Abstract
Background
The mortality risk of coronavirus disease 2019 (COVID-19) is higher in patients with older age, and many elderly patients are reported to require advanced respiratory support.
Methods
We reviewed medical records of 98 patients aged ≥ 65 years who were hospitalized with COVID-19 during a regional outbreak in Daegu/Gyeongsangbuk-do province of Korea. The outcome measures were in-hospital mortality and the treatment with mechanical ventilation (MV) or high-flow nasal cannula (HFNC).
Results
The median age of the patients was 72 years; 55.1% were female. Most (74.5%) had at least one underlying condition. Overall case fatality rate (CFR) was 20.4%, and median time to death after admission was 8 days. The CFR was 6.1% among patients aged 65–69 years, 22.7% among those aged 70–79 years, and 38.1% among those aged ≥ 80 years. The CFR among patients who required MV was 43.8%, and the proportion of patients received MV/HFNC was 28.6%. Nosocomial acquisition, diabetes, chronic lung diseases, and chronic neurologic diseases were significant risk factors for both death and MV/HFNC. Hypotension, hypoxia, and altered mental status on admission were also associated with poor outcome. CRP > 8.0 mg/dL was strongly associated with MV/HFNC (odds ratio, 26.31; 95% confidence interval, 7.78–88.92; P < 0.001), and showed better diagnostic characteristics compared to commonly used clinical scores.
Conclusion
Patients aged ≥ 80 years had a high risk of requiring MV/HFNC, and mortality among those severe patients was very high. Severe initial presentation and laboratory abnormalities, especially high CRP, were identified as risk factors for mortality and severe hospital course.
Keywords: COVID-19, Outcome, Elderly, Risk Factors
Graphical Abstract
INTRODUCTION
Coronavirus disease 19 (COVID-19) is an infectious disease caused by a novel coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Approximately 3 months after the first report in Wuhan, China, the number of cases exceeded 400,000 by late March.1 High viral shedding early in the disease course and slow progression make the effort for containment extremely difficult, and a large surge of cases has been observed in Europe and North America.2,3 Its clinical course ranges from asymptomatic infection to acute respiratory distress syndrome (ARDS) and death.4 Although most patients undergo mild febrile illness, a relatively large proportion of patients need hospitalization and respiratory support such as high-flow nasal cannula (HFNC) or mechanical ventilation (MV).5 Case fatality rates (CFRs) vary significantly by country, as the magnitude and velocity of surge greatly affect the care of patients. However, severe cases and mortality are consistently reported among the elderly, and patients aged ≥ 60 years comprise the majority of fatal cases in both China and Italy.6,7 Despite the importance of old age with respect to outcome, there have been no reports specifically aimed at examining the clinical characteristics and treatment outcomes in elderly patients with COVID-19 who require hospitalization. Information on the outcomes in this population, especially the need for MV/HFNC that requires specialized machines and considerable resources, is necessary for the public health response and planning.
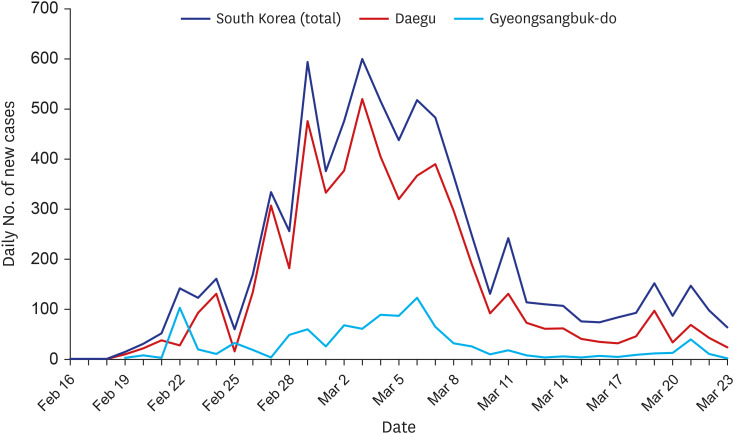
Since February 19, 2020, a regional outbreak of COVID-19 occurred in the Daegu/Gyeongsangbuk-do province of Korea (Fig. 1). Owing to an expanded testing capacity and rapid public health response, most patients are considered diagnosed and monitored. Although the healthcare capacity has been overstretched during the outbreak, the CFR observed in the area is substantially lower than the CFRs reported in China and Italy, suggesting that the healthcare system has been largely capable of providing adequate care for patients.8,9 The clinical data from Daegu/Gyeongsangbuk-do province would provide useful information regarding the characteristics of COVID-19 in a situation that is different from the other two gravely affected countries.
Fig. 1. Daily number of new cases in Daegu/Gyeongsangbuk-do and in Korea.
Thus, we conducted a retrospective study to elucidate the clinical characteristics and risk factors for mortality and the need for MV/HFNC in elderly patients hospitalized with COVID-19.
METHODS
Study population and data sources
We obtained medical records of patients aged ≥ 65 years who were admitted with laboratory-confirmed COVID-19 in four hospitals between February 18 and March 4, 2020. The end date was set to ensure that all patients were observed for at least 14 days after admission, as the median time from onset to MV was 10.5 days in a previous report and 75th percentile of time to death after symptom onset was reported to be 14 days.9,10 All patients were residents in the Daegu/Gyeongsangbuk-do province, and diagnosis of COVID-19 was made using a real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay of a nasopharyngeal swab or sputum according to the national guidelines.
Electronic medical records were reviewed to extract demographic characteristics, comorbidities, clinical features and laboratory findings on the day of admission, clinical course, treatment, and outcome. Patients were followed until death or discharge from hospital, whichever came first.
Study outcomes and definitions
The outcome measures were all-cause in-hospital death and MV/HFNC. We did not include care in intensive care units (ICUs) as an outcome measure as many mechanically ventilated patients were treated outside an ICU due to a shortage of ICU beds. The severity of the clinical course was evaluated through the highest respiratory support required during the hospital stay. They were categorized into none, supplementary oxygen (via nasal prong or facial mask), HFNC, MV, and extracorporeal membrane oxygenation. Noninvasive positive pressure ventilation was not administered to any of our study patients. Nosocomial acquisition was defined as a diagnosis of COVID-19 during admission in an acute-care hospital or a long-term care facility for other unrelated illnesses. The modified early warning score (MEWS) and national early warning score 2 (NEWS2) were calculated as previously described.11,12
Statistical analysis
Patient characteristics were summarized and compared among outcomes using Student's t-test or Mann-Whitney U test for continuous variables and χ2 or Fisher's exact test for categorical variables, as appropriate. In-hospital mortality of the two groups was compared using the Kaplan-Meier curve. A receiver operating characteristic curve was used to evaluate the accuracy of the prognostic factors. All tests were two-tailed, and significance was assessed at P < 0.05. R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for the analyses.
Ethics statement
The study was approved by the Institutional Review Board of the Samsung Medical Center (SMC 2020-03-116-001) with waived informed consent.
RESULTS
We identified 98 patients hospitalized with COVID-19 who were aged ≥ 65 years. Fifty-four patients (55.1%) were female, and the median age was 72 (interquartile range [IQR], 68–79; range, 65–93) years. Most patients (74.5%) had underlying conditions; hypertension (52.0%), diabetes (27.6%), cardiovascular diseases (16.3%), chronic neurologic disease (14.3%), and malignancy (11.2%) were common comorbidities. Eight patients (8.2%) had chronic lung disease, and six patients (6.1%) had chronic kidney disease. None of the patients had end-stage renal disease requiring dialysis before their COVID-19 diagnosis. Lopinavir/ritonavir or darunavir/ritonavir was administered to 75 patients (76.5%) and hydroxychloroquine to 59 patients (60.2%). Systemic glucocorticoids were administered to 28 patients (28.6%). The median time of follow-up since admission was 18 (IQR, 13–22) days.
Risk factors for mortality
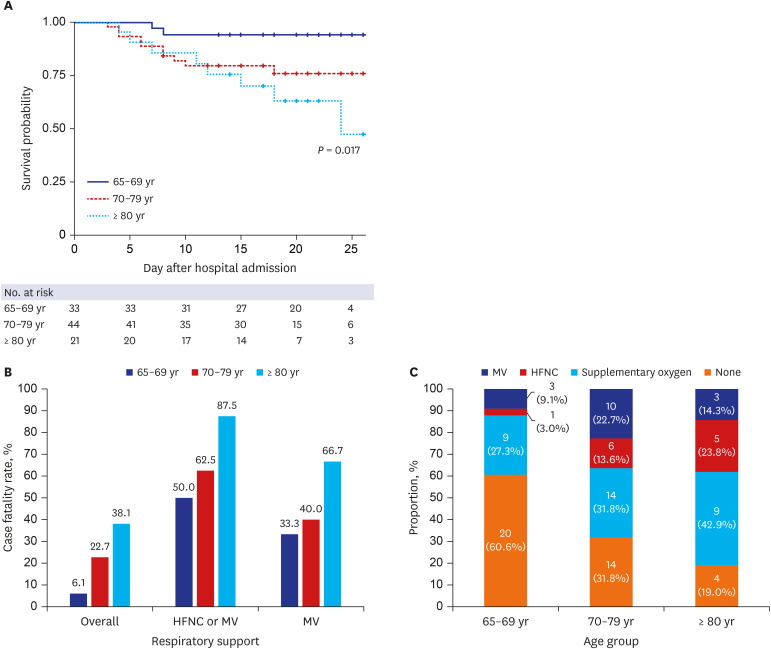
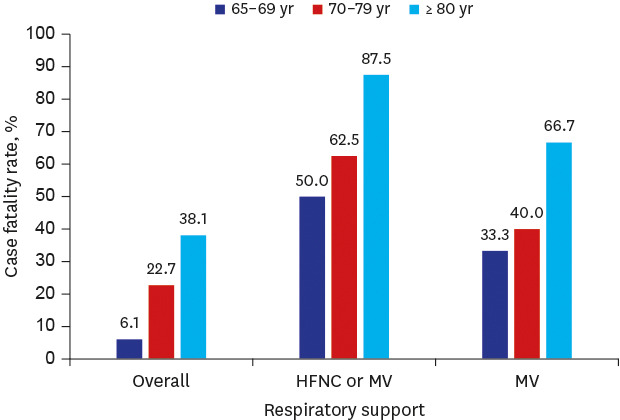
Twenty patients died during their hospital stay, and the overall CFR was 20.4% (Table 1). The median time to death after admission was 8 (IQR, 5–11) days. The CFR among male patients was significantly higher than that among female patients (31.8% vs. 11.1%, P = 0.023). Age was a significant predictor of mortality (Fig. 2A); the CFR was 6.1% among patients aged 65–69 years, 22.7% among those aged 70–79 years, and 38.1% among those aged ≥ 80 years died. The substantial effect of age on outcome was observed consistently when CFR was examined according to severity. The CFR among patients ≥ 80 years of age who required HFNC or MV was 87.5%, substantially higher than that of lower age groups (Fig. 2B). In addition, the time to death was longer in patients aged ≥ 80 years (median, 11 days; IQR, 6–15 days) than in patients aged 70–79 years (median, 7 days; IQR, 4–8 days), although the difference was not statistically significant (P = 0.141). The overall CFR among patients who required MV was 43.8% (n = 7/16). All 12 patients whose highest respiratory support was HFNC, who declined to be intubated, died.
Table 1. Characteristics of the patients by mortality.
| Characteristics | Survival (n = 78) | Mortality | ||||
|---|---|---|---|---|---|---|
| Death (n = 20) | OR (95% CI) | P value | ||||
| Female, sex | 48 (61.5) | 6 (30.0) | 0.27 (0.09–0.77) | 0.023 | ||
| Age, yr | 71.0 (67.0–78.0) | 77.0 (73.0–83.5) | 0.003 | |||
| 65–69 | 31 (39.7) | 2 (10.0) | 0.012 | |||
| 70–79 | 34 (43.6) | 10 (50.0) | ||||
| ≥ 80 | 13 (16.7) | 8 (40.0) | ||||
| Nosocomial acquisition | 5 (6.4) | 7 (35.0) | 7.86 (2.16–28.57) | 0.002 | ||
| Comorbidities | ||||||
| Any | 53 (67.9) | 20 (100.0) | N/A | 0.008 | ||
| Diabetes | 16 (20.5) | 11 (55.0) | 4.74 (1.68–13.38) | 0.005 | ||
| Hypertension | 38 (48.7) | 13 (65.0) | 1.95 (0.70–5.42) | 0.294 | ||
| Chronic lung disease | 3 (3.8) | 5 (25.0) | 8.33 (1.80–38.68) | 0.008 | ||
| Cardiovascular disease | 13 (16.7) | 3 (15.0) | 0.88 (0.23–3.45) | > 0.999 | ||
| Chronic renal disease | 3 (3.8) | 3 (15.0) | 4.41 (0.82–23.78) | 0.097 | ||
| Chronic liver disease | 1 (1.3) | 2 (10.0) | 8.56 (0.73–99.61) | 0.105 | ||
| Chronic neurologic disease | 6 (7.7) | 8 (40.0) | 8.00 (2.36–27.16) | 0.001 | ||
| Cancer | 7 (9.0) | 4 (20.0) | 2.54 (0.66–9.71) | 0.228 | ||
| Immunosuppressant use | 0 (0.0) | 1 (5.0) | N/A | 0.204 | ||
| Clinical manifestations on admission | ||||||
| Heart rate | 84.7 ± 16.9 | 94.0 ± 17.6 | 0.032 | |||
| ≥ 90 | 29 (37.2) | 10 (50.0) | 1.69 (0.63–4.55) | 0.430 | ||
| Systolic blood pressure, mmHg | 138.1 ± 25.3 | 114.0 ± 24.4 | < 0.001 | |||
| < 110 | 8 (10.3) | 8 (40.0) | 5.83 (1.84–18.53) | 0.004 | ||
| Vasopressor use | 4 (5.1) | 4 (20.0) | 4.63 (1.04–20.47) | 0.052 | ||
| Respiratory rate (n = 94) | 20.0 (18.0–20.5) | 23.0 (20.0–28.0) | < 0.001 | |||
| ≥ 20 | 52/75 (69.3) | 19/19 (100.0) | N/A | 0.005 | ||
| Oxygen saturation by pulse oximetry on room air, % (n = 77) | 96.0 (93.5–98.0) | 90.5 (85.0–95.0) | 0.010 | |||
| < 95 | 19/59 (32.2) | 11/18 (61.1) | 3.31 (1.11–9.88) | 0.054 | ||
| Body temperature, °C | 37.0 (36.5–37.9) | 37.3 (36.6–37.8) | 0.375 | |||
| Altered mental status | 7 (9.0) | 11 (55.0) | 12.40 (3.83–40.11) | < 0.001 | ||
| MEWS | 2.0 (1.0–3.0) | 4.0 (3.0–5.0) | < 0.001 | |||
| NEWS2 | 1.0 (0.0–2.0) | 4.5 (2.0–6.0) | 0.001 | |||
| Laboratory findings on admission | ||||||
| White blood cell count, /mm3 | 5,135.0 (4,140.0–6,730.0) | 7,855.0 (6,610.0–14,445.0) | < 0.001 | |||
| Neutrophil count, /mm3 | 3,403.8 (2,430.0–4,790.0) | 6,451.2 (5,260.1–11,532.2) | < 0.001 | |||
| > 4,500, /mm3 | 21 (26.9) | 16 (84.2) | 14.48 (3.83–54.78) | < 0.001 | ||
| Lymphocyte count, /mm3 | 1,251.4 (895.7–1,510.0) | 636.4 (519.1–858.6) | < 0.001 | |||
| < 900 | 20 (25.6) | 15 (78.9) | 10.88 (3.23–36.63) | < 0.001 | ||
| Blood urea nitrogen, mg/dL | 15.7 (12.0–20.0) | 36.4 (16.5–45.0) | < 0.001 | |||
| > 20 | 20 (25.6) | 14 (70.0) | 6.77 (2.29–19.99) | 0.001 | ||
| Serum creatinine, mg/dL | 0.8 (0.7–1.0) | 1.4 (1.0–2.1) | < 0.001 | |||
| > 1.0 | 22 (28.2) | 17 (85.0) | 14.42 (3.84–54.14) | < 0.001 | ||
| Lactate dehydrogenase (n = 77), IU/L | 507.0 (449.0–677.0) | 599.0 (557.0–744.0) | 0.053 | |||
| > 600 | 21/68 (30.9) | 4/9 (44.4) | 1.79 (0.44–7.35) | 0.461 | ||
| C-reactive protein, mg/dL | 2.6 (0.6–7.1) | 12.8 (9.6–18.6) | < 0.001 | |||
| > 8.0 | 19 (24.4) | 18 (90.0) | 27.95 (5.93–131.63) | < 0.001 | ||
| Radiologic findings on admission | ||||||
| Presence of infiltrate | 60 (76.9) | 20 (100.0) | N/A | 0.020 | ||
| Bilateral infiltrate | 46 (59.0) | 20 (100.0) | N/A | 0.001 | ||
| Treatment | ||||||
| Lopinavir/ritonavir or darunavir/ritonavir | 57 (73.1) | 18 (90.0) | 3.32 (0.71–15.53) | 0.145 | ||
| Hydroxychloroquine | 47 (60.3) | 12 (60.0) | 0.99 (0.36–2.70) | > 0.999 | ||
| Corticosteroid | 13 (16.7) | 15 (75.0) | 15.00 (4.64–48.54) | < 0.001 | ||
| Clinical course | ||||||
| Highest respiratory support | < 0.001 | |||||
| None | 38 (48.7) | 0 (0.0) | ||||
| Supplementary oxygen | 31 (39.7) | 1 (5.0) | ||||
| High-flow nasal cannula | 0 (0.0) | 12 (60.0) | ||||
| Mechanical ventilation | 9 (11.5) | 7 (35.0) | ||||
| Continuous renal-replacement therapy | 3 (3.8) | 1 (5.0) | ||||
| Outcome at the last follow-up | ||||||
| Discharged alive | 12 (15.4) | |||||
| Being admitted and stable | 61 (78.2) | |||||
| Being admitted and on mechanical ventilator | 5 (6.4) | |||||
Data are presented as mean ± standard deviation, median (interquartile range) or number (%).
OR = odds ratio, CI = confidence interval, N/A = not available, MEWS = modified early warning score, NEWS2 = national early warning score 2.
Fig. 2. Mortality and the highest respiratory support by age group.
(A) Survival curve of study patients by age group. (B) Case fatality rate by the highest respiratory support and age group. (C) Highest respiratory support by age group. Number of patients and proportions among each age group were shown.
HFNC = high flow nasal cannula, MV = mechanical ventilation.
Nosocomial acquisition was also a significant risk factor for mortality (odds ratio [OR], 7.86; 95% confidence interval [CI], 2.16–28.57; P = 0.002). All patients who died had at least one underlying condition. Among comorbidities, diabetes (OR, 4.74; 95% CI, 1.68–13.38; P = 0.005), chronic lung diseases (OR, 8.33; 95% CI, 1.80–38.68; P = 0.008), and chronic neurologic diseases (OR, 8.00; 95% CI, 2.36–27.16; P = 0.001) were significantly associated with mortality. Severe manifestations on the day of admission were also associated with an increased risk of death. Systolic blood pressure < 110 mmHg (OR, 5.83; 95% CI, 1.84–18.53; P = 0.004), oxygen saturation by pulse oximetry < 95% on room air (OR, 3.31; 95% CI, 1.11–9.88; P = 0.054), and altered mental status (OR, 12.40; 95% CI, 3.83–40.11; P < 0.001) were significant predictive factors for mortality. In addition, all patients who died presented with respiration rates ≥ 20/min on admission, except one patient who had to be intubated before vital signs could be recorded. MEWS and NEWS2 scores were both significantly higher in patients who died later.
Among laboratory findings, a higher white blood cell count (P < 0.001), lymphocyte count < 900/mm3 (OR, 10.88; 95% CI, 3.23–36.63; P < 0.001), blood urea nitrogen > 20 mg/dL (OR, 6.77; 95% CI, 2.29–19.99; P = 0.001), serum creatinine > 1.0 mg/dL (OR, 14.42; 95% CI, 3.84–54.14; P < 0.001), and C-reactive protein (CRP) > 8.0 mg/dL (OR, 27.95; 95% CI, 5.93–131.63; P < 0.001) were associated with mortality.
Risk factors for advanced respiratory support
The overall proportion of patients received MV/HFNC was 28.6%. Older patients were more likely to need MV or HFNC; among patients aged 65–69 years, 12.1% received MV/HFNC, but 36.9% of patients aged ≥ 70 years needed MV/HFNC (Fig. 2C). Similarly, the proportion of patients who required supplementary oxygen was also higher in the older age groups.
Diabetes (OR, 2.75; 95% CI, 1.07–7.05; P = 0.058), chronic lung diseases (OR, 4.86; 95% CI, 1.08–21.93; P = 0.041), and chronic neurologic diseases (OR, 4.27; 95% CI, 1.32–13.77; P = 0.021) were associated with the need for MV/HFNC (Table 2). Patients who presented with hypotension (OR, 8.41; 95% CI, 2.57–27.49; P < 0.001), tachypnea or hypoxemia (OR, 3.25; 95% CI, 1.13–9.33; P = 0.048), or altered mental status (OR, 45.33; 95% CI, 9.22–222.96; P < 0.001) were more likely to require MV/HFNC during their subsequent hospital stay. All patients who required vasopressors also needed MV/HFNC (n = 8). MEWS and NEWS2 were significantly higher in those who received MV/HFNC. Among laboratory findings on admission, a high white blood cell count, lymphocyte < 900/mm3 (OR, 10.48; 95% CI, 3.73–29.43; P < 0.001), blood urea nitrogen > 20 mg/dL (OR, 6.08; 95% CI, 2.34–15.76; P < 0.001), serum creatinine > 1.0 mg/dL (OR, 6.71; 95% CI, 2.53–17.78; P < 0.001), and CRP > 8.0 mg/dL (OR, 26.31; 95% CI, 7.78–88.92; P < 0.001) were predictive factors for higher respiratory support.
Table 2. Characteristics of the patients according to the highest respiratory support required.
| Characteristics | Mechanical ventilation | High flow nasal cannula or mechanical ventilation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 82) | Yes (n = 16) | OR (95% CI) | P value | No (n = 70) | Yes (n = 28) | OR (95% CI) | P value | |||
| Female sex | 47 (57.3) | 7 (43.8) | 0.58 (0.20–1.71) | 0.470 | 43 (61.4) | 11 (39.3) | 0.41 (0.17–1.00) | 0.077 | ||
| Age, yr | 72.0 (67.0–79.0) | 74.0 (70.0–78.0) | 0.467 | 71.0 (67.0–78.0) | 75.5 (71.5–82.0) | 0.026 | ||||
| 65–69 | 30 (36.6) | 3 (18.8) | 0.293 | 29 (41.4) | 4 (14.3) | 0.037 | ||||
| 70–79 | 34 (41.5) | 10 (62.5) | 28 (40.0) | 16 (57.1) | ||||||
| ≥ 80 | 18 (22.0) | 3 (18.8) | 13 (18.6) | 8 (28.6) | ||||||
| Nosocomial acquisition | 10 (12.2) | 2 (12.5) | 1.03 (0.20–5.21) | > 0.999 | 5 (7.1) | 7 (25.0) | 4.33 (1.24–15.10) | 0.035 | ||
| Comorbidities | ||||||||||
| Any | 61 (74.4) | 12 (75.0) | 1.03 (0.30–3.55) | > 0.999 | 49 (71.0) | 24 (85.7) | 2.57 (0.79–8.33) | 0.207 | ||
| DM | 20 (24.4) | 7 (43.8) | 2.41 (0.80–7.31) | 0.132 | 15 (21.4) | 12 (42.9) | 2.75 (1.07–7.05) | 0.058 | ||
| Hypertension | 40 (48.8) | 11 (68.8) | 2.31 (0.74–7.24) | 0.234 | 34 (48.6) | 17 (60.7) | 1.64 (0.67–3.99) | 0.388 | ||
| Chronic lung disease | 6 (7.3) | 2 (12.5) | 1.81 (0.33–9.89) | 0.613 | 3 (4.3) | 5 (17.9) | 4.86 (1.08–21.93) | 0.041 | ||
| Cardiovascular disease | 13 (15.9) | 3 (18.8) | 1.22 (0.31–4.91) | 0.722 | 13 (18.6) | 3 (10.7) | 0.53 (0.14–2.01) | 0.546 | ||
| Chronic renal disease | 3 (3.7) | 3 (18.8) | 6.08 (1.11–33.41) | 0.053 | 3 (4.3) | 3 (10.7) | 2.68 (0.51–14.16) | 0.349 | ||
| Chronic liver disease | 2 (2.4) | 1 (6.2) | 2.67 (0.23–31.31) | 0.418 | 1 (1.4) | 2 (7.1) | 5.31 (0.46–61.05) | 0.196 | ||
| Chronic neurologic disease | 12 (14.6) | 2 (12.5) | 0.83 (0.17–4.14) | > 0.999 | 6 (8.6) | 8 (28.6) | 4.27 (1.32–13.77) | 0.021 | ||
| Cancer | 6 (7.3) | 5 (31.2) | 5.76 (1.50–22.09) | 0.016 | 5 (7.1) | 6 (21.4) | 3.55 (0.98–12.77) | 0.071 | ||
| Immunosuppressant use | 1 (1.2) | 0 (0.0) | N/A | > 0.999 | 0 (0.0) | 1 (3.6) | N/A | 0.286 | ||
| Clinical manifestations on admission | ||||||||||
| Heart rate | 86.6 ± 17.1 | 86.6 ± 19.1 | 0.998 | 84.2 ± 16.3 | 92.8 ± 18.7 | 0.027 | ||||
| ≥ 90 | 31 (37.8) | 8 (50.0) | 1.65 (0.56–4.83) | 0.527 | 24 (34.3) | 15 (53.6) | 2.21 (0.91–5.39) | 0.125 | ||
| Systolic blood, mmHg | 137.9 ± 25.3 | 109.1 ± 21.5 | < 0.001 | 141.6 ± 24.1 | 112.3 ± 21.8 | < 0.001 | ||||
| < 110 | 9 (11.0) | 7 (43.8) | 6.31 (1.89–21.08) | 0.004 | 5 (7.1) | 11 (39.3) | 8.41 (2.57–27.49) | < 0.001 | ||
| Vasopressor use | 2 (2.4) | 6 (37.5) | 24.00 (4.25–135.39) | < 0.001 | 0 (0.0) | 8 (28.6) | N/A | < 0.001 | ||
| Respiratory rate (n = 94) | 20.0 (18.0–22.0) | 23.0 (20.0–30.0) | 0.004 | 20.0 (18.0–20.0) | 23.0 (20.0–30.0) | < 0.001 | ||||
| ≥ 20 | 59/82 (72.0) | 12/12 (100.0) | N/A | 0.035 | 47/70 (67.1) | 24/24 (100.0) | N/A | 0.003 | ||
| Oxygen saturation by pulse oximetry on room air (n = 77), % | 95.0 (92.5–97.5) | 92.0 (85.0–98.0) | 0.389 | 96.0 (94.0–98.0) | 90.5 (85.0–96.5) | 0.013 | ||||
| < 95 | 25/68 (36.8) | 5/9 (55.6) | 2.15 (0.53–8.75) | 0.300 | 18/57 (31.6) | 12/20 (60.0) | 3.25 (1.13–9.33) | 0.048 | ||
| Body temperature, °C | 37.1 (36.5–37.9) | 37.2 (36.5–37.8) | 0.825 | 37.0 (36.5–37.9) | 37.2 (36.6–37.8 | 0.359 | ||||
| Altered mental status | 11 (13.4) | 7 (43.8) | 5.02 (1.55–16.24) | 0.009 | 2 (2.9) | 16 (57.1) | 45.33 (9.22–222.96) | < 0.001 | ||
| MEWS | 2.0 (1.0–3.0) | 3.0 (2.0–3.5) | 0.026 | 2.0 (1.0–2.0) | 3.5 (2.5–5.0) | < 0.001 | ||||
| NEWS2 | 1.0 (0.0–3.0) | 2.0 (1.0–5.0) | 0.281 | 1.0 (0.0–2.0) | 4.5 (2.0–5.5) | 0.001 | ||||
| Laboratory findings on admission | ||||||||||
| White blood cell count, /mm3 | 5,180.0 (4,140.0–6,960.0) | 9,105.0 (6,690.0–14,200.0) | 0.001 | 4,975.0 (4,090.0–6,200.0) | 8,480.0 (6,690.0–14,095.0) | < 0.001 | ||||
| Neutrophil count, /mm3 | 3,480.0 (2,430.0–4,870.0) | 7,858.4 (5,201.3–10,034.1) | < 0.001 | 3,215.0 (2,320.0–4,236.8) | 6,584.6 (5,557.0–11,501.6) | < 0.001 | ||||
| > 4,500, /mm3 | 25 (30.5) | 12 (80.0) | 9.12 (2.37–35.17) | 0.001 | 14 (20.0) | 23 (85.2) | 23.00 (6.84–77.33) | < 0.001 | ||
| Lymphocyte count, /mm3 | 1,235.0 (834.0–1,510.0) | 598.3 (514.4–1,164.3) | 0.003 | 1,294.3 (930.0–1,770.0) | 636.4 (519.1–908.7) | < 0.001 | ||||
| < 900 | 25 (30.5) | 10 (66.7) | 4.56 (1.41–14.72) | 0.017 | 15 (21.4) | 20 (74.1) | 10.48 (3.73–29.43) | < 0.001 | ||
| Blood urea nitrogen, mg/dL | 16.0 (12.0–22.0) | 23.1 (15.2–41.0) | 0.026 | 15.0 (12.0–19.0) | 25.9 (15.2–42.9) | < 0.001 | ||||
| > 20 | 25 (30.5) | 9 (56.2) | 2.93 (0.98–8.75) | 0.090 | 16 (22.9) | 18 (64.3) | 6.08 (2.34–15.76) | < 0.001 | ||
| Serum creatinine, mg/dL | 0.9 (0.8–1.1) | 1.2 (0.7–2.2) | 0.061 | 0.8 (0.7–1.0) | 1.2 (0.9–2.0) | 0.001 | ||||
| > 1.0 | 29 (35.4) | 10 (62.5) | 3.05 (1.01–9.23) | 0.080 | 19 (27.1) | 20 (71.4) | 6.71 (2.53–17.78) | < 0.001 | ||
| Lactate dehydrogenase (n = 77), IU/L | 510.0 (454.0–686.0) | 628.5 (517.0–1,010.0) | 0.113 | 502.0 (449.0–686.0) | 590.0 (556.0–741.0) | 0.088 | ||||
| > 600 | 21/69 (30.4) | 4/8 (50.0) | 2.29 (0.52–10.02) | 0.426 | 20/64 (31.2) | 5/13 (38.5) | 1.38 (0.40–4.73) | 0.747 | ||
| C-reactive protein, mg/dL | 2.8 (0.7–8.9) | 12.8 (8.3–16.4) | < 0.001 | 2.2 (0.4–5.7) | 12.2 (8.7–16.4) | < 0.001 | ||||
| > 8.0 | 24 (29.3) | 13 (81.2) | 10.47 (2.74–40.09) | < 0.001 | 13 (18.6) | 24 (85.7) | 26.31 (7.78–88.92) | < 0.001 | ||
| Radiologic findings on admission | ||||||||||
| Presence of infiltrate | 64 (78.0) | 16 (100.0) | N/A | 0.038 | 52 (74.3) | 28 (100.0) | N/A | 0.007 | ||
| Bilateral infiltrate | 50 (61.0) | 16 (100.0) | N/A | 0.006 | 38 (54.3) | 28 (100.0) | N/A | < 0.001 | ||
| Treatment | ||||||||||
| Lopinavir/ritonavir or darunavir/ritonavir | 60 (73.2) | 15 (93.8) | 5.50 (0.69–44.13) | 0.108 | 49 (70.0) | 26 (92.9) | 5.57 (1.21–25.64) | 0.032 | ||
| Hydroxychloroquine | 48 (58.5) | 11 (68.8) | 1.56 (0.50–4.90) | 0.628 | 42 (60.0) | 17 (60.7) | 1.03 (0.42–2.53) | > 0.999 | ||
| Corticosteroid | 18 (22.0) | 10 (62.5) | 5.93 (1.90–18.51) | 0.002 | 8 (11.4) | 20 (71.4) | 19.38 (6.44–58.32) | < 0.001 | ||
| Outcome | ||||||||||
| Discharged alive | 12 (14.6) | 0 (0.0) | < 0.001 | 12 (17.1) | 0 (0.0) | < 0.001 | ||||
| Being admitted and stable | 57 (69.5) | 4 (25.0) | 57 (81.4) | 4 (14.3) | ||||||
| Being admitted and on mechanical ventilator | 0 (0.0) | 5 (31.2) | 0 (0.0) | 5 (17.9) | ||||||
| Death | 13 (15.9) | 7 (43.8) | 1 (1.4) | 19 (67.9) | ||||||
Data are presented as mean ± standard deviation, median (interquartile range) or number (%).
OR = odds ratio, CI = confidence interval, DM = diabetes mellitus, N/A = not available, MEWS = modified early warning score, NEWS2 = national early warning score 2.
A high CRP level (> 8.0 mg/dL) showed the highest risk for a severe clinical course in our patients, so its diagnostic characteristics was compared with those of two commonly used prognostication scores (Table 3). High CRP levels showed higher sensitivity, specificity, and positive predictive value in predicting the need for MV/HFNC. The negative predictive values were comparable. The area under the receiver operating characteristic curve was also larger for high CRP level.
Table 3. Diagnostic characteristics of MEWS, NEWS2, and CRP > 8.0 mg/dL for predicting the use of high flow nasal cannula or mechanical ventilation. 95% CIs are shown in parentheses.
| Criteria | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Area under ROC curve |
|---|---|---|---|---|---|
| MEWS ≥ 3 | 0.75 (0.58–0.92) | 0.76 (0.66–0.86) | 0.51 (0.35–0.68) | 0.90 (0.82–0.98) | 0.754 (0.652–0.855) |
| NEWS2 ≥ 2 | 0.80 (0.63–0.98) | 0.65 (0.53–0.77) | 0.44 (0.28–0.61) | 0.90 (0.81–0.99) | 0.725 (0.615–0.834) |
| CRP > 8.0 mg/dL | 0.86 (0.73–0.99) | 0.81 (0.72–0.91) | 0.65 (0.50–0.80) | 0.93 (0.87–1.00) | 0.836 (0.755–0.916) |
MEWS = modified early warning score, NEWS2 = national early warning score 2, CRP = C-reactive protein, CI = confidence interval, ROC = receiver operating characteristic.
DISCUSSION
In our study of patients aged ≥ 65 years with COVID-19, a high mortality rate and severe clinical course frequently requiring advanced respiratory support were observed. The CFR (20.4%) in our patients was markedly higher than the overall mortality of COVID-19 in Korea (approximately 1.4%).13 Approximately 29% needed MV or HFNC, and the CFR among that subgroup was very high (67.9%). Most patients had at least one underlying condition, which complicated the clinical course.
Age was the most important preexisting risk factor for mortality and MV/HFNC. In particular, patients aged ≥ 80 years had a 38.1% chance of receiving MV/HFNC. Among them, only one patient survived but was still on a mechanical ventilator at the time of data entry. The effect of older age on mortality has also been reported in China and Italy, which is consistent with our findings.6,7 Furthermore, our data demonstrated the resources required to manage elderly patients with COVID-19. Combined with the relative risk of infection by age group and population distribution, our results provide critical information needed by healthcare facilities and public health authorities to prepare ventilators and HFNC machines to meet the expected demand. However, it should also be noted that no patients who used HFNC without further planning for MV survived in our study. The interpretation of our results is limited by the small number, but the limited role of HFNC alone may be taken into consideration when resources are extremely overwhelmed. As previous studies from China reported a lower mortality rate of patients treated with HFNC, there exists the possibility that our observation is specific to elderly patients.14,15
Nosocomial acquisition and the presence of comorbidities were identified as important risk factors for mortality. Our results suggest that outbreaks in hospitals and long-term care facilities would result in grave consequences, which has been observed in the United States.16 One interesting finding in our study is the lack of association between hypertension and mortality. Previous large-scale epidemiological data from the Chinese Center for Disease Control and Prevention reported that patients with hypertension had a high risk of death, similar to those in patients with chronic lung disease.6 Other studies also showed that hypertension is associated with mortality or ICU care,5,15,17 but conflicting reports also exist.10,18 In our study, hypertension was not a statistically significant risk factor in elderly patients, of whom about a half had hypertension. Isolated hypertension is generally not regarded as an important prognostic factor in infectious diseases; thus, the possibility of confounding should be examined in future studies.
A severe initial presentation, namely hypotension, tachypnea, hypoxia, or altered mental status, was indeed associated with a poor outcome. Two commonly used prognostication scores (MEWS and NEWS2) also correlated well with mortality. Among laboratory findings, leukocytosis, lymphopenia, and high CRP levels were associated with mortality and the need for MV/HFNC. Such an association has been reported in previous studies on the overall population and in critically ill patients.10,14,15 Furthermore, we observed a very high degree of association with CRP; its OR for mortality was 25.33, and the OR for MV/HFNC was 25.08. When the cutoff was set at 8.0 mg/dL, elevated CRP had better diagnostic characteristics than those of MEWS and NEWS2. These two scores measure vital signs only, so a high CRP could be a useful addition for initial triage. Neutrophilia, lymphopenia, and elevated lactate dehydrogenase or D-dimer have been associated with severe course and mortality in previous reports, but a strong association of CRP was also reported in one study that specifically examined the risk factors for ARDS and death.5 However, it is unclear whether this association reflects the degree of cytokine storm that leads to ARDS or the severity of viral infection.19 Nonetheless, this study suggests that respiratory support might be prepared in advance for patients with high CRP as well as with MEWS ≥ 3 or NEWS ≥ 2.
Our study has several limitations. First, it was a retrospective study with a relatively small number of patients. The possibility of confounding cannot be excluded. Multivariable analysis using logistic regression was attempted, but an adequate model could not be constructed because of the small number and high collinearity between variables. Second, our study subjects consisted of hospitalized patients; thus, those deemed to be sufficiently fit for home isolation were not included. This explains the high mortality and severity observed in our cohort. Therefore, our results are not generalizable to mild cases. Finally, although we limited our study to patients with a follow-up duration of ≥ 14 days, a substantial proportion of patients were still hospitalized at the time of data entry. Although the risk of death was low after 14 days of admission in patients (Fig. 2A), further follow-up is necessary. Despite these limitations, we believe that our results provide valuable information on the clinical outcomes and resource requirements of care for elderly patients with COVID-19 who have been shown to be the most vulnerable. We thought that waiting for the negative conversion of RT-PCR and subsequent discharge of patients would add little value to our results and delay the delivery of these important data.
In a retrospective study on elderly patients hospitalized with COVID-19, a high need for MV/HFNC and poor outcomes were observed. Patients aged ≥ 80 years had a high risk of requiring MV/HFNC, and mortality among those patients with severe disease was extremely high. A severe initial presentation and laboratory abnormalities were identified as risk factors for mortality and severe hospital course. In addition to high MEWS or NEWS2 scores, high CRP level was strongly associated with severity, suggesting its role in triage and prognostication.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Peck KR, Chang HH.
- Data curation: Lee JY, Kim HA, Hyun M, Rhee JY, Jang S, Kim JY, Chang HH.
- Formal analysis: Huh K.
- Methodology: Kim HA, Huh K, Rhee JY, Peck KR, Chang HH.
- Writing - original draft: Huh K.
- Writing - review & editing: Huh K, Peck KR, Chang HH.
References
- 1.World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report - 65. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.Kim JY, Ko JH, Kim Y, Kim YJ, Kim JM, Chung YS, et al. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J Korean Med Sci. 2020;35(7):e86. doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 8.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35(10):e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention. Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35(12):e132. doi: 10.3346/jkms.2020.35.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–526. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 12.Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-illness Severity in the NHS. Updated Report of a Working Party. London: Royal College of Physicians; 2017. [Google Scholar]
- 13.Korea Centers for Disease Control and Prevention. Updates on COVID-19 in Republic of Korea (25 March 2020) Cheongju: Korea Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 14.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 19.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]