficult for both patients and their treating physicians to diagnose and manage their disease. The degree of risk and associated clinical outcomes for people with asthma, however, are not clearly understood on the basis of available data.
Published studies have concentrated on hospitalized patients with COVID-19, which makes it difficult to determine whether asthma is a risk factor for COVID-19 or increases COVID-19–related morbidity. The primary objective of the current study was to determine the prevalence of asthma and comorbidities associated with asthma in inpatients and outpatients with COVID-19. Second, we tested the risk of COVID-19–related hospitalization among those with asthma compared with those without asthma. Finally, we examined the association of corticosteroid use in patients with asthma and COVID-19.
Methods
Identification of patients with COVID-19
This retrospective study was conducted across 10 hospitals affiliated with Northwestern Medicine, one of the largest health systems in Chicago and surrounding Illinois suburbs. Study patients were identified by automated chart review using Northwestern Medicine’s Enterprise Data Warehouse, an electronic repository of inpatient and outpatient health records of more than 6.6 million distinct patients (from Illinois and surrounding states) seen within the health system. This study was approved by the Northwestern University Feinberg School of Medicine’s Institutional Review Board.
Patients of all ages (including 2 patients <18 years old) were included in this study if they were evaluated between March 1, 2020, and April 15, 2020, within Northwestern Medicine and had received the International Classification of Disease, Tenth Revision (ICD-10) diagnosis code for COVID-19 (U07.1). Presumed COVID-19 patients (U07.2) without laboratory RT-PCR were not included in this study. Of the 1837 patients identified with COVID-19, 295 were excluded because the presence of SARS-CoV-2 was not confirmed. Mortality in our study cohort was determined up to April 30, 2020.
Identification of asthma among patients with COVID-19
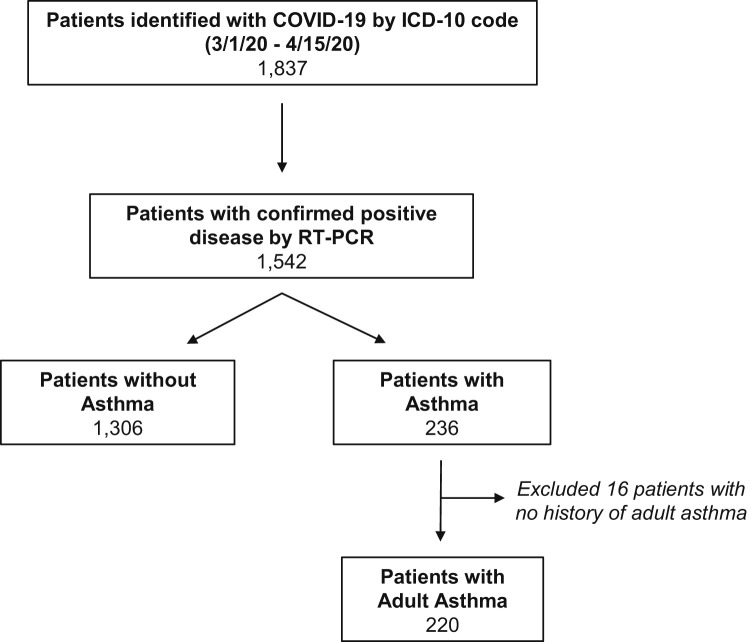
Data collected from RT-PCR–confirmed COVID-19 patients (N = 1542) were subsequently stratified on the basis of the presence (N = 236) or absence (N = 1306) of comorbid asthma as assessed by International Classification of Disease, Ninth Revision or ICD-10 codes (any 493.xx or J45.xx) (Fig 1 ). Manual chart review of all patients with asthma was then performed to confirm a diagnosis of asthma. The criteria used to classify asthma included either a physician diagnosis of asthma or self-reported history of asthma. Patients with a diagnosis of childhood asthma (N = 16) but no diagnosis of asthma as an adult were excluded.
Fig 1.
Algorithm for identifying patients with COVID-19 and patients with asthma. Patients with COVID-19 were identified using the ICD-10 diagnosis code and COVID-19 PCR. Patients with asthma were identified by International Classification of Disease diagnosis code and confirmed by chart review.
Identification of clinical characteristics and comorbidities
Automated chart review was performed to identify clinical characteristics including age, sex, race/ethnicity, smoking status, and obesity (body mass index [BMI] ≥30). International Classification of Disease, Ninth Revision and ICD-10 codes were used to identify clinical comorbidities including HTN, DM, obstructive sleep apnea (OSA), coronary artery disease (CAD), COPD, allergic rhinitis, rhinosinusitis, and immunodeficiency. Immunodeficiency was defined as the presence of common variable immunodeficiency, antibody deficiency, or IgA deficiency (see Table E1 in this article’s Online Repository at www.jacionline.org).
Assessment of asthma medications
For each patient with asthma, a manual chart review was performed to document a prescription of inhaled corticosteroids (ICS), combination ICS plus long-acting β-agonists (ICS/LABA), and/or systemic corticosteroids at the time of the diagnosis of COVID-19 or hospitalization.
Identification of laboratory values
When available, laboratory measurements including white blood cell counts, absolute eosinophil counts, absolute lymphocyte counts, platelet counts as well as ferritin, lactate dehydrogenase, D-dimer, creatinine, and C-reactive protein levels were evaluated in each study patient at the time of COVID-19 diagnosis. If more than 1 laboratory value was available, the first value obtained up to 4 weeks after the diagnosis of COVID-19 was used for this study.
Statistical analysis
Demographic data and clinical characteristics were computed for all included participants and compared using chi-square tests. Differences in laboratory values were compared using nonparametric Mann-Whitney tests or Kruskal-Wallis test, where appropriate. Poisson regression models were used to calculate the relative risk (RR) of hospital admission (inpatient with or without intensive care unit [ICU] vs outpatient). The association between asthma and COVID-19 hospitalization was determined in patients with COVID-19 (N = 1526). Model covariables included (1) age, sex, and race/ethnicity (model 1) and (2) age, sex, race/ethnicity, smoking status, and comorbidities (model 2). Comorbidities included obesity, HTN, DM, OSA, CAD, COPD, allergic rhinitis, rhinosinusitis, and immunodeficiency. Similar models were used for the analysis sample of only patients with COVID-19 with asthma (N = 220) in which the association between ICS use and hospitalization was tested. There were only a small number of patients (N = 15) among 220 patients with asthma receiving systemic corticosteroids. In a sensitivity analysis, we repeated the analysis after excluding these 15 patients to examine whether systemic corticosteroids may have any impact on the association of using ICS with the risk of hospitalization. Data were displayed and statistics were performed using SAS statistical software version 9.4 (SAS Institute, Inc, Cary, NC) and GraphPad Prism 8 (GraphPad Software, La Jolla, Calif).
Results
Prevalence of asthma among patients with COVID-19
An automated electronic review of patient medical records identified 1837 patients with an ICD-10 diagnosis code of COVID-19 in our system between March 1, 2020, and April 15, 2020. Of these, 1542 (84%) had confirmed disease by RT-PCR and were included in subsequent analyses (Table I ). Most patients with COVID-19 (N = 1306) did not have asthma. Of the 236 patients with comorbid COVID-19 and asthma by International Classification of Disease code, 16 patients did not have a diagnosis of adult asthma on further chart review. Our final analysis thus included 1526 patients with COVID-19, of which 220 (14.4%) had asthma (Fig 1).
Table I.
Demographic and clinical characteristics of patients with COVID-19 confirmed by RT-PCR and stratified by asthma status
| Characteristic, n (%) | All patients, 1526 (100) | Nonasthma, 1306 (86) | Asthma, 220 (14.4) | P value∗ |
|---|---|---|---|---|
| Age (y) | .05 | |||
| <40 | 414 (27.1) | 351 (26.9) | 63 (28.6) | |
| 40-69 | 844 (55.3) | 713 (54.6) | 131 (59.6) | |
| ≥70 | 268 (17.6) | 242 (18.5) | 26 (11.8) | |
| Sex | <.0001 | |||
| Female | 808 (53) | 652 (49.9) | 156 (70.9) | |
| Race/ethnicity | <.0001 | |||
| Non-Hispanic African American | 358 (23.5) | 280 (21.4) | 78 (35.5) | |
| Non-Hispanic white | 643 (42.1) | 548 (42) | 95 (43.2) | |
| Hispanic or Latino | 324 (21.2) | 296 (22.7) | 28 (12.7) | |
| Non-Hispanic Asian | 70 (4.6) | 63 (4.8) | 7 (3.2) | |
| Other | 201 (13.2) | 182 (13.9) | 19 (8.6) | |
| Smoking status | <.0001 | |||
| Current smoker | 53 (3.5) | 43 (3.3) | 10 (4.5) | |
| Former smoker | 336 (22) | 285 (21.8) | 51 (23.2) | |
| Never smoker | 897 (58.8) | 748 (57.3) | 149 (67.7) | |
| Unknown | 240 (15.7) | 230 (17.6) | 10 (4.6) | |
| Hospitalization | 853 (55.9) | 738 (56.5) | 115 (52.3) | .242 |
| Mortality† | 72 (4.7) | 64 (4.9) | 8 (3.6) | .413 |
Values in boldface indicate statistical significance.
P value indicated is for the comparison between asthma and nonasthma groups using χ2 test.
Mortality data in this cohort were determined up to April 30, 2020.
Demographic and clinical characteristics of patients with COVID-19 with and without asthma
We assessed and compared various demographic and clinical characteristics in patients with COVID-19 with and without comorbid asthma (Table I). Most (55.3%) patients with COVID-19 were between ages 40 and 69 years regardless of asthma status. Slightly more than half (53%) of all patients with COVID-19 were female, with a significant female predominance in the asthma cohort (70.9%). The primary race/ethnicities of the total COVID-19 cohort were non-Hispanic white (42.1%), non-Hispanic African American (23.5%), and Hispanic or Latino (21.2%). Within those with asthma, the percentage of patients identifying as non-Hispanic African American was 35.5%, which was significantly higher compared with 21.4% in the nonasthma cohort. Although Hispanics comprised a significant proportion of the asthma cohort (12.7%), their representation was even higher in the nonasthma group (22.7%). Hospitalization rate and mortality did not significantly differ between patients with COVID-19 with or without asthma.
Comparison of clinical comorbidities of patients with COVID-19 with and without asthma
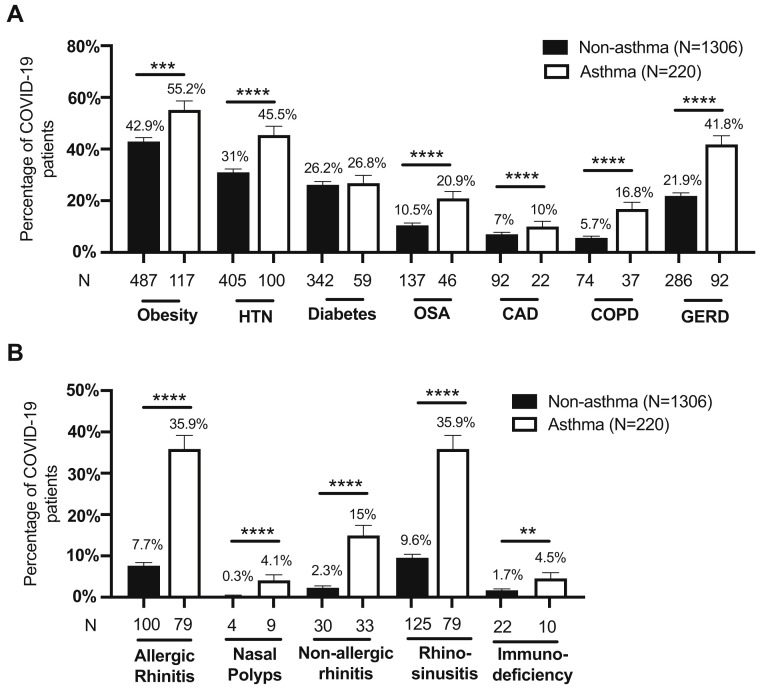
Next, we determined the prevalence of various comorbidities in patients with COVID-19 on the basis of their asthma status (Fig 2 ). Rates of obesity, HTN, OSA, CAD, COPD, and gastroesophageal reflux disease were significantly increased in the cohort of patients with COVID-19 with asthma compared with patients with COVID-19 without asthma (Fig 2, A). Patients with COVID-19 with asthma also had a higher prevalence of allergic rhinitis, rhinosinusitis, and immunodeficiencies (Fig 2, B).
Fig 2.
Prevalence of comorbid diseases in patients with COVID-19 stratified by asthma status. (A) Comorbid diseases associated with metabolic syndrome, heart disease, and chronic lung diseases, and (B) allergic diseases were evaluated. Immunodeficiency includes patients with a diagnosis of immunodeficiency, antibody deficiency, or IgA deficiency. Obesity was determined on the basis of reported BMI (≥30). For 2 patients who were younger than 20 years, the weight-for-age percentile was used instead of BMI. Bars represent mean ± SEM. Statistical comparisons were performed using chi-square tests. GERD, Gastroesophageal reflux disease. A total of 180 patients had missing BMI values. ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
Assessment of laboratory data at the time of COVID-19 diagnosis by asthma status
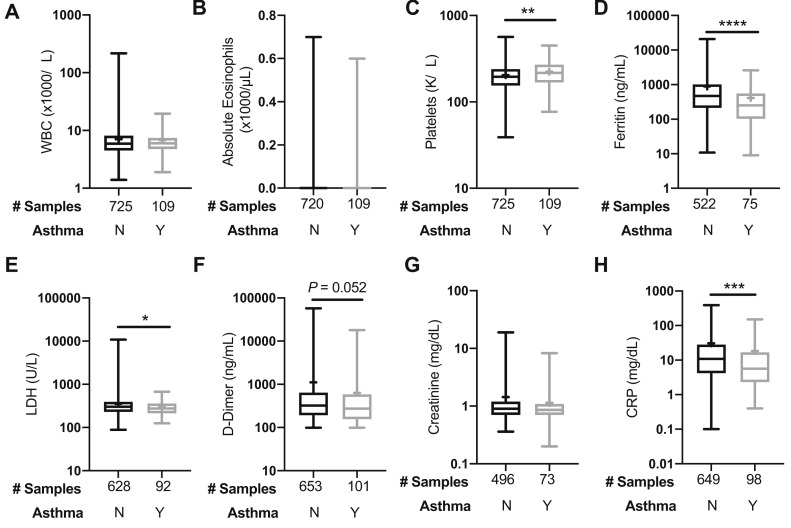
Results of various laboratory tests were collected for all hospitalized patients at the time of their COVID-19 diagnosis. If more than 1 laboratory value was available, we used the first value for up to 4 weeks after the diagnosis (Fig 3 ). Complete blood cell counts showed a white blood cell count and eosinophil count within normal limits, which did not differ significantly between patients with and without asthma (Fig 3, A and B). Platelet counts were significantly lower in the nonasthma subgroup versus the asthma subgroup (P = .006) (Fig 3, C). Ferritin, lactate dehydrogenase, and C-reactive protein, which have been described as markers of COVID-19 severity,15 were all significantly lower in patients with COVID-19 with asthma compared with patients with COVID-19 without asthma (P < .0001, .048, .0004, respectively). D-dimer was also lower in patients with asthma compared with patients without asthma although this was not statistically significant (P = .052). Absolute lymphocyte counts (×1000/μL) (median [Q1-Q3]) were lower in the ICU asthmatic patients with COVID-19 (0.8 [0.7-1.2]) compared with both non-ICU hospitalized (1.2 [0.8-1.6]) and outpatient asthmatic patients (1.2 [1.0-1.7]) (P = .03).
Fig 3.
Laboratory values at the time of COVID-19 diagnosis in hospitalized patients with a concurrent diagnosis of asthma compared with nonasthma. (A) WBCs, (B) absolute eosinophils, (C) platelets, (D) ferritin, (E) LDH, (F) D-dimer, (G) creatinine, and (H) CRP laboratory values are plotted using a box and whisker plot. The box extends from the 25th to 75th percentiles. The line within the box denotes median and a “+” is shown at the mean. Whiskers represent minimum and maximum values. “Y” (Yes) denotes the group with asthma, and “N” (No) denotes the nonasthma group. Statistical analysis was performed with nonparametric Mann-Whitney 2-tailed tests. CRP, C-Reactive protein; LDH, lactate dehydrogenase; WBC, white blood cell. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
Relative risks for COVID-19–associated hospitalization due to asthma
We used 2 different models to evaluate whether asthma was associated with an increased risk of hospitalization for COVID-19. After adjusting for baseline age, sex, and race/ethnicity (model 1), it was found that patients with asthma did not have a higher risk of COVID-19–related hospitalization compared with patients without asthma (RR, 1.01; 95% CI, 0.83-1.24) (Table II , model 1). When further adjusted for multiple risk factors including smoking, obesity, CAD, DM, HTN, OSA, COPD, allergic rhinitis, rhinosinusitis, and immunodeficiency (model 2), there was still no difference in the RR of hospitalization between the asthma and nonasthma cohorts (RR, 0.96; 95% CI, 0.77-1.19) (Table II, model 2).
Table II.
Adjusted RR (95% CI) for COVID-19–related hospital admission from March 1 to April 15, 2020, by asthma status
| Baseline risk factor profile | Asthma vs nonasthma, RR (95% CI) | P value |
|---|---|---|
| Model 1 | ||
| Adjusted for age, sex, and race/ethnicity | 1.01 (0.83-1.24) | .90 |
| Model 2 | ||
| Adjusted for age, sex, race/ethnicity, smoking, obesity, CAD, diabetes, HTN, OSA, COPD, allergic rhinitis, rhinosinusitis, and immunodeficiency | 0.96 (0.77-1.19) | .71 |
Using model 1, we assessed the individual risk of age, sex, or race/ethnicity on COVID-19–related hospitalization. In this analysis, younger age (<40 years) was associated with a lower RR of hospitalization (RR, 0.34; 95% CI, 0.27-0.42). Patients of Hispanic or Latino ethnicity (RR, 1.44; 95% CI, 1.21-1.72) or non-Hispanic African American (1.23; 95% CI, 1.03-1.46) race had a significantly higher risk of COVID-19–related hospitalization compared with non-Hispanic white patients (see Table E2 in this article’s Online Repository at www.jacionline.org). These demographic risks for hospitalization were present irrespective of asthma status. Even when adjusting for comorbidities using model 2, Hispanics continued to be at increased risk of hospitalization due to COVID-19 (RR, 1.35; 95% CI, 1.12-1.63; Table E2). However, in this model, non-Hispanic African Americans no longer had a significantly elevated RR of hospitalization compared with non-Hispanic white patients (see Table E2). Age (≥70 years), male sex, and comorbid diagnoses of diabetes (RR, 1.16; 95% CI, 1.00-1.36) and OSA (RR, 1.23; 95% CI, 1.01-1.49) also elevated the RR of COVID-19 hospitalization regardless of asthma status (Table E2, model 2).
Rhinosinusitis was associated with a significantly lower risk of hospitalization compared with the absence of rhinosinusitis (RR, 0.78; 95% CI, 0.61-0.99) (Table E2, model 2). Patients with allergic rhinitis also showed a trend toward lower hospitalization although not statistically significant (RR, 0.83; 95% CI, 0.64-1.07). These associations with rhinosinusitis and allergic rhinitis were observed in patients with COVID-19 with or without asthma.
Relative risks for COVID-19–associated hospitalization due to corticosteroid use
We also explored the relationship between ICS and the risk of hospitalization in patients with COVID-19 with asthma using 2 different statistical models. More than half (52%, N = 114) the patients with COVID-19 with asthma were not prescribed either ICS or ICS/LABA at the time of diagnosis (see Table E3 in this article’s Online Repository at www.jacionline.org), whereas among those with asthma, 11.8% and 36.4% had documentation of ICS (N = 26) or ICS/LABA (N = 80), respectively, at the time of COVID-19 diagnosis. The breakdown of inhaler use among patients with COVID-19 by the level of medical care is shown in Fig 4 . Although the percentage of patients with COVID-19 with asthma stratified by ICS use and level of medical care was not statistically different (P = .10), the proportion of patients not using ICS or ICS/LABA was highest (57.1%) in the outpatient group and lowest (31.6%) in the ICU group. The proportion of patients using ICS/LABA was lowest in the outpatient group (28.6%) and highest in the ICU group (57.9%).
Fig 4.
Percentage of patients with COVID-19 with asthma using inhaled or oral corticosteroids by the level of care. Percentage of patients with COVID-19 with asthma (1) not taking ICS, (2) using ICS alone, or (3) using ICS/LABA at the time of COVID-19 diagnosis. Oral steroids were used by 15 of 220 patients with asthma: outpatient (N = 7), inpatient – no ICU (N = 8), and inpatient – ICU (N = 0). Bars represent mean ± SEM. Statistics were analyzed using chi-square test (P = .10).
In general, among patients with COVID-19 with asthma, the risk for hospitalization was not significantly different between those with documentation of ICS or ICS/LABA prescriptions in their medical records and those who were not prescribed maintenance inhalers (model 1: RR, 1.22; 95% CI, 0.84-1.76; model 2: RR, 1.39; 95% CI, 0.90-2.15) (Table III ). The individual baseline risk factors used to adjust for RR assessing ICS use and COVID-19–related hospital admission are listed in Table E4 in this article’s Online Repository at www.jacionline.org.
Table III.
Asthma-specific adjusted RR (95% CI) for COVID-19–related hospital admission by ICS use
| Asthma-specific baseline risk factor profile | ICS ± LABA vs no ICS ± LABA, RR (95% CI) | P value |
|---|---|---|
| Model 1 | ||
| Adjusted for age, sex, and race/ethnicity | 1.22 (0.84-1.76) | .30 |
| Model 2 | ||
| Adjusted for age, sex, race/ethnicity, smoking, obesity, CAD, diabetes, HTN, OSA, COPD, allergic rhinitis, rhinosinusitis, and immunodeficiency | 1.39 (0.90-2.15) | .13 |
Fifteen patients with asthma were receiving systemic corticosteroids at the time of COVID-19 diagnosis. Of these 15 patients, 13 patients had been prescribed a short course of prednisone for an asthma exacerbation in the 2 weeks before their COVID-19 diagnosis. Systemic corticosteroid use before COVID-19 diagnosis was not different between the outpatient and inpatient managed subgroups. We repeated the regression model to determine the impact of ICS on COVID-19 hospitalization risk after removing the 15 patients prescribed systemic corticosteroids. The findings were nearly identical, and the use of ICS did not increase or decrease the risk of COVID-19 hospitalization in patients with asthma and COVID-19 (RR, 1.47; 95% CI, 0.93-2.32). Only 1 patient was receiving an asthma-related biologic (omalizumab). This patient required an ICU stay and was intubated for COVID-19 but was successfully discharged after 16 days of hospitalization.
Discussion
To our knowledge, this is the first comprehensive cohort study of patients with COVID-19 and comorbid asthma. In this study, asthma was present in 14.4% of patients with COVID-19, which included both hospitalized and nonhospitalized patients. Compared with the general US and metropolitan Chicago population, which is estimated to have an asthma prevalence of 8% to 9% and 9.5%, respectively, asthma is enriched in our COVID-19 population.4 , 5 , 16 Among only hospitalized patients with COVID-19 in this cohort, the prevalence of asthma was 13.5%, which supports recently published US data observing asthma prevalence of between 7.4% and 17% in COVID-19 hospitalized patients.2 , 12, 13, 14 This is in stark contrast to the low prevalence of asthma (≤1%) noted in China.10 , 11 Geographic differences in the frequency of asthma or methods of ascertainment may be contributing to these heterogeneous findings.
Importantly, despite the high prevalence of asthma in our study, we observed no significant difference in risk of hospitalization or mortality due to COVID-19 in patients with or without asthma. The overall mortality rate (4.7%) in our COVID-19 population aligned closely with the national mortality rate of 6.0% during this time period as published on the Johns Hopkins Coronavirus Resource Center (May 6, 2020). In this cohort, the mortality rate (3.6%) in the COVID-19 population with asthma at the time of this study was not different than the mortality rate in the COVID-19 population without asthma (4.9%).
Well-established comorbidities that are associated with COVID-19 were present in this cohort of patients with asthma (Fig 2). Interestingly, patients with asthma and COVID-19, compared with patients with COVID-19 without asthma, had an increased prevalence of multiple comorbidities. Previous studies have shown that obesity, OSA, and gastroesophageal reflux disease are associated with asthma.17, 18, 19 In the general COVID-19 cohort, DM and OSA were associated with a higher risk of hospitalization; however, this was no longer true when evaluating the asthma subgroup alone. Further investigation is needed to determine why these comorbidities, despite being more prevalent in patients with asthma, do not appear to worsen COVID-19–related outcomes.
Dramatic racial disparities have been reported during the COVID-19 pandemic and this was true in our study. Non-Hispanic African Americans made up almost one-quarter of our overall COVID-19 cohort despite the 6.1% prevalence of African Americans in our health care system. Moreover, African Americans were disproportionately higher in the asthma group (36%) compared with the nonasthma group (21%). Of the patients with COVID-19 with asthma in this study, 12.7% were Hispanic or Latino. These data are in contrast to the national findings. According to the CDC, African Americans and Hispanics comprise 9.6% and 6.0% of the adult asthma population, respectively.5 After controlling for age, sex, and race, African Americans had a higher risk of COVID-19–related hospitalization in the general COVID-19 cohort. Depending on the model used, the risk of COVID-19–related hospitalization was even higher in an adjusted analysis for the Hispanic or Latino population (35%-44%).
The assessment of laboratory values demonstrates that patients with asthma had significantly lower levels of ferritin, C-reactive protein, and lactate dehydrogenase, compared with patients without asthma. These are markers of disease severity in COVID-19. This is the first report to our knowledge to describe a potential decreased inflammatory burden in patients with COVID-19 with comorbid asthma, despite these patients having higher levels of other comorbid diseases compared with patients without asthma. These findings suggest that underlying immune modulation due to either asthma or asthma treatment may have a mitigating effect on COVID-19, but more studies are needed to understand this.
Interestingly, asthma did not increase the risk of hospitalization after adjusting for covariates. This is notable because it has been anticipated that underlying chronic lung diseases such as asthma, which are typically triggered by a viral illness, would place these patients at increased risk of severe exacerbations.20 The role of ICS treatment in patients with asthma and COVID-19 is not established and has brought concern to many patients.21 , 22 Almost half (48%) the patients with asthma were using ICS before COVID-19 in our study. After controlling for baseline risk factors, the use of ICS did not increase the risk of COVID-19–related hospitalization. In this study, only 15 patients were prescribed systemic corticosteroids before diagnosis, so this limits our ability to make any conclusion specifically regarding oral corticosteroid use in COVID-19. However, it is reassuring that in the model assessing the risk of ICS, oral corticosteroids did not change the risk of hospitalization.
It has been postulated that type 2 immune modulation decreases the expression of angiotensin-converting enzyme 2, the known receptor for COVID-19 cellular entry.23, 24, 25 Jackson et al26 published early data that suggest that patients with allergic asthma have decreased angiotensin-converting enzyme 2 expression in nasal and bronchial epithelial cells. Peters et al27 observed that ICS use was associated with the reduction in expression of both angiotensin-converting enzyme 2 and TMPRSS2 (transmembrane protease serine 2; a host serine protease critical to spike protein priming for cell entry) in patients with asthma from the Severe Asthma Research Program cohort. A separate, preliminary, in vitro study with ciclesonide showing viral suppression of SARS-CoV-2 begets the question of whether certain ICS commonly used by patients with asthma could provide clinical protection.28 These experimental studies in patients without COVID-19 suggest a potential protective role for ICS. Although our real-world data on ICS use in patients with COVID-19 do not show a lower risk of hospitalization, it is reassuring because we did not see an increase in hospitalization in patients who were receiving an ICS. Interestingly, we found that patients with rhinosinusitis and allergic rhinitis, which are predominantly type 2 inflammatory diseases, have reduced risk of COVID-19–related hospitalization. Assessing whether intranasal corticosteroids are protective in patients with COVID-19, especially in those with allergic rhinitis and rhinosinusitis, needs further investigation.
There are several limitations to our study. Data were obtained retrospectively so we are limited to drawing associations rather than causal inferences. Our study population and some of the variables used for analyses were based on International Classification of Disease codes, which may have miscaptured data. To minimize this, we performed chart reviews for the asthma cohort to confirm the diagnosis of both asthma and COVID-19, prescribed medications, and the level of care required for COVID-19. Also, because of the study design, we cannot assume adherence with the prescribed medications. An additional limitation of our study is that we did not assess the contribution of asthma severity or control to COVID-19–related hospitalization because we were limited by our study design. Although we cannot make inferences on the basis of asthma severity, COVID-19–associated level of care (ICU vs non-ICU) was not significantly different between patients prescribed ICS or ICS/LABA and those not on ICS or ICS/LABA. Our findings are based on data collected between March 1 and April 15 (with the exception of mortality assessed until April 30, 2020) and might change as additional data are collected after the study period. Although it may be possible that patients with asthma were more likely to be tested because asthma is a chronic lung disease, our asthma prevalence data were similar to the prevalence data reported by the Morbidity and Mortality Weekly Report from the CDC during this study period.13 Finally, widespread COVID-19 testing was not available during our data collection period so selected patients may represent a bias toward more severe COVID-19 disease.
Conclusions
We found that asthma prevalence was 14% in our cohort of patients with COVID-19. Despite a high prevalence of comorbid diseases that are associated with COVID-19 severity, it is reassuring that neither asthma nor the use of ICS was associated with an increased risk of COVID-19 hospitalization. With this in mind, physicians need to be vigilant of older patients, those with comorbidities (especially DM and OSA based on this study), African Americans, and Hispanics who present with COVID-19 symptoms because they are at increased risk of hospitalization. This is true in the general population as well as in patients with asthma, according to this study. Further investigation is necessary to understand the possible protective role of type 2 inflammation in asthma and COVID-19.
Clinical Implications.
The prevalence of asthma among patients with COVID-19 was 14.4% versus the national asthma prevalence of 8% to 9%. Asthma and ICS use were not associated with risk of hospitalization due to COVID-19.
Footnotes
This work was supported by the Chronic Rhinosinusitis Integrative Studies Program 2 (grant no. NIH P01AI145818) and the Ernest Bazley Foundation.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
ICD-9 and ICD-10 classification codes
| ICD code | Code diagnosis |
|---|---|
| U07.1 | COVID-19 |
| V15.82, Z87.891 | Former smoker (N = 336) |
| 305.1, F17.200 | Current smoker (N = 53) |
| Any 493.x, any J45.x | Asthma (N = 220) |
| 496, 491.xx, 492.xx, any J44.x | Chronic obstructive pulmonary disease (N = 111) |
| 494.0, 494.1, any J47.x | Bronchiectasis (N = 19) |
| 530.81, 530.11, K21.0, K21.9 | Gastroesophageal reflux disease (N = 378) |
| 461.9, 473.8, 473.9, J01.90, J32.9 | Rhinosinusitis (N = 204) |
| 471.xx, J33.9 | Nasal polyposis (N = 13) |
| Any 477.x, any J30.x | Allergic rhinitis (N = 179) |
| 327.23, G47.33 | Obstructive sleep apnea (N = 183) |
| 472, J31.0 | Nonallergic rhinitis (N = 63) |
| 297.06, D83.9, 279.xx, D80.6, D80.3 | Common variable immunodeficiency, antibody deficiency, IgA deficiency (N = 32) |
| 250, E11.9 | Diabetes mellitus (N = 401) |
| 414.01, 125.10 | Coronary artery disease (N = 114) |
| 401.9, R03.0 | Hypertension (N = 505) |
ICD, International Classification of Disease; ICD-10, International Classification of Disease, Tenth Revision.
Table E2.
Individual baseline risk factors and associated adjusted RR (95% CI) for COVID-19–related hospital admission
| Individual baseline risk factors | Hospital admission |
|||
|---|---|---|---|---|
| Model 1 |
Model 2 |
|||
| RR | 95% CI | RR | 95% CI | |
| Asthma vs nonasthma | 1.01 | 0.83-1.24 | 0.96 | 0.77-1.19 |
| Age (y) | ||||
| <40 | 0.34 | 0.27-0.42 | 0.50 | 0.38-0.64 |
| 40-69 | 0.66 | 0.56-0.78 | 0.76 | 0.64-0.91 |
| ≥70 | 1 (reference) | 1 (reference) | ||
| Sex | ||||
| Female | 0.82 | 0.71-0.94 | 0.86 | 0.75-0.99 |
| Race/ethnicity | ||||
| Non-Hispanic African American | 1.23 | 1.03-1.46 | 1.11 | 0.93-1.32 |
| Non-Hispanic white | 1 (reference) | 1 (reference) | ||
| Hispanic or Latino | 1.44 | 1.21-1.72 | 1.35 | 1.12-1.63 |
| Non-Hispanic Asian | 0.93 | 0.63-1.35 | 0.96 | 0.65-1.42 |
| Other | 0.94 | 0.71-1.26 | 1.07 | 0.80-1.43 |
| Smoking status | ||||
| Current smoker | 1.10 | 0.75-1.61 | ||
| Former smoker | 1.06 | 0.89-1.25 | ||
| Never smoker | 1 (reference) | |||
| Other/unknown | 1.35 | 1.08-1.70 | ||
| Concurrent diagnoses | ||||
| Obesity (BMI ≥ 30)∗ | 1.10 | 0.95-1.27 | ||
| Hypertension | 1.14 | 0.97-1.33 | ||
| DM | 1.16 | 1.00-1.36 | ||
| OSA | 1.23 | 1.01-1.49 | ||
| CAD | 1.02 | 0.80-1.29 | ||
| COPD | 1.18 | 0.93-1.50 | ||
| Allergic rhinitis | 0.83 | 0.64-1.07 | ||
| Rhinosinusitis | 0.78 | 0.61-0.99 | ||
| Immunodeficiency | 1.14 | 0.75-1.75 | ||
Values in boldface indicate RR is statistically significant.
BMI data were not available for 180 study patients.
Table E3.
Descriptive analysis of patients with COVID with asthma stratified by ICS use
| Characteristic, n (%) | No maintenance inhalers, 114 (51.8) | ICS or ICS/LABA, 106 (48.2) | Total, 220 (100) | χ2P value |
|---|---|---|---|---|
| Age (y) | .162 | |||
| <40 | 37 (58.7) | 26 (41.3) | 63 | |
| 40-69 | 61 (46.6) | 70 (53.4) | 131 | |
| ≥70 | 16 (61.5) | 10 (38.5) | 26 | |
| Sex | .961 | |||
| Male | 33 (51.6) | 31 (48.4) | 64 | |
| Female | 81 (51.9) | 75 (48.1) | 156 | |
| Race/ethnicity | .873 | |||
| Non-Hispanic African American | 44 (56.4) | 34 (43.6) | 78 | |
| Non-Hispanic white | 46 (48.4) | 49 (51.6) | 95 | |
| Hispanic or Latino | 14 (14.0) | 14 (50.0) | 28 | |
| Non-Hispanic Asian | 4 (57.1) | 3 (42.9) | 7 | |
| Other | 6 (50.0) | 6 (50.0) | 12 | |
| Smoking status | .787 | |||
| Current smoker | 4 (40.0) | 6 (60.0) | 10 | |
| Former smoker | 28 (54.9) | 23 (45.1) | 51 | |
| Never smoker | 76 (51.0) | 73 (49.0) | 149 | |
| Unknown | 5 (83.3) | 1 (16.7) | 6 | |
| Concurrent diagnoses | ||||
| Obesity (BMI ≥ 30) | 65 (55.6) | 52 (44.4) | 117 | .301 |
| HTN | 50 (50.0) | 50 (50.0) | 100 | .622 |
| DM | 34 (57.6) | 25 (42.4) | 59 | .297 |
| OSA | 20 (43.5) | 26 (56.5) | 46 | .203 |
| CAD | 13 (59.1) | 9 (40.9) | 22 | .472 |
| COPD | 13 (35.1) | 24 (64.9) | 37 | .026 |
| Allergic rhinitis | 25 (31.6) | 54 (68.4) | 79 | <.0001 |
| Rhinosinusitis | 30 (38.0) | 49 (62.0) | 79 | .002 |
| Nasal polyps | 2 (22.2) | 7 (77.8) | 9 | .07 |
| GERD | 44 (47.8) | 48 (52.2) | 92 | .315 |
| Oral steroid use | 15 | |||
| Biologics | 1∗ |
Values in boldface indicate statistical significance. GERD, Gastroesophageal reflux disease.
Omalizumab.
Table E4.
Individual baseline risk factors and associated asthma-specific adjusted RR (95% CI) for COVID-19–related hospital admission
| Individual baseline risk factors | Hospital admission |
|||
|---|---|---|---|---|
| Model 1 |
Model 2 |
|||
| RR | 95% CI | RR | 95% CI | |
| ICS vs non-ICS | 1.22 | 0.84-1.76 | 1.39 | 0.9-2.15 |
| Age (y) | ||||
| <40 | 0.31 | 0.17-0.59 | 0.43 | 0.20-0.91 |
| 40-69 | 0.59 | 0.36-0.95 | 0.66 | 0.38-1.15 |
| ≥70 | 1 (reference) | 1 (reference) | ||
| Sex | ||||
| Female | 0.99 | 0.66-1.49 | 1.10 | 0.71-1.70 |
| Race/ethnicity | ||||
| Non-Hispanic African American | 1.24 | 0.82-1.88 | 1.20 | 0.76-1.91 |
| Non-Hispanic white | 1 (reference) | 1 (reference) | ||
| Hispanic or Latino | 1.42 | 0.80-2.51 | 1.28 | 0.69-2.36 |
| Non-Hispanic Asian | 0.89 | 0.27-2.88 | 1.11 | 0.33-3.82 |
| Other | 1.12 | 0.44-2.85 | 1.19 | 0.45-3.15 |
| Smoking status | ||||
| Current smoker | 1.50 | 0.57-4.00 | ||
| Former smoker | 1.31 | 0.82-2.10 | ||
| Never smoker | 1 (reference) | |||
| Other/unknown | 1.83 | 0.63-5.30 | ||
| Concurrent diagnoses | ||||
| Obesity (BMI ≥ 30)∗ | 1.23 | 0.80-1.89 | ||
| HTN | 1.25 | 0.79-2.00 | ||
| DM | 1.29 | 0.82-2.03 | ||
| OSA | 0.96 | 0.59-1.57 | ||
| CAD | 1.37 | 0.76-2.46 | ||
| COPD | 1.07 | 0.63-1.80 | ||
| Allergic rhinitis | 0.92 | 0.56-1.49 | ||
| Rhinosinusitis | 0.83 | 0.52-1.33 | ||
| Immunodeficiency | 0.39 | 0.12-1.30 | ||
Values in boldface indicate RR is statistically significant.
BMI data were not available for 180 study patients.
References
- 1.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y., Liu Y. Recent trends in current asthma prevalence among US adults, 2009-2018. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.04.041. S2213-2198:30398-6. [DOI] [PubMed] [Google Scholar]
- 5.NHIS, Centers for Disease Control and Prevention Most recent national asthma data. 2020.. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm Available at: Accessed May 6, 2020.
- 6.Sears M.R. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008;122:662–668. doi: 10.1016/j.jaci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Wark P.A., Tooze M., Powell H., Parsons K. Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission. Respirology. 2013;18:996–1002. doi: 10.1111/resp.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko F.W., Chan P.K., Chan R.W.Y., Chan K.P., Ip A., Kwok A. Molecular detection of respiratory pathogens and typing of human rhinovirus of adults hospitalized for exacerbation of asthma and chronic obstructive pulmonary disease. Respir Res. 2019;20:210. doi: 10.1186/s12931-019-1181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng X.Y., Xu Y.J., Guan W.J., Lin L.F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol. 2018;163:845–853. doi: 10.1007/s00705-017-3700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan [published online ahead of print April 12, 2020]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed]
- 12.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323:2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020;95:304-7. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chicago Department of Public Health, Chicago Health Atlas Asthma: adults who have been diagnosed with asthma. 2018.. https://www.chicagohealthatlas.org/indicators/asthma Available at:
- 17.Cazzola M., Calzetta L., Bettoncelli G., Novelli L., Cricelli C., Rogliani P. Asthma and comorbid medical illness. Eur Respir J. 2011;38:42–49. doi: 10.1183/09031936.00140310. [DOI] [PubMed] [Google Scholar]
- 18.ten Brinke A., Sterk P.J., Masclee A.A., Spinhoven P., Schmidt J.T., Zwinderman A.H. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26:812–818. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 19.Hekking P.P., Amelink M., Wener R.R., Bouvy M.L., Bel E.H. Comorbidities in difficult-to-control asthma. J Allergy Clin Immunol Pract. 2018;6:108–113. doi: 10.1016/j.jaip.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Johnston S.L. Overview of virus-induced airway disease. Proc Am Thoracic Soc. 2005;2:150–156. doi: 10.1513/pats.200502-018AW. [DOI] [PubMed] [Google Scholar]
- 21.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8:436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpin D.M.G., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55 doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson DJ, Busse WW, Bacharier LB, Kattan M, O’Connor GT, Wood RA, et al. Association of respiratory allergy, asthma and expression of the SARS-CoV-2 receptor, ACE2 [published online ahead of print April 22, 2020]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed]
- 27.Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids [published online ahead of print April 29, 2020]. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed]
- 28.Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15 [published online ahead of print March 12, 2020]. bioRxiv. 10.1101/2020.03.11.987016. [DOI]