Abstract
Background
Since its first detection in December 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 infection has spread rapidly around the world. Although there have been several studies investigating prognostic factors for severe COVID-19, there have been no such studies in Korea.
Methods
We performed a retrospective observational study of 110 patients with confirmed COVID-19 hospitalized at a tertiary hospital in Daegu, Korea. Demographic, clinical, laboratory, and outcome data were collected and analyzed. Severe disease was defined as a composite outcome of acute respiratory distress syndrome, intensive care unit care, or death.
Results
Diabetes mellitus (odds ratio [OR], 19.15; 95% confidence interval [CI], 1.90–193.42; P = 0.012), body temperature ≥ 37.8°C (OR, 10.91; 95% CI, 1.35–88.36; P = 0.025), peripheral oxygen saturation < 92% (OR, 33.31; 95% CI, 2.45–452.22; P = 0.008), and creatine kinase-MB (CK-MB) > 6.3 (OR, 56.84; 95% CI, 2.64–1,223.78, P = 0.010) at admission were associated with higher risk of severe COVID-19. The likelihood of development of severe COVID-19 increased with an increasing number of prognostic factors.
Conclusion
In conclusion, we found that diabetes mellitus, body temperature ≥ 37.8°C, peripheral oxygen saturation < 92%, and CK-MB > 6.3 are independent predictors of severe disease in hospitalized COVID-19 patients. Appropriate assessment of prognostic factors and close monitoring to provide the necessary interventions at the appropriate time in high-risk patients may reduce the case fatality rate of COVID-19.
Keywords: COVID-19, Severe Disease, Prognostic Factor, Korea
Graphical Abstract
INTRODUCTION
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in December 2019 in Wuhan, Hubei Province, China.1 This disease has spread rapidly to other regions around the world, including the Western Pacific, Europe, Eastern Mediterranean, Americas, and Southeast Asia. The World Health Organization declared COVID-19 a pandemic on March 12, 2020. By April 19, 2020, approximately 2.28 million cases had been diagnosed with 155,124 deaths worldwide.
Although 81% of COVID-19 cases are mild, 14% are severe, and 5% are critical. The fatality rate is about 50.0% in critical cases.2 A number of factors associated with severe COVID-19 have been identified from China. Older age, male sex, presence of comorbidities, low oxygen saturation, and abnormal lab findings (high lactate dehydrogenase [LDH], high procalcitonin, low CD4 cell count, low albumin level) were shown to be risk factors for severe COVID-19.3,4,5,6,7,8
However, patient- and disease-related factors vary from region to region, and these factors may be associated with the clinical severity of COVID-19. There have been no studies regarding prognostic factors for severe disease in COVID-19 patients in Korea. This study was performed to identify prognostic factors for severe disease in patients with COVID-19 in Daegu, Korea.
METHODS
Study design and participants
This was a retrospective observational study of 110 patients with confirmed COVID-19 at Yeungnam University Medical Center, Daegu, Korea, from February 19, 2020 to April 15, 2020. During the study period, all adult patients (age ≥ 18 years) with COVID-19 who were hospitalized via the emergency room or outpatient department were eligible for inclusion.
Data collection and definitions
Demographic, clinical, laboratory, treatment, and outcome data were collected from the electronic medical records of the participants. Demographic and clinical data included age, sex, comorbidities, symptoms and vital signs on admission, and treatment in the hospital. Laboratory data consisted of complete blood count, blood biochemistry, and infection-related biomarkers. Peripheral oxygen saturation was measured by pulse oximetry immediately on hospitalization of the patient. In-hospital case fatality rate was monitored until the final date of follow-up. The data were collected and analyzed by all authors.
Severe disease was defined as a composite outcome of acute respiratory distress syndrome (ARDS), intensive care unit care, or death. ARDS was diagnosed according to the Berlin definition.9 SARS-CoV-2 infection was confirmed by real-time reverse transcription polymerase chain reaction assay of nose and/or throat swap samples.
Statistical analysis
Continuous variables are expressed as means ± standard deviation and were compared by Student's t-test or the Mann-Whitney U test. Categorical variables are described as number (%) and were compared by the χ2 test or Fisher's exact test. Univariable logistic regression analysis was performed to identify prognostic factors of severe COVID-19. Multivariable logistic regression analysis was conducted with variables that showed P < 0.05 in univariable analysis. We excluded variables from the univariable analysis if the number of events was too small for calculation and if there was no marked difference between two groups. In all analyses, two-tailed P < 0.05 was taken to indicate statistical significance. All statistical analyses were performed using SPSS software (ver. 24.0; SPSS Inc., Chicago, IL, USA).
Ethics statement
This study was conducted in accordance with the tenets of the Declaration of Helsinki and was reviewed and approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (YUH IRB 2020-03-057). The requirement for informed consent was waived because of the retrospective study design. The final follow-up date was April 15, 2020.
RESULTS
Demographic and clinical characteristics
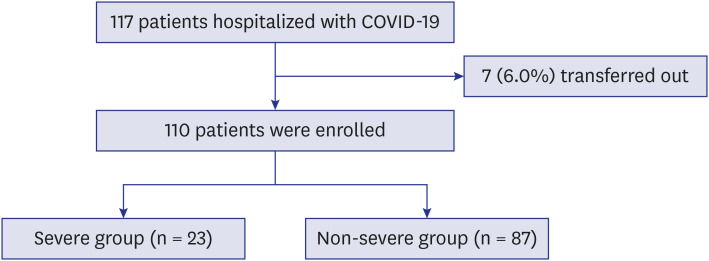
After excluding seven patients who were transferred to other hospitals, 110 hospitalized patients with confirmed COVID-19 were included in this study (Fig. 1). Baseline characteristics of all patients are summarized in Table 1. The mean age was 56.9 ± 17.0 and 62 patients (56.4%) were women. Forty-nine patients (44.5%) had comorbidities, of which hypertension was the most common (33.6%) followed by diabetes mellitus (26.4%). The most frequently presenting symptoms were fever (56.4%) and cough (52.7%).
Fig. 1. Flow chart.
COVID-19 = coronavirus disease 2019.
Table 1. Baseline characteristics of the study participants with COVID-19.
| Characteristics | All patients (n = 110) | Severe patients (n = 23) | Non-severe patients (n = 87) | P value | |
|---|---|---|---|---|---|
| Age, yr | 56.9 ± 17.0 | 68.0 ± 11.9 | 53.9 ± 17.0 | < 0.001 | |
| Sex | 0.051 | ||||
| Male | 48 (43.6) | 14 (60.9) | 34 (39.1) | ||
| Female | 62 (56.4) | 9 (39.1) | 53 (60.9) | ||
| Comorbidities | 49 (44.5) | 13 (57) | 36 (57) | 0.144 | |
| Cardiovascular disease | 10 (9.1) | 1 (4.3) | 9 (10.3) | 0.352 | |
| Cerebrovascular disease | 4 (3.6) | 1 (4.3) | 3 (3.4) | 0.614 | |
| Chronic lung disease | 4 (3.6) | 2 (8.7) | 2 (2.3) | 0.192 | |
| Dementia | 4 (3.6) | 2 (8.7) | 2 (2.3) | 0.614 | |
| Diabetes mellitus | 29 (26.4) | 14 (60.9) | 15 (17.2) | < 0.001 | |
| Hypertension | 37 (33.6) | 12 (52.2) | 25 (28.7) | 0.033 | |
| Connective tissue disease | 1 (0.9) | 0 (0) | 1 (1.1) | 0.791 | |
| Liver disease | 1 (0.9) | 0 (0) | 1 (1.1) | 0.192 | |
| Malignancy | 6 (5.5) | 1 (4.3) | 5 (5.7) | 0.633 | |
| Parkinson's disease | 1 (0.9) | 0 (6.7) | 1 (1.1) | 0.209 | |
| Symptoms on admission | |||||
| Fever | 62 (56.4) | 14 (60.9) | 48 (55.2) | 0.402 | |
| Cough | 58 (52.7) | 10 (43.4) | 48 (55.2) | 0.222 | |
| Sputum | 38 (34.5) | 6 (26.1) | 32 (36.8) | 0.241 | |
| Dyspnea | 37 (33.6) | 13 (56.5) | 24 (27.6) | 0.010 | |
| Diarrhea | 11 (10.0) | 1 (4.3) | 10 (11.5) | 0.453 | |
| Nausea/vomiting | 3 (2.7) | 1 (4.3) | 2 (2.3) | 0.509 | |
| Sore throat | 13 (11.8) | 0 (0) | 13 (14.9) | 0.066 | |
| Chest pain | 5 (4.5) | 0 (0) | 5 (5.7) | 0.582 | |
| Altered smell or taste loss | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Skin lesion | 1 (0.9) | 1 (4.3) | 0 (0) | 0.209 | |
| Vital signs on admission | |||||
| Body temperature, °C | 37.2 ± 0.7 | 37.9 ± 1.0 | 37.3 ± 0.7 | 0.002 | |
| Heart rate, beats/min | 86.0 ± 13.8 | 87.6 ± 12.7 | 85.5 ± 14.1 | 0.532 | |
| Respiratory rate | 21.0 ± 2.8 | 23.1 ± 4.7 | 20.4 ± 1.7 | 0.013 | |
| Systolic BP, mmHg | 128.1 ± 18.6 | 134.9 ± 18.6 | 126.3 ± 18.4 | 0.058 | |
| Peripheral oxygen saturation, % | 94.1 ± 5.7 | 87.7 ± 7.4 | 95.8 ± 3.6 | < 0.001 | |
| Radiologic findings | |||||
| Chest X-ray only | 34 (30.9) | 6 (26.1) | 28 (32.2) | ||
| Chest X-ray and CT | 76 (69.1) | 17 (73.9) | 59 (67.8) | ||
| Unilateral pneumonia | 17 (15.5) | 1 (4.3) | 16 (18.4) | 0.078 | |
| Bilateral pneumonia | 46 (41.8) | 13 (56.5) | 33 (37.9) | ||
| Multiple ground-glass opacity | 39 (35.5) | 9 (39.1) | 30 (34.5) | ||
| Treatment in hospital | |||||
| Antibiotics | 108 (98.2) | 23 (100) | 85 (97.7) | 0.624 | |
| Lopinavir/ritonavir | 106 (96.4) | 22 (95.7) | 84 (96.6) | 0.614 | |
| Hydroxychloroquine | 91 (82.7) | 23 (100) | 68 (78.2) | 0.011 | |
| Glucocorticoid | 21 (19.1) | 15 (65.2) | 6 (16.6) | < 0.001 | |
| Clinical outcomes | |||||
| Death | 8 (7.3) | 8 (34.8) | 0 (0) | 1.000 | |
| Causes of mortality | |||||
| Respiratory failure | 4 (3.6) | 4 (17.4) | 0 (0) | 1.000 | |
| Septic shock | 4 (3.6) | 4 (17.4) | 0 (0) | 1.000 | |
Data are presented as the mean ± standard deviation or number (%).
COVID-19 = coronavirus disease 2019, BP = blood pressure, CT = computed tomography.
The patients in the severe group were significantly older than the patients in the non-severe group (68.0 ± 11.9 vs. 53.9 ± 17.0, respectively, P < 0.001). The severe group was significantly more likely to have diabetes mellitus (60.9% vs. 17.2%, respectively, P = 0.001) and hypertension (52.2% vs. 28.7%, respectively, P = 0.033). On admission, body temperature (37.9°C ± 1.0°C vs. 37.3°C ± 0.7°C, respectively, P = 0.002) and respiration rate (23.1 ± 4.7 vs. 20.4 ± 1.7 breaths per minute, respectively, P = 0.013) were significantly higher in the severe group than the non-severe group. Peripheral oxygen saturation was significantly lower in the severe group than the non-severe group (87.7 ± 7.4 vs. 95.8 ± 3.6, respectively, P < 0.001). It was difficult to detect meaningful differences in radiologic findings between patients in the severe group and those who were not.
Laboratory findings
Laboratory findings on hospital admission are summarized in Table 2. In complete blood counts, white blood cell count (8.2 ± 3.4 vs. 6.3 ± 3.2, respectively, P = 0.017) and neutrophil count (7.1 ± 3.4 vs. 4.1 ± 3.0, respectively, P < 0.001) were higher in the severe group than the non-severe group. Lymphocyte count (0.7 ± 0.3 vs. 1.6 ± 0.7, respectively, P < 0.001) and platelet count (184.7 ± 75.3 vs. 259.8 ± 104.6, respectively, P = 0.002) were significantly lower in the severe group than the non-severe group. With regard to blood chemistry, albumin level was significantly lower in the severe group than the non-severe group (3.1 ± 0.4 vs. 3.9 ± 0.5 g/dL, respectively, P < 0.001). Concentrations of aspartate aminotransferase, total bilirubin, blood urea nitrogen, LDH, and creatine kinase-MB (CK-MB) were significantly higher in the severe group than the non-severe group. With regard to infection-related markers, C-reactive protein level was significantly higher in the severe group than the non-severe group (15.5 ± 8.8 vs. 3.3 ± 6.2 mg/L, respectively, P < 0.001), although procalcitonin level was not significantly different between the two groups (2.2 ± 5.6 vs. 0.1 ± 0.1 ng/mL, respectively, P = 0.094). Three cases of bacterial co-infection (2 cases of Klebsiella pneumonia, 1 case of Clostridium difficile) were identified in the non-severe group. Duration of viral shedding was not different between the two groups.
Table 2. Laboratory findings on admission in patients with COVID-19.
| Variables | All patients (n = 110) | Severe group (n = 23) | Non-severe group (n = 87) | P value | ||
|---|---|---|---|---|---|---|
| Complete blood count (normal range) | ||||||
| White blood cell count, ×109/L (4–10) | 6.7 ± 3.3 | 8.2 ± 3.4 | 6.3 ± 3.2 | 0.017 | ||
| < 4 | 16 (14.5) | 1 (4.3) | 15 (17.2) | 0.007 | ||
| 4–10 | 81 (73.6) | 15 (65.2) | 66 (75.9) | |||
| > 10 | 13 (11.8) | 7 (30.4) | 6 (6.9) | |||
| Neutrophil count, ×109/L (1.8–6.3) | 4.7 ± 3.3 | 7.1 ± 3.4 | 4.1 ± 3.0 | < 0.001 | ||
| > 6.3 | 24 (21.8) | 13 (56.5) | 11 (12.6) | < 0.001 | ||
| Lymphocyte count, ×109/L (1.1–3.2) | 1.4 ± 0.7 | 0.7 ± 0.3 | 1.6 ± 0.7 | < 0.001 | ||
| < 0.8 | 24 (21.8) | 15 (65.2) | 9 (10.3) | < 0.001 | ||
| Hemoglobin, g/dL (12–16) | 13.0 ± 1.5 | 13.2 ± 1.8 | 12.9 ± 1.4 | 0.385 | ||
| < 12 | 22 (20.0) | 4 (17,4) | 18 (20.7) | 0.491 | ||
| Platelets, ×109/L (140–440) | 244.1 ± 103.5 | 184.7 ± 75.3 | 259.8 ± 104.6 | 0.002 | ||
| < 140 | 19 (17.3) | 10 (43.5) | 9 (10.3) | < 0.001 | ||
| Blood chemistry (normal range) | ||||||
| Albumin, g/dL (3.5–5) | 3.7 ± 0.6 | 3.1 ± 0.4 | 3.9 ± 0.5 | < 0.001 | ||
| < 3.5 | 38 (35.2) | 20 (87) | 18 (21.2) | < 0.001 | ||
| Alanine aminotransferase, IU/L (0–40) | 36.6 ± 46.6 | 54.5 ± 84.1 | 31.9 ± 28.9 | 0.215 | ||
| > 40 | 31 (28.2) | 13 (56.6) | 18 (20.7) | 0.001 | ||
| Aspartate aminotransferase, IU/L (10–35) | 45.1 ± 47.8 | 81.3 ± 83.3 | 35.5 ± 26.0 | 0.016 | ||
| > 35 | 29 (26.4) | 6 (26.1) | 23 (22.9) | 0.973 | ||
| Total bilirubin, mg/dL (0.1–1.2) | 0.9 ± 0.5 | 1.1 ± 0.6 | 0.8 ± 0.4 | 0.014 | ||
| > 1.2 | 18 (16.8) | 7 (30.4) | 11 (13.1) | 0.054 | ||
| Blood urea nitrogen, mg/dL (8–23) | 15.6 ± 9.0 | 21.7 ± 15.6 | 13.9 ± 5.4 | 0.027 | ||
| > 23 | 13 (11.8) | 8 (34.8) | 5 (5.7) | 0.001 | ||
| Creatinine, mg/dL (0.5–0.9) | 0.9 ± 0.5 | 1.1 ± 0.9 | 0.8 ± 0.2 | 0.174 | ||
| > 0.9 | 36 (32.7) | 11 (47.8) | 25 (28.7) | 0.083 | ||
| Creatinine phosphokinase, IU/L (1–145) | 108.4 ± 155.2 | 152.2 ± 182.0 | 95.0 ± 145.0 | 0.150 | ||
| > 145 | 9 (10.6) | 4 (20.0) | 5 (7.7) | 0.127 | ||
| Lactate dehydrogenase, IU/L (150–550) | 625.9 ± 331.7 | 996.7 ± 497.3 | 527.7 ± 171.9 | < 0.001 | ||
| > 550 | 51 (48.6) | 21 (95.5) | 30 (36.1) | < 0.001 | ||
| CK-MB, ng/mL (0.6–6.3) | 3.2 ± 3.3 | 5.3 ± 4.1 | 1.9 ± 1.8 | 0.003 | ||
| > 6.3 | 7 (15.6) | 6 (33.3) | 1 (3.7) | 0.012 | ||
| Coagulation function | ||||||
| Prothrombin time, sec (10.4–13.3) | 13.0 ± 17.4 | 16.3 ± 26.7 | 10.7 ± 4.2 | 0.278 | ||
| > 13.3 | 10 (20.4) | 7 (35.0) | 3 (10.3) | 0.068 | ||
| D-dimer, μg/mL (0–0.5) | 4.4 ± 16.5 | 8.6 ± 25.3 | 1.4 ± 1.8 | 0.029 | ||
| > 0.5 | 29 (76.3) | 16 (100.0) | 13 (59.1) | 0.005 | ||
| Infection biomarkers (normal range) | ||||||
| C-reactive protein, mg/dL (0–0.5) | 5.8 ± 8.4 | 15.5 ± 8.8 | 3.3 ± 6.2 | < 0.001 | ||
| > 0.5 | 68 (66.7) | 21 (100) | 47 (58.0) | < 0.001 | ||
| Procalcitonin, ng/mL (0–0.5) | 0.49 ± 2.6 | 2.2 ± 5.6 | 0.1 ± 0.1 | 0.094 | ||
| > 0.5 | 7 (6.5) | 6 (27.3) | 1 (1.2) | < 0.001 | ||
| Co-infection | ||||||
| Bacteria | 3 (2.7) | 0 (0) | 3 (3.4) | 1.000 | ||
| Other viruses | 0 | 0 | 0 | 1.000 | ||
| Viral shedding | ||||||
| Duration of viral shedding, days | 33.1 ± 10.0 | 34.2 ± 10.1 | 32.9 ± 10.0 | 0.659 | ||
Data are presented as the mean ± standard deviation or number (%).
COVID-19 = coronavirus disease 2019, CK-MB = creatine-kinase MB.
Prognostic factors for severe COVID-19
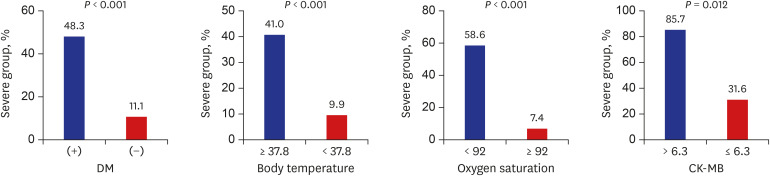
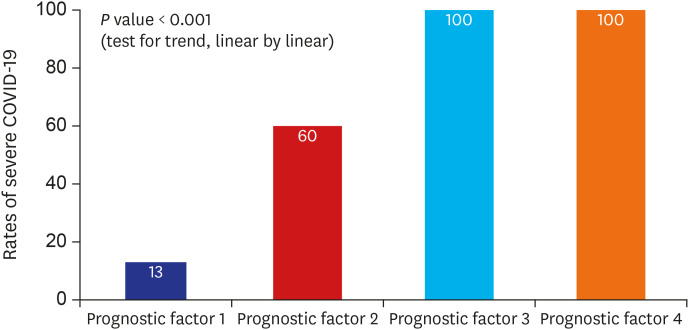
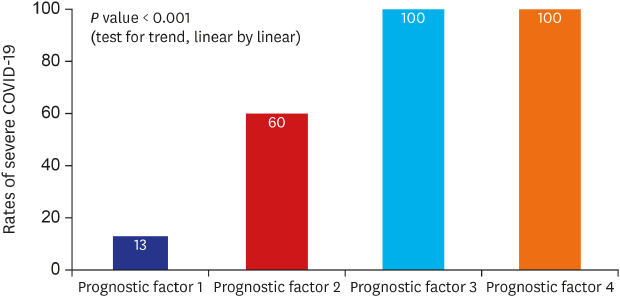
Multivariable analysis using variables with P < 0.05 in univariable analysis (Table 3) and the final logistic regression model demonstrated that diabetes mellitus (odds ratio [OR], 19.15; 95% confidence interval [CI], 1.90–193.42; P = 0.012), body temperature ≥ 37.8°C (OR, 10.91; 95% CI, 1.35–88.36; P = 0.025), peripheral oxygen saturation < 92% (OR, 33.31; 95% CI, 2.45–452.22; P = 0.008), and CK-MB > 6.3 (OR, 56.84; 95% CI, 2.64–1,223.78; P = 0.010) on admission were associated with greater risk of severe COVID-19 (Table 4). The rates of severe disease increased for patients with diabetes mellitus, body temperature ≥ 37.8°C, peripheral oxygen saturation ≤ 92%, and CK-MB > 6.3 (Fig. 2). The likelihood of development of severe COVID-19 increased with increasing number of prognostic factors (P < 0.001, test for trend) (Fig. 3).
Table 3. Univariable analysis of prognostic factors for severe COVID-19.
| Prognostic factors | OR (95% CI) | P value | |
|---|---|---|---|
| Age, yr | 1.07 (1.03–1.11) | 0.001 | |
| 0–39 | Reference | ||
| 40–49 | 0.00 (0.0–0.0) | 1.000 | |
| 50–59 | 0.40 (0.03–4.70) | 0.463 | |
| 60–69 | 3.46 (0.65–18.29) | 0.145 | |
| ≥ 70 | 8.14 (1.57–42.33) | 0.013 | |
| Male, sex | 2.43 (0.95–6.22) | 0.065 | |
| Diabetes mellitus | 7.47 (2.73–20.40) | < 0.001 | |
| Hypertension | 2.71 (1.06–6.93) | 0.038 | |
| Body temperature, °C | 2.54 (1.35–4.78) | 0.004 | |
| < 37.8 | Reference | ||
| ≥ 37.8 | 6.36 (2.32–17.43) | < 0.001 | |
| Respiratory rate | 1.42 (1.14–1.76) | < 0.001 | |
| ≤ 20 | Reference | ||
| > 20 | 8.11 (2.94–22.36) | < 0.001 | |
| Peripheral oxygen saturation, % | 0.75 (0.66–0.85) | < 0.001 | |
| ≥ 92 | Reference | ||
| < 92 | 17.71 (5.82–53.87) | < 0.001 | |
| Albumin | 0.03 (0.01–0.13) | < 0.001 | |
| ≥ 3.5 | Reference | ||
| < 3.5 | 24.82 (6.63–92.92) | < 0.001 | |
| Total bilirubin | 3.19 (1.22–8.35) | 0.018 | |
| ≤ 1.2 | Reference | ||
| > 1.2 | 2.71 (1.05–7.00) | 0.040 | |
| CK-MB | 1.52 (1.12–2.01) | 0.007 | |
| ≤ 6.3 | Reference | ||
| > 6.3 | 13.0 (1.41–120.27) | 0.024 | |
COVID-19 = coronavirus disease 2019, OR = odds ratio, CI = confidence interval, CK-MB = creatine-kinase MB.
Table 4. Multivariable logistic regression analysis of prognostic factors for severe COVID-19.
| Prognostic factors | Multivariable model 1a | Multivariable model 2b | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, yr | |||||
| ≥ 70 | 3.37 (0.26–43.58) | 0.352 | |||
| Male, sex | 2.98 (0.23–38.81) | 0.404 | |||
| Diabetes mellitus | 6.53 (2.26–18.87) | 0.001 | 19.15 (1.90–193.42) | 0.012 | |
| Hypertension | 1.44 (0.49–4.26) | 0.508 | 1.00 (0.09–10.78) | 0.999 | |
| Body temperature ≥ 37.8°C | 8.27 (2.62–26.09) | < 0.001 | 10.91 (1.35–88.36) | 0.025 | |
| Peripheral oxygen saturation < 92% | 15.39 (4.83–48.98) | < 0.001 | 33.31 (2.45–452.22) | 0.008 | |
| Albumin < 3.5 | 27.21 (6.98–106.10) | < 0.001 | 5.36 (0.49–59.32) | 0.171 | |
| Total bilirubin > 1.2 | 2.53 (0.92–6.97) | 0.073 | 5.92 (0.69–51.01) | 0.106 | |
| CK-MB > 6.3 | 13.00 (1.41–120.27) | 0.024 | 56.84 (2.64–1,223.78) | 0.010 | |
COVID-19 = coronavirus disease 2019, OR = odds ratio, CI = confidence interval, CK-MB = creatine-kinase MB.
aAdjusted for age, sex; bAdjusted for age, sex, diabetes mellitus, hypertension, body temperature, peripheral oxygen saturation, albumin, total bilirubin, and CK-MB.
Fig. 2. Comparison of rates of severe COVID-19 using categorical variables.
DM = diabetes mellitus, COVID-19 = coronavirus disease 2019, CK-MB = creatine-kinase MB.
Fig. 3. Rate of patients with severe COVID-19 according to the presence of prognostic factors.
COVID-19 = coronavirus disease 2019.
DISCUSSION
Among the 110 patients with COVID-19, 23 (20.9%) had severe disease and the in-hospital case fatality rate was 7.3% in this study. We showed that the presence of diabetes mellitus, body temperature ≥ 37.8°C, peripheral oxygen saturation < 92%, and CK-MB > 6.3 were independent predictors of severe disease in hospitalized COVID-19 patients. To our knowledge, this is the first study to evaluate the prognostic factors of severe COVID-19 in Korea.
Diabetes mellitus is a major public health issue, with an estimated global prevalence of 9.3% in 2019.10 A population-based cohort study showed that type 2 diabetes increased the risk of death associated with pneumonia, and hyperglycemia on admission was associated with increased mortality for both diabetic and nondiabetic patients with community-acquired pneumonia.11 Yang et al.12 reported that diabetes and ambient hyperglycemia are independent risk factors for death and morbidity in SARS patients. Diabetes also results in immune dysregulation and more severe and prolonged lung pathology in Middle East respiratory syndrome.13 The main mechanisms underlying the poorer clinical outcomes in cases of infections associated with diabetes mellitus are as follows: 1) decreased T lymphocyte response; 2) decreased neutrophil function; 3) disorders of humoral immunity; and 4) depression of the antioxidant system.14 In a recent study, COVID-19 patients without other comorbidities but with diabetes were shown to be at greater risk of severe disease as assessed by organ damage, inflammatory factors, and hypercoagulability. In addition, COVID-19 patients with diabetes are at high risk for disease progression.15 The results of the present study suggested that the progression of COVID-19 is influenced by diabetes mellitus. Physicians should pay close attention to whether diabetic patients with COVID-19 show rapid clinical deterioration.
Body temperature is one of the variables included in the pneumonia severity index16 and systemic inflammatory response syndrome (SIRS),17 which can predict clinical outcomes in pneumonia. The febrile response is thought to be mediated by endogenous factors, called endogenous pyrogens. Pyrogenic cytokines, such as tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, and interferons (IFNs), are released in response to exogenous stimuli, such as bacterial or viral products and toxins.18 In COVID-19, the levels of the proinflammatory cytokines IL-1β, IL-4, IL-6, IL-10, IFN-γ, and TNF-α are significantly higher in severe cases than in mild cases.19 The results of the present study suggested that critical COVID-19 patients have elevated levels of inflammatory cytokines, which increase body temperature.
Low peripheral oxygen saturation was shown to be an independent prognostic factor for severe COVID-19.8 Many COVID-19 patients experience rapid respiratory failure and hypoxemia without any signs of dyspnea, which is referred to as silent hypoxemia.20 This unique characteristic of COVID-19 makes it difficult to predict clinical deterioration accurately using traditional scores, such as quick sequential organ failure assessment and SIRS. As COVID-19 is a highly contagious infectious disease, medical staff tend to have less contact with patients. Therefore, the discovery of a worsening condition in patients may be delayed. From this viewpoint, peripheral oxygen saturation measured immediately upon hospitalization through pulse oximetry can be used as a convenient and accurate marker for the prediction of clinical deterioration in COVID-19.
Cardiac injury is associated with death, and severity of COVID-19 is associated with acute cardiac injury. In the systemic review of the 28 studies with 4,189 confirmed COVID-19 patients, severe COVID-19 infection was associated with high cardiac injury related markers, such as troponin, CK-MB, and myoglobin. And also cardiac injury in COVID-19 were related with higher mortality (OR, 3.85; 95% CI, 2.13–6.96; P < 0.001).21 It has been known that, the SARS-CoV-2 invades human cells via the receptor angiotensin converting enzyme II (ACE2). ACE2 is expressed in the lung, heart, esophagus, kidney, bladder, and ileum. Thus, organs that express ACE2 are vulnerable to SARS-CoV-2 infection.22 Therefore, the measurement of cardiac damage markers on admission is needed in patients with COVID-19, which predict the prognosis of COVID-19.
There are several Chinese studies demonstrating the risk factors for severity of COVID-19. Li et al.5 reported that elder age, hypertension, high cytokine levels, and high LDH levels were associated with severe COVID-19 inpatients in Wuhan. A study in Anhui, China revealed that low fingertip oxygen saturation, and decreased CD4 cell count were independent risk factors for severe COVID-19 patients.8 Diabetes, and maximum body temperature admission were risk factors for progression of COVID-19.15,23 The predictors of severe disease progression on Korean patients and those in Chinese patients were not much different.
This study had several limitations. First, this was a retrospective study conducted in a single center in Korea, which subjected only hospitalized patients. Therefore, these results cannot be generalized to all COVID-19 patients. Second, antiviral agents and corticosteroid usage were not included as variables in this study. Our research focused on the baseline clinical characteristics and laboratory findings related to worsening of the patients' condition due to severe disease and not on treatment. Third, selection bias could not be avoided because population-based data were not used. The disease severity of patients may vary between hospitals in the same region. Fourth, proinflammatory cytokines and early CD8+ T-cell response that can be associated with disease severity, were not measured in this study.
In conclusion, we found that the presence of diabetes mellitus, body temperature ≥ 37.8°C, peripheral oxygen saturation < 92%, and CK-MB > 6.3 are independent predictors of severe disease in hospitalized COVID-19 patients. The likelihood of progression to severe COVID-19 increased with an increasing number of prognostic factors. Appropriate assessment of prognostic factors and close monitoring to provide the necessary interventions at the appropriate time in high-risk patients may reduce the case fatality rate of COVID-19.
Footnotes
Funding: This study was supported by a research grant from the Daegu Medical Association COVID-19 Scientific Committee in 2020.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jang JG, Ahn JH.
- Data curation: Jang JG, Hur J, Choi EY, Hong KS, Lee W, Ahn JH.
- Investigation: Jang JG, Ahn JH.
- Supervision: Hur J, Choi EY, Hong KS, Lee W.
- Writing - original draft: Jang JG, Ahn JH.
- Writing - review & editing: Jang JG, Ahn JH.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Li W, Shi X, Chen Z, Jiang B, Liu J, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020 doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei YY, Wang RR, Zhang DW, Tu YH, Chen CS, Ji S, et al. Risk factors for severe COVID-19: Evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 11.Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sørensen HT. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care. 2007;30(9):2251–2257. doi: 10.2337/dc06-2417. [DOI] [PubMed] [Google Scholar]
- 12.Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 13.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20):e131774. doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 18.Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clin Infect Dis. 2000;31(Suppl 5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- 19.Hong KS, Lee KH, Chung JH, Shin KC, Choi EY, Jin HJ, et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61(5):431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46(5):837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li JW, Han TW, Woodward M, Anderson CS, Zhou H, Chen YD, et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]