Abstract
Background
Information regarding the incidence and characteristics of COVID-19 pneumonia amongst pregnant women is scarce.
Methods
Single-centre experience with 32 pregnant women diagnosed with COVID-19 between March 5 to April 5, 2020 at Madrid, Spain.
Findings
COVID-19 pneumonia was diagnosed in 61·5% (32/52) women. Only 18·7% (6/32) had some underlying condition (mostly asthma). Supplemental oxygen therapy was required in 18 patients (56·3%), with high-flow requirements in six (18·7%). Eight patients (25·0%) fulfilled the criteria for acute distress respiratory syndrome. Invasive mechanical ventilation was required in two patients (6·2%). Tocilizumab was administered in five patients (15·6%). Delivery was precipitated due to COVID-19 in three women (9·4%). All the newborns had a favourable outcome, with no cases of neonatal SARS-CoV-2 transmission. Severe cases of pneumonia requiring supplemental oxygen were more likely to exhibit bilateral alveolar or interstitial infiltrates on chest X-ray (55·6% vs. 0·0%; P-value = 0·003) and serum C-reactive protein (CRP) levels >10 mg/dL (33·0% vs. 0·0%; P-value = 0·05) at admission than those with no oxygen requirements.
Interpretation
Pregnant women with COVID-19 have a high risk of developing pneumonia, with a severe course in more than half of cases. The presence of bilateral kung infiltrates and elevated serum CRP at admission may identify women at-risk of severe COVID-19 pneumonia.
Funding
Instituto de Salud Carlos III (COV20/00,181), Spanish Ministry of Science and Innovation.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Pregnancy, Pneumonia, Risk stratification
Abbreviations: ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computerized tomography; ePaO2/FiO2, estimated arterial oxygen/fraction of inspired oxygen ratio; HCQ, hydroxychloroquine; ICU, intensive care unit; IFN-β, interferon-β; IQR, interquartile range; IMV, invasive mechanical ventilation; IV, intravenous; LPV/r, lopinavir/ritonavir; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TCZ, tocilizumab; URTI, upper respiratory tract infection
Research in context.
Evidence before this study
We searched PubMed database for articles published up to April 27, 2020, by using the keywords “novel coronavirus”, “2019 novel coronavirus”, “2019-nCoV”, “pneumonia”, “SARS-CoV-2″ OR “coronavirus” AND “pregnancy” OR “maternal infection”, for articles published in both Chinese and English. A total 108 cases of COVID-19 in pregnancy have been published in form of case reports and four case series (including a maximum of 16 cases each). From the data available from these reports it was not possible to extrapolate the rate of COVID-19 pneumonia amongst pregnant women with SARS-CoV-2 infection (either symptomatic or asymptomatic), concluding a very low global rate of severe disease, even in case series focused on pneumonia.
Added value of this study
We offer a thorough analysis of the clinical profile and outcome of 52 pregnant women with COVID-19. Pneumonia was diagnosed in more than 60% of symptomatic women. More than half of them required supplemental oxygen therapy, with 25% fulfilling the criteria for acute respiratory distress syndrome. Invasive mechanical ventilation was required in 2 cases (6•2%). We found that severe cases were more likely to exhibit bilateral alveolar or interstitial infiltrates and higher serum C-reactive protein (CRP) levels at admission.
Implications of all the available evidence
In contrast to previous reports, in the present single-centre series of pregnant women with COVID-19 we have observed a notable risk of developing pneumonia, which have a severe course in more than half of the cases. We were also able to characterize a risk profile based on radiological findings and initial serum CRP levels that could be useful to identify pregnant women at high risk and, eventually, to improve therapeutic management and outcomes in this specific population.
Alt-text: Unlabelled box
1. Introduction
Due to physiological and anatomical changes, pregnant women are considered more vulnerable to severe viral respiratory infections [1,2]. During the 2009 H1N1 influenza pandemic, in which early treatment with oseltamivir was demonstrated to decrease the rate of complications, pregnant women developed severe pneumonia in up to 20% of the cases [3]. The causative agent of the now termed coronavirus disease 2019 (COVID-19) is a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) against which no effective antiviral treatment is yet available. Therefore, severe cases are expected to occur amongst the pregnant population during the current COVID-19 pandemic, as previously described for SARS-CoV [4].
First reports of pregnant women diagnosed with COVID-19 pneumonia coming from China suggested that the clinical picture was similar to that observed amongst similarly-aged patients, with favourable outcomes and a mild course [5], [6], [7]. Further communications, however, described severely ill cases requiring intensive care unit (ICU) admission [8,9], although scarce details were provided on the clinical course and therapeutic management.
Preliminary reports outside China warn about a higher incidence of severe COVID-19 pneumonia in pregnant women [10,11]. The city of Madrid has experienced a high community transmission rate for SARS-CoV-2, with 59,199 cases diagnosed from March 1 to April 22, 2020 [12]. A notable number of severe and fatal episodes of pneumonia have been observed throughout this period, with mortality rates exceeding 10% [13], much higher than those reported from China [14]. An analysis of COVID-19 pneumonia prevalence and severity in potentially high-risk groups as pregnant women in such a high-incidence area seems advisable.
The present study was aimed to measure the incidence of pneumonia in pregnant women with symptomatic COVID-19 in the context of the first pandemic wave in Madrid and describe clinical characteristics and serial outcome parameters in patients with pneumonia to determine high and low-risk clinical profiles.
2. Materials and methods
2.1. Study population and design
The present retrospective cohort study was performed at the Department of Obstetrics of the University Hospital “12 de Octubre” (Madrid, Spain), which attends around 4000 deliveries per year. The inclusion criterion comprised adult (≥18 years) pregnant women diagnosed with COVID-19 from March 5 to April 5, 2020. The local Clinical Research Ethics Committee approved the study protocol (ref. no. 20/202) and granted a waiver of informed consent due to its retrospective observational design.
Patients were enroled at the time of diagnosis of COVID-19 and followed up to April 14, 2020. Clinical characteristics at presentation and serial assessment (days 0, 1, 2, 3, 5, 7, 10 and 14) of vital signs, respiratory status, laboratory values and radiological findings, antiviral and immunomodulatory drugs administered, obstetric management (delivery induction or non-programmed caesarean section), and women and newborn outcomes were obtained from electronic medical records using a standardized case report form. All laboratory and imaging investigations were performed as part of standard of care.
Respiratory function was assessed by means of the estimated partial pressure of arterial oxygen/ fraction of inspired oxygen (ePaO2/FiO2) ratio, which was calculated on the basis of the pulse oximetry saturation/fraction of inspired oxygen (SpO2/FiO2) ratio (SpO2/FiO2 = 64 + 0·84 x PaO2/FiO2) [15]. Acute respiratory distress syndrome (ARDS) was classified as mild, moderate or severe according to ePaO2/FiO2 ratio thresholds of 300, 200 and 100, respectively [16]. Supplemental oxygen requirement was considered when the patient needed at least 48 h of FiO2 ≥28%. Impaired liver function was defined by aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) levels >40 IU/L.
A six-point ordinal scale was used to analyse dynamic changes in the respiratory status: 1·- not hospitalized; 2·- hospitalized, not requiring supplemental oxygen; 3·- hospitalized, requiring low-flow supplemental oxygen (FiO2 <40%); 4·- hospitalized, requiring high-flow supplemental oxygen (FiO2 ≥40%) or non-invasive mechanical ventilation; 5·- hospitalized, requiring invasive mechanical ventilation (IVM), extracorporeal membrane oxygenation, or both; and 6·- death.
2.2. Patient management
Since the beginning of March 2020, a specific protocol for the management of potential cases of COVID-19 in pregnant women was established that included the evaluation of all symptomatic patients and determining outpatient or hospital management according to the clinical status and the diagnosis or not of pneumonia (Appendix S1). The diagnosis of COVID-19 was made by real time reverse transcription polymerase chain reaction (RT-PCR) in nasopharyngeal swab or sputum samples [17]. Such procedures were performed in a specific room in the Obstetrics Emergency Department. Chest X-ray was performed in all pregnant women with persistent cough and/or dyspnoea and/or chest pain, whereas computerized tomography (CT) scan was not systematically ordered for the diagnosis of pneumonia.
The diagnosis of COVID-19 pneumonia required the detection of SARS-CoV-2 by RT-PCR and the presence of infiltrates in the chest X-ray. The diagnosis of upper respiratory tract infection (URTI) was reserved for women with no radiological findings and/or absence of cough, dyspnoea and chest pain.
Patients were admitted in the presence of severity criteria (e.g. dyspnoea or diagnosis of pneumonia) and/or due to obstetric indication. Daily follow-up was performed during hospital admission or by means of telephone visits for at least 14 days from symptom onset. All women with suspected or confirmed SARS-CoV-2 infection with viable newborn at the time of diagnosis (over 22 weeks) were admitted in a COVID-19-dedicated obstetric ward with daily assessment by an Infectious Diseases consultant, whereas those at less than 22 weeks were managed in the Internal Medicine ward.
Treatment was administered following clinical practice guidelines for COVID-19 proposed by the Spanish Ministry of Health and local protocols [18]. In detail, co-formulated lopinavir/ritonavir (LPV/r) (200/100 mg twice daily orally for up to 14 days) and/or hydroxychloroquine (HCQ) (400 mg twice orally for the first 24 h, followed by 200 mg twice daily for 5–10 days) were the alternatives to be prescribed to patients with pneumonia. Empirical antibiotics were associated if bacterial superinfection was suspected. Immunomodulatory agents were considered in patients with bilateral (or progressive) interstitial or alveolar infiltrates, elevated inflammatory parameters, and rapid progression of respiratory failure. A multidisciplinary committee that included clinical specialties involved and the Department of Pharmacy was established to facilitate and standardize therapeutic decisions on the use of intravenous (IV) tocilizumab (TCZ). Corticosteroid therapy could be used at different dosing regimens (IV methylprednisolone 0·5–1 mg/Kg daily for ≤5 days or as boluses of 0.5-1 mg/kg daily for 3 days). All patients received thromboprophylaxis with low-molecular weight heparin.
2.3. Foetal monitoring and management of delivery
Ultrasound examination was performed to assess foetal well-being, whereas external foetal heart rate monitoring was carried out daily during hospital admission. COVID-19 was not an absolute indication to finish the pregnancy (induction or caesarean section), but was rather reserved for obstetric reasons, maternal clinical worsening or suspected foetal distress.
2.4. Statistical analysis
The present study adheres to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. Clinical data were extracted by chart review from electronic medical records and entered into a database specifically designed for this study in an anonymized way. Variables regarding demographics, obstetric status, co-morbidity, clinical presentation of COVID-19, therapeutic management and outcome were collected and subjected to descriptive analysis. Quantitative data were shown as the mean ± standard deviation (SD) or the median with interquartile range (IQR). Qualitative variables were expressed as absolute and relative frequencies. Two different comparative analysis were performed: (a) women with COVID-19 pneumonia versus URTI; and (b) women with COVID-19 pneumonia with or without oxygen therapy requirements. Categorical variables were compared using the χ2 test. Student's t-test or Mann-Whitney U test were applied for continuous variables, as appropriate. All the significance tests were two-tailed. The threshold for significance was set at a P-value <0·05. Statistical analysis was performed with SPSS version 20.0 (IBM Corp, Armonk, NY), and graphs were generated with Prism version 6.0 (GraphPad Software Inc, La Jolla, CA).
2.5. Role of the funding source
No study sponsor had any role in study design; collection, analysis, or interpretation of data; or in writing this paper or the decision to submit for publication. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
3. Results
Sixty-five pregnant women were diagnosed with COVID-19 during the study period. Thirteen of them (20·0%) diagnosed at the time or after delivery were excluded. Therefore, 52 women were finally included in the present study. COVID-19 pneumonia was diagnosed in 32 (61·5%) patients, whereas the remaining 20 cases were categorized as URTI. Chest X-ray was performed in 40·0% (8/20) of patients. Malaise and dyspnoea were significantly more frequent in women developing pneumonia, whereas coryza-like symptoms were more common amongst women with milder infection (Table S1).
Demographics and clinical characteristics of 32 patients with pneumonia are shown in Table 1. The most common ethnicity was Hispanic (65·6% [21/32]), which constitutes a significantly higher percentage as compared to pregnant women delivering at our centre during two representative months of 2019 (34·1% [206/604]; P-value = 0·0006). Most women were in their third trimester of pregnancy, and relevant comorbidities was observed in 18·7% (6/32), mainly asthma (12·5% [4/32]).
Table 1.
Clinical characteristics of pregnant women with COVID-19 pneumonia (n = 32).
| Baseline characteristics at diagnosis | |
| Age, years (mean ± SD) | 32 ± 7 |
| Gestational age, weeks [median (IQR)] | 29 (25–34) |
| Gestational trimester [n (%)] | |
| First | 1 (3·1) |
| Second | 9 (28·1) |
| Third | 22 (68·8) |
| Underlying conditions [n (%)] | |
| None | 26 (81·3) |
| Asthma | 4 (12·5) |
| Obesity | 1 (3·1) |
| Multiple sclerosis | 1 (3·1) |
| Gestational diabetes | 2 (6·3) |
| Hispanic ethnicity [n (%)] | 21 (65·6) |
| Clinical presentation | |
| Cough [n (%)] | 31 (96·9) |
| Dyspnoea [n (%)] | 25 (78·1) |
| Fever [n (%)] | 20 (62·5) |
| Fatigue [n (%)] | 15 (46·9) |
| Myalgia [n (%)] | 13 (40·6) |
| Headache [n (%)] | 9 (28·1) |
| Coryza [n (%)] | 5 (15·6) |
| Chest pain [n (%)] | 4 (12·5) |
| diarrhea [n (%)] | 1 (3·1) |
| Anosmia and/or dysgeusia [n (%)] | 1 (3·1) |
| Time from symptoms onset, days [median (IQR)] | 7 (5–8) |
| Clinical data at the time of pneumonia diagnosis | |
| AST, IU/L·(mean ± SD) | 37·6 ± 30 |
| ALT, IU/L (mean ± SD) | 34·5 ± 25 |
| LDH, IU/L (mean ± SD) | 296 ± 82 |
| Lymphocyte count, x 10⁹/L (mean ± SD) | 0.96 ± 0.361 |
| CRP, mg/dL (mean ± SD) | 7·4 ± 5·3 |
| Radiological findings [n (%)] | |
| Bilateral ground-glass opacities | 15 (46·9) |
| Bilateral alveolar infiltrates | 9 (28·2) |
| Unilateral alveolar infiltrate | 4 (12·5) |
| Unilateral ground-glass opacity | 3 (9·4) |
| Bilateral interstitial infiltrates | 1 (3·1) |
| Management of pneumonia | |
| Hospital admission [n (%)] | 29 (90·6) |
| Antiviral therapy [n (%)] | |
| Hydroxychloroquine monotherapy | 22 (68·8) |
| Hydroxychloroquine plus LPV/r | 8 (25·0) |
| No antiviral treatment | 2 (6·3) |
| Antibiotic therapy [n (%)] | |
| Azithromycin | 12 (37·5) |
| Amoxicillin-clavulanate | 11 (34·4) |
| Ceftriaxone | 9 (28·1) |
| Other beta-lactam | 1 (3·1) |
| Immunomodulatory therapy [n (%)] | |
| Tocilizumab | 5 (15·6) |
| Steroids | 3 (9·4) |
| Colchicine | 1 (3·1) |
| Outcomes | |
| Length of hospital admission, days [median (IQR)] | 8 (5–10) |
| Supplemental oxygen requirement FiO2 ≥28% for ≥2 days·[n (%)] | 18 (56·3) |
| Length of oxygen therapy, days [median (IQR)] | 7 (4–8) |
| Supplemental oxygen requirement FiO2 ≥48% for ≥2 days [n (%)] | 6 (18·7) |
| Length of oxygen therapy, days [median (IQR)] | 5 (4–8) |
| ARDS [n (%)] | 8 (25) |
| ICU admission and IMV [n (%)] | 2 (6·2) |
| COVID-19-related foetal delivery at admission [n (%)] | 3 (9·4) |
| All-cause mortality [n (%)] | 0 (0·0) |
ARDS: acute respiratory distress syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP: C-reactive protein; FiO2, fraction of inspired oxygen; IQR: interquartile range; LDH, lactate dehydrogenase; LPV/r: lopinavir/ritonavir; SD: standard deviation.
Cough was virtually present in all the patients with pneumonia, fever was reported in only 62·5% (20/32) of cases, and fatigue and myalgias were reported by nearly half of the women. At the time of diagnosis of COVID-19 pneumonia 78·1% (25/32) patients referred dyspnoea. In the initial chest X-ray, most patients (78·1% [25/32]) presented bilateral infiltrates. The most common radiological findings were bilateral ground-glass opacities (46·9% [15/32]), followed by bilateral or unilateral alveolar infiltrates (40·6% [13/32]). Laboratory parameters are also shown in Table 1. Although the majority of the patients required hospital admission for a median of 8 days (IQR: 5–10), three women were managed as outpatients.
Monotherapy with HCQ was the most common therapeutic regimen used (68·8% [22/32]), associated with LPV/r in 25·0% (8/32) of cases. LPV/r had to be prematurely discontinued in two women due to severe gastrointestinal symptoms. Most patients received antibiotics (78·1% [25/32]), mainly azithromycin, amoxicillin-clavulanate and ceftriaxone. No electrocardiographic abnormalities during the course of therapy were observed. Immunomodulatory therapy was administered in 18·8% (6/32) of cases that experienced a rapid worsening of respiratory symptoms (Table 2). In detail, five patients (15·6%) received TCZ at a median of 3 days from hospital admission.
Table 2.
Clinical characteristics of pregnant patients developing severe COVID-19 pneumonia that received immunomodulatory therapy.
| Case | Age (years) | Comorbidity | Radiological pattern | Type of therapy | Criteria for immunomodulatory therapy | Outcome |
|---|---|---|---|---|---|---|
| #1 | 39 | None | Bilateral alveolar infiltrates | TCZ (2 doses)a, day 3• | CPR: 12•7 mg/dL, abrupt respiratory function deterioration (ePaO2/ FiO2: 332), overwhelming fatigue, worsening of radiologic infiltrates | Clinical and radiological improvement, no supplemental oxygen requirements by day 5 after the initiation of therapy• |
| #2 | 28 | None | Bilateral ground-glass opacities | Tocilizumab (single dose)b, day 6• | CPR: 11•5 mg/dL, abrupt respiratory function deterioration (ePaO2/ FiO2: 288) with high-flow oxygen requirements | Clinical and radiological improvement, no supplemental oxygen requirements by day 4 after the initiation of therapy• |
| #3 | 33 | None | Bilateral ground-glass opacities | Metilprednisolonec, day 1; TCZ (single dose)b, day 3 | CPR: 15 mg/dL, abrupt respiratory function deterioration (ePaO2/ FiO2: 288) with high-flow oxygen requirements, worsening of radiologic infiltrates | Clinical and radiological improvement, no supplemental oxygen requirements by day 5 after the initiation of therapy• |
| #4 | 38 | Asthma | Bilateral alveolar infiltrates | TCZ (2 doses)a, day 3 | CPR: 8•8 mg/dL, abrupt respiratory function deterioration (ePaO2/ FiO2: 172) with high-flow oxygen requirements, worsening of radiologic infiltrates | IMV, urgent caesarean section (preterm, 28 weeks), ICU admission and IMV for 2 days after delivery, further clinical and radiological improvement, no supplemental oxygen requirements by day 15 after the initiation of therapy• |
| #5 | 40 | Asthma | Bilateral alveolar infiltrates | Metilprednisolonec, day 3 | CPR: 23•7 mg/dL, abrupt respiratory function deterioration (ePaO2/ FiO2: 318) with high-flow oxygen requirements• | Urgent caesarean section (term, 39 weeks), clinical improvement, no supplemental oxygen requirements by day 3 after the initiation of therapy• |
| #6 | 37 | None | Unilateral ground-glass opacity | TCZ 2 dosesa, day 3 | CPR: 21•8 mg/dL, abrupt respiratory function deterioration (ePaO2/ FiO2:175) with high-flow oxygen requirements, worsening of radiologic infiltrates• | Slow clinical and radiological improvement, no supplemental oxygen requirements by day 5 after the initiation of therapy• |
CRP: C-reactive protein; ePaO2/FiO2: estimated arterial oxygen/fraction of inspired oxygen ratio; ICU: intensive care unit; IMV: invasive mechanical ventilation; TCZ: tocilizumab.
First TCZ dose of 600 mg, second dose 400 mg.
Single TCZ dose of 400 mg.
Metilprednisolone at 1 mg/Kg/day for 3 days.
The median follow-up was 19 days (IQR: 12–22). Overall, 56·3% (18/32) of patients required supplemental oxygen for a median of 7 days (IQR: 4–8). Therapy with high-flow oxygen was initiated in six patients (18·8%) at a median of 3 days after admission (IQR: 1–7) for a median duration of 5 days (IQR: 4–8). The criteria for ARDS were fulfilled in 25·0% (8/32) women, and IMV was required in two cases (6·2%) (Table 1).
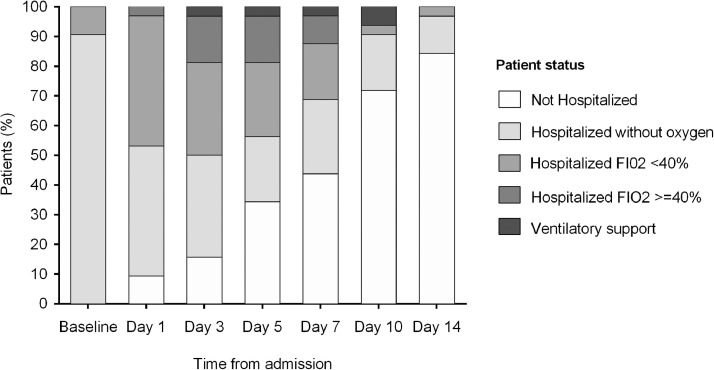
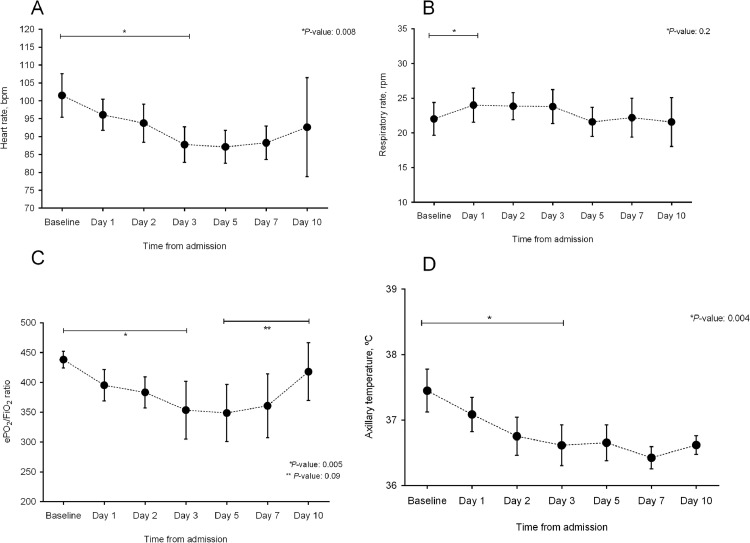
Patient status according to the five-point ordinal scale at different times over time is shown in Fig. 1. The evolution of vital signs (heart rate, respiratory rate, ePO2/FiO2 ratio, and axillary temperature) and analytical parameters is showed in Figs. 2 and S1, respectively.
Fig. 1.
Evolution of respiratory status from day 1 though 14 following admission in the overall cohort of pregnant patients with COVID-19 pneumonia.
Fig. 2.
Evolution of vital signs: (a) heart rate; (b) respiratory rate; (c) ePO2/FiO2; and (d) axillary temperature. Dots represent median values and bars indicate 95% confidence intervals.
Of note, the six patients with more severe pneumonia requiring high-flow oxygen developed an abrupt worsening of their clinical condition, generally on the third day of admission.
At the time of writing, delivery had occurred in 18·8% (6/32) of women with COVID-19 pneumonia, through vaginal delivery in only one case (Table S2). In three patients (9·4%), delivery was considered to be precipitated by COVID-19 (through urgent caesarean section in all of them), with one case of severe preterm delivery. Main characteristics of newborns are described in Table S2. No cases of neonatal SARS-CoV-2 transmission were observed.
A comparative analysis was performed between women with COVID-19 pneumonia with or without supplemental oxygen requirement (18 [56·3%] and 14 [43·7%] patients, respectively) (Table 3). More severe cases were characterized by a higher rate of bilateral alveolar or interstitial infiltrates (55·6% versus 0·0%, P-value = 0·003), dyspnoea (100·0% versus 50·0%; P-value = 0·003), and myalgia (61·1% versus 14·3%; P-value = 0·02). In addition, impaired liver function (i.e. AST and/or ALT levels >40 IU/L) (66·7% versus 21·4%; P-value = 0·03) and increased CRP levels (33% versus 0·0%; P-value = 0·05) were more frequent in severe cases. The comparison between these two distinctive clinical profiles of COVID-19 pneumonia is graphically shown in Figure S2 and Figure S3. There were no deaths in the present series and all the women were finally discharged, including both critical cases.
Table 3.
Comparison between pregnant women with COVID-19 pneumonia with or without supplemental oxygen requirements.
| No supplemental oxygen requirement (n = 14) | Supplemental oxygen requirement (n = 18) | P-value | |
|---|---|---|---|
| Baseline characteristics at diagnosis | |||
| Age, years (mean ± SD) | 31•3 ± 7•7 | 32•6 ± 6•6 | 0•6 |
| Gestational age, weeks [median (IQR)] | 28•3 ± 7•9 | 28•9 ± 7•2 | 0•8 |
| Asthma and/or obesity [n (%)] | 2 (14•3) | 3 (16•7) | 0•7 |
| Hispanic ethnicity [n (%)] | 7 (50) | 14 (77•8) | 0•2 |
| Clinical presentation | |||
| Cough [n (%)] | 13 (93) | 18 (100) | 0•9 |
| Dyspnoea [n (%)] | 7 (50) | 18 (100) | 0•003 |
| Fever [n (%)] | 8 (57•1) | 12 (66•7) | 0•8 |
| Fatigue [n (%)] | 4 (28•6) | 11 (61•1) | 0•14 |
| Myalgia [n (%)] | 2 (14•3) | 11 (61•1) | 0•02 |
| Headache [n (%)] | 2 (14•3) | 7 (38•9) | 0•2 |
| Coryza [n (%)] | 3 (21•4) | 2 (11•1) | 0•7 |
| Chest pain [n (%)] | 1 (7•1) | 3 (16•7) | 0•8 |
| Time from symptoms onset, days (mean ± SD) | 6•2 ± 4•2 | 6•8 ± 2•3 | 0•6 |
| Clinical data at the time of pneumonia diagnosis | |||
| Initial ePaO2/FiO2 (mean ± SD) | 456 ± 4•8 | 426 ± 44•6 | 0•012 |
| AST, IU/L•(mean ± SD) | 23•5 ± 12 | 48•6 ± 35 | 0•011 |
| ALT, IU/L (mean ± SD) | 23•3 ± 5•7 | 43•2 ± 34 | 0•02 |
| AST and/or ALT >40 IU/L [n (%)] | 14 (21•4) | 18 (66•7) | 0•03 |
| LDH, IU/L (mean ± SD) | 270 ± 56 | 315 ± 93 | 0•13 |
| Lymphocyte count, x 10⁹/L (mean ± SD) | 1.03 ± 0.39 | 0.91 ± 0.33 | 0•3 |
| CRP, mg/dL (mean ± SD) | 4•3 ± 2•9 | 9•7 ± 5•6 | 0•002 |
| CPR levels >5 mg/dL [n (%)] | 6 (42•9) | 14 (77•8) | 0•09 |
| CPR levels >10 mg/dL [n (%)] | 0 (0•0) | 6 (33•0) | 0•05 |
| Radiological findings [n (%)] | |||
| Unilateral infiltrates | 7 (50•0) | 0 (0•0) | 0•003 |
| Bilateral ground-glass opacities | 7 (50•0) | 8 (44•4) | 0•9 |
| Bilateral alveolar or interstitial infiltrates | 0 (0•0) | 10 (55•6) | 0•003 |
| Management of pneumonia | |||
| Antiviral therapy [n (%)] | |||
| Hydroxychloroquine monotherapy | 10 (71•4) | 12 (66•7) | 0•8 |
| Hydroxychloroquine plus LPV/r | 2 (14•3) | 6 (33•3) | 0•4 |
| No antiviral treatment | 2 (14•3) | 0 (0•0) | 0•3 |
| Antibiotic therapy [n (%)] | |||
| Azithromycin | 2 (14•3) | 10 (55•6) | 0•04 |
| Amoxicillin-clavulanate | 5 (35•7) | 6 (33•3) | 0•9 |
| Ceftriaxone | 1 (7•1) | 8 (44•4) | 0•05 |
| Immunomodulatory therapy [n (%)] | |||
| Total | 0 (0•0) | 6 (33•3) | 0•05 |
| Tocilizumab | 0 (0•0) | 5 (27•7) | 0•09 |
| Steroids | 0 (0•0) | 3 (16•7) | 0•3 |
| Outcomes | |||
| Length of hospital admission, days [median (IQR)] | 5•64 (3•14) | 10•24 (4•4) | 0•006 |
| Peak LDH level, IU/L (mean ± SD) | 291 ± 93 | 386 ± 93± | 0•17 |
| Lowest lymphocyte count, x 10⁹ cells/L (mean ± SD) | 0.96 ± 0.35 | 0.84 ± 0.834 | 0•29 |
| Peak CRP level, mg/dl (mean ± SD) | 4•8 ± 2•7 | 11•2 ± 5•2 | <0•0001 |
| Lowest ePaO2/FiO2 (mean ± SD) | 443 ± 29 | 284 ± 74 | <0•0001 |
| ARDS [n (%)] | 0 (0•0) | 8 (44•4) | 0•01 |
| ICU admission and IMV [n (%)] | 0 (0•0) | 2 (11•1) | 0•5 |
| COVID-19-related foetal delivery at admission [n (%)] | 2 (14•3) | 4 (22•3) | 0•9 |
ARDS: acute respiratory distress syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP: C-reactive protein; ePaO2/FiO2: estimated arterial oxygen/fraction of inspired oxygen ratio; IQR: interquartile range; LDH, lactate dehydrogenase; LPV/r: lopinavir/ritonavir; SD: standard deviation.
4. Discussion
We report herein one of the first series of COVID-19 in pregnant women with low comorbidity burden. The most relevant finding in this series is that 61·5% (32 out of 52) of symptomatic pregnant women with COVID-19 eventually developed pneumonia, although this percentage could have been even higher since not all patients underwent chest X-ray, and more sensitive techniques such as CT scan were not performed. It is also remarkable that more than half of these patients experienced a severe course requiring supplemental oxygen therapy and as much as 25·0% developed ADRS (although ICU admission and IMV was needed in only two women). Whether this severity profile is worse than that of similarly aged non-pregnant women is difficult to ascertain, since specific clinical data are not available for these age ranges. In general terms, younger patients seem to have a more favourable course of COVID-19, being advanced age a major risk factor for severe and fatal disease [19,20]. In a recent study that analysed a large cohort comprised of 1591 ICU-admitted COVID-19 patients in Lombardy, women aged between 20–45 years represented only 0·7% [21]. From the analysis of Spanish registry data, we could conclude that 10% of confirmed COVID-19 cases pertained to women aged between 30–40 years, with rates of pneumonia and ICU admission of 25% and 1·2%, respectively [13]. Similar findings have been recently published from the US [22], suggesting that, in contrast to what has been previously reported [5,6,8], there seems to be an excess of severity associated to COVID-19 during pregnancy.
We were able to define two different clinical profiles in terms of outcome. Around 40% of women developed a mild disease with stable respiratory function. Such a benign course could be anticipated at admission on the basis of certain features such as unilateral infiltrates on chest X-ray, lack of dyspnoea and myalgias, and only moderately elevated CPR levels. Knowledge of this low-risk profile at diagnosis could inform decision making to allow for outpatient management or early hospital discharge, of crucial relevance in the case of healthcare system overload.
On the other hand, pregnant women in our cohort presenting with bilateral alveolar infiltrates, impaired liver function tests and elevated inflammatory parameters were more prone to develop severe disease with worsening of respiratory function, usually around the third day of admission. Whereas bilateral ground-glass opacities are the most frequently described radiological finding in COVID-19, bilateral alveolar consolidations have been reported to be associated to more severe disease [20,23]. Elevated CPR levels has been also proposed as a surrogate of the state of hypercytokinemia involved in immune-related lung injury observed in patients with severe COVID-19 and ARDS [24]. This high-risk group would therefore benefit from close foetal monitoring, high-flow oxygen therapy, and immunomodulatory therapy. The potential role for immunomodulation in severe COVID-19 [25] prompted the inclusion of TCZ (an anti-interleukin 6-receptor monoclonal antibody) in national and local guidelines as off-label alternative for selected cases [18]. Supporting evidence, however, is still limited [26], particularly during pregnancy. However, TCZ therapy in pregnant women with rheumatologic conditions appears to be safe [27]. Five women in our series received TCZ, with favourable response and no relevant adverse events.
In previous reports, urgent caesarean section was performed in virtually all pregnant women with symptomatic COVID-19, probably due to initial uncertainties regarding the outcome and potential SARS-CoV-2 foetal transmission [5], [6], [7], [8]. In our experience we restricted such an approach to those cases with evident maternal or foetal risk, resulting in a rate of caesarean section below 10%.
An intriguing finding was the significant overrepresentation of Hispanic American women, mostly within the most severe cases. The potential influence of ethnicity in the incidence, severity and outcome of COVID-19 is being proposed on the basis of certain reports suggesting ethnic-associated differences [28]. Most women of Hispanic macro-ethnicity were Amerindians that moved from a different nutritional background, being more prone to develop truncal obesity and sustained low- grade inflammation [29], which exerts a deleterious impact on the course of SARS-CoV-2 infection [30]. Nevertheless, whether the observed ethnicity-related increased risk depends on differential nutritional habits or other factors (such as genetic predisposition or social crowding) remains to be clarified. Unfortunately, the present study was not powered or designed to address this question due to the lack of data on nutritional or socioeconomic status of participants. Therefore, specifically aimed studies should be conducted in future.
The main limitation of our study is the small sample size, which prevented us from performing multivariate models for a more accurate definition of risk profiles. Therefore, although we believe that the present report may be useful for the management of the overall pregnant population at risk for COVID-19, caution must be applied before extrapolating our findings. In addition, since asymptomatic women were not tested for SARS-CoV-2, the incidence of COVID-19 in this specific population could not be estimated.
In conclusion, the present single-centre experience suggests that COVID-19 during pregnancy is associated to more severe cases of pneumonia than previously described. The extent of pulmonary infiltrates at admission together with clinical data and laboratory values suggestive of systemic inflammation can help to individualize therapeutic management and, eventually, reduce associated risks for the mother and the newborn.
Acknowledgments
Transparency declaration
The authors have no conflicts of interest to disclose.
Funding
This study was supported by the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (COVID-19 research call COV20/00181) and by the Network for Research in Infectious Diseases (REIPI [RD16/0016]) also from Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation and co‐financed by the European Development Regional Fund “A way to achieve Europe”, Operative Program Intelligent Growth 2014‐2020. M.F.R. holds a research contract “Miguel Servet” (CP18/00073) and M.R.R. a research training contract “Río Hortega” (CM17/00098), both from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation.
Authors’ contributions
R.S.J. and P.B. were involved in study concept and design. R.S.J. was in charge of drafting the manuscript. M.F.R. and J.M.A. participated in the critical revision of the manuscript. R.S.J, P.B, M.F.R. and J.M.A. were involved in data analysis and interpretation. P.B, E.B, I.M, L.F, A.G and A.G.B. were responsible for data collection. M.D.F. performed microbiological procedures. F.L.M, M.L, P.H.J, J.T.S, M.R.R, L.C, I.R.G, A.L, P.H.J, A.G, C.L and A.G.B. made substantial revisions to the final version of the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100407.
Appendix. Supplementary materials
References
- 1.Goodnight W.H., Soper D.E. Pneumonia in pregnancy. Crit Care Med. 2005;33(10 Suppl):S390–S397. doi: 10.1097/01.ccm.0000182483.24836.66. [DOI] [PubMed] [Google Scholar]
- 2.O'Day M.P. Cardio-respiratory physiological adaptation of pregnancy. Semin Perinatol. 1997;21(4):268–275. doi: 10.1016/s0146-0005(97)80069-9. [DOI] [PubMed] [Google Scholar]
- 3.Creanga A.A., Johnson T.F., Graitcer S.B. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115(4):717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz D.A., Graham A.L. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses. 2020;12(2) doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu N., Li W., Kang Q. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N., Han L., Peng M. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaigham M., Andersson O. Maternal and Perinatal Outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Wang Y., Zeng Y. Critically ill pregnant patient with COVID-19 and neonatal death within two hours of birth. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breslin N., Baptiste C., Gyamfi-Bannerman C. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrazzi E., Frigerio L., Savasi V. Vaginal delivery in SARS-CoV-2 infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020 doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministerio de Sanidad, Centro de Coordinación de Alertas y Emergencias Sanitarias, España· Actualización n° 83· Enfermedad por el coronavirus (COVID-19)·2020·https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_83_COVID-19.pdf
- 13.Instituto de Salud Carlos III Centro Nacional de Epidemiología· Informe sobre la situación de COVID-19 en España n° 24 (21 de abril de 2020)·2020·https://www·isciii·es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20n%C2%BA%2024·%20Situaci%C3%B3n%20de%20COVID-19%20en%20Espa%C3%B1a%20a%2021%20de%20abril%20de%202020·pdf (accessed april 18, 2020
- 14.Grech V. Unknown unknowns - COVID-19 and potential global mortality. Early Hum Dev. 2020;144 doi: 10.1016/j.earlhumdev.2020.105026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Rice T.W., Wheeler A.P., Bernard G.R., Hayden D.L., Schoenfeld D.A., Ware L.B. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 16.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Petruzzi G., De Virgilio A., Pichi B. COVID-19: nasal and oropharyngeal swab. Head Neck. 2020 doi: 10.1002/hed.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministerio de Sanidad Ministerio de Sanidad, Centro de Coordinación de Alertas y Emergencias Sanitarias· Documento técnico de manejo clínico de pacientes con enfermedad por el nuevo coronavirus (COVID-19) (versión 3 de Marzo de 2020)2020https://www·mscbs·gob·es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Protocolo_manejo_clinico_COVID-19.pdf (accessed April 21, 2020
- 19.Wu C., Chen X., Cai Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.·Grasselli G., Zangrillo A., Zanella A. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020MMWR Morbidity and mortality weekly report2020;69(12): 343–6 [DOI] [PMC free article] [PubMed]
- 23.Inui S., Fujikawa A., Jitsu M. Chest CT Findings in Cases from the Cruise Ship “Diamond Princess” with Coronavirus Disease 2019 (COVID-19) Radiol Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.·Giamarellos-Bourboulis E.J., Netea M.G., Rovina N. Complex Immune Dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., Zhao Y., Zhang F. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clinical Immunol (Orlando, Fla) 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.·Hoeltzenbein M., Beck E., Rajwanshi R. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheum. 2016;46(2):238–245. doi: 10.1016/j.semarthrit.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 28.·Khunti K., Singh A.K., Pareek M. Hanif W· Is ethnicity linked to incidence or outcomes of covid-19. BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 29.Yu L., Li Y., Du C. Pattern recognition receptor-mediated chronic inflammation in the development and progression of obesity-related metabolic diseases. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/5271295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiappetta S., Sharma A.M., Bottino V., Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes. 2020;(2005) doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.