Abstract
Background
High-dose intravenous steroids are the first-line treatment for patients with moderate-to-severe and active Graves' ophthalmopathy (GO). We aimed to investigate the response rate of methylprednisolone (MPD) treatment among Korean patients with active moderate-to-severe GO and to identify predictive factors of treatment response.
Methods
This is a retrospective observational study. We included 54 active moderate-to-severe GO patients treated with 4.5 g intravenous MPD over 12 weeks between November 2011 and November 2018. Response was defined as an improvement in at least two of five indicators (clinical activity score [CAS], soft-tissue involvement, exophthalmos, diplopia, and visual acuity) at immediate and 3 months after treatment completion. We examined predictive factors for response using logistic regression analysis.
Results
Twenty-four (44.4%) and 22 (40.7%) patients showed response at immediate and 3 months after intravenous (IV) steroid treatment. Of the five ophthalmic parameters, all patients in the responsive group (100.0%) showed a decrease in CAS and 90.9% showed less soft tissue involvement after IV steroid treatment. Among variables, the sum of extraocular muscle width was positively (odds ratio [OR], 1.163; 95% confidence interval [CI], 0.973–1.389; P = 0.096) associated with treatment response. While, the OR of age was 0.918 (95% CI, 0.856–0.985; P = 0.017) and thyrotropin binding inhibitory immunoglobulin (TBII) was 0.921 (95% CI, 0.864–0.982; P = 0.012).
Conclusion
In Korean active moderate-to-severe GO patients, intravenous steroid treatment is not as effective as previously reported. Parameters associated with CAS and soft-tissue involvement were found to be influenced by IV MPD treatment. Extraocular muscle enlargement, younger age and lower TBII are predictive factors for a good steroid treatment response.
Keywords: Graves' Ophthalmopathy, Glucocorticoids, Clinical Activity Score, Response Rate
Graphical Abstract
INTRODUCTION
Graves' ophthalmopathy (GO) is the most frequent extrathyroidal manifestation among patients with Graves' disease. Almost 50% of patients with Graves' disease report eye symptoms such as a dry and gritty sensation, excessive tearing, photophobia, double vision, and pain.1 Three to five percent of patients with GO experience severe disease with intense pain, inflammation, and sight-threatening corneal ulceration or compressive optic neuropathy.2 Therefore, it is important to classify patients with GO by the level of activity and severity when planning treatment for patients with GO. The European Group on Graves' Orbitopathy (EUGOGO) recommends that the activity and severity of GO be assessed according to standardized criteria to select the most appropriate treatment for individual patients with GO.3 Activity is evaluated using the clinical activity score (CAS).4 Patients with a CAS of ≥ 3 of 7 items are classified as having active GO. The severity of GO can be classified as mild, moderate-to-severe, or sight threatening.3
As recommended by EUGOGO,3 high-dose intravenous (IV) steroids are the first-line treatment for patients with moderate-to-severe and active GO. In several randomized clinical studies, the response rate in the IV steroids group was about 70%–80%.5 However, each study used a different dose of IV steroids, and some studies6,7,8 even used more cumulative doses (6–10 g) of methylprednisolone (MPD) than recommended (4.5 g). Although there are differences in ethnic-specific features and symptoms of GO, little is known about the response rate of patients in the Korean population to IV steroid treatment. Furthermore, there are few established prognostic factors that can predict IV steroid treatment response.
Based on these uncertainties, we aimed to investigate the response rate of 4.5 g IV MPD treatment among Korean patients with active moderate-to-severe GO and to identify factors that can predict response to 4.5 g IV MPD treatment.
METHODS
Subjects
We included patients who were diagnosed with active moderate-to-severe GO and treated with 4.5 g IV MPD at Chung-Ang University Hospital between November 2011 and November 2018. Active GO was defined as GO with a CAS of ≥ 3 of 7 items.9 The severity of GO was also classified according to the guidelines of EUGOGO.9 IV MPD was administered by one endocrinologist for 12 weeks. For the first 6 weeks, 0.5 g of MPD was injected weekly on the same day, and 0.25 g was injected in the same manner for the remaining 6 weeks. Liver function and blood glucose levels were monitored regularly during IV MPD treatment. Patients who were followed up at our hospital for at least 3 months after the end of IV steroid treatment were included in this study to identify cases that worsened immediately after IV steroid treatment.
We excluded patients who received any immunosuppressive therapy or radiotherapy before IV MPD treatment, as well as patients younger than 20 years; pregnant patients; hepatitis carriers; and patients with a past medical history of other eye diseases such as glaucoma, diabetic retinopathy, maculopathy, and strabismus.
During the study period, 54 patients were treated with 4.5 g IV MPD. We examined the subjects' height, body weight, gender, age at MPD treatment, smoking status, duration of GO before MPD treatment, and history of use of anti-thyroid drugs or levothyroxine. Thyroid function test results (thyroid stimulating hormone [TSH] and free thyroxine [T4] levels), thyrotropin binding inhibitory immunoglobulin (TBII) and total cholesterol level were assessed before IV MPD treatment.
Ophthalmic assessment and response evaluation
Ophthalmic examinations were performed before and immediately after IV MPD treatment and at 3 months after IV MPD treatment. All ophthalmic examinations were performed by one ophthalmologist. The proptosis status, margin reflex distance 1 (MRD1, the vertical distance between the center of the pupil and the center of the upper eyelid margin), palpebral fissure height, intraocular pressure, diplopia status, visual acuity, and NOSPECS classification (N = No Symptoms or signs, O = Only symptoms, S = Soft tissue involvement, P = Proptosis, E = Extraocular muscle involvement, C = Corneal involvement, S = Sight loss due to optic nerve compression) and CAS scores were assessed and recorded at each visit. Proptosis was assessed via Hertel exophthalmometry (Oculus, Arlington, VA, USA). Palpebral fissure height was recorded as the sum of MRD1 and MRD2 (the distance between the center of the pupil and the center of lower eyelid margin). MRD1 and palpebral fissure height were measured using the custom-made PC-based software EAS (Eyelid Analysis Software; Biomedical Research Institute, Seoul, Korea) after photographs were taken in the primary position.10 Diplopia was assessed using the Gorman score.11 Visual acuity was assessed using the Snellen chart. The modified CAS was assessed by assigning a point to each of the following seven items (spontaneous retrobulbar pain, pain on attempted upward or downward gaze, redness of eyelids, redness of the conjunctiva, inflammation of caruncle and/or plica, swelling of eyelids, and conjunctival edema).4 The modified NOSPECS score was used to assess the severity of GO.12 Contiguous 1-mm sections of extraocular muscles for orbital computed tomography scans were obtained from the patients. The thickness of each extraocular muscle (superior, inferior, lateral, and medial rectus) was measured using ImageJ (https://imagej.nih.gov/ij). The horizontal diameters of the medial and lateral rectus and the vertical diameters of the superior and inferior rectus muscles were measured on a series of images; the largest diameter of the middle section of each muscle was selected. The sum of the thickness of the four (superior, inferior, lateral, and medial rectus) extraocular muscles was also calculated.
Response was defined as the presence of at least two of the following five ophthalmic parameters: 1) reduction in proptosis by at least 2 mm, 2) reduction in any of the class 2 NOSPECS signs by at least one grade, 3) improvement in CAS by at least 2 points or CAS < 3/7, 4) improvement in visual acuity on Snellen line and 5) decrease in the Gorman score (from constant to inconstant, inconstant to intermittent, or intermittent to absent).
We divided the study subjects into two groups: “responsive” and “nonresponsive” groups, according to the overall response at immediate and 3 months after IV MPD treatment. Patients who received additional radiation therapy shortly after the end of IV MPD treatment were classified as nonresponsive when assessing response at 3 months after IV MPD because radiation therapy was performed mainly when there was no change after IV MPD treatment or when symptoms worsened immediately after IV MPD treatment. Twelve patients received radiation therapy after IV MPD treatment.
Statistical analysis
Continuous variables are presented as means ± standard deviations and analyzed using Student's t-test or the Mann-Whitney U test based on the result of the normality test. Categorical variables were analyzed using Pearson's χ2 test. Binary logistic regression analysis was performed to analyze the effect of each clinical variable on the binary response of IV treatment. For analyzing combined effect of more than two variables on the IV steroid response, multivariate logistic regression analysis was performed using backward stepwise procedures as variable selection method to minimize Akaike information criterion (AIC). All statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Analysis items with P < 0.05 were considered statistically significant.
Ethics statement
The protocol of this retrospective observational study was approved by the Institutional Review Board (IRB) of Chung-Ang University Hospital (IRB No. 1905-004-16263). Informed consent was not required for this study considering its retrospective design.
RESULTS
Baseline characteristics of the responsive and nonresponsive groups
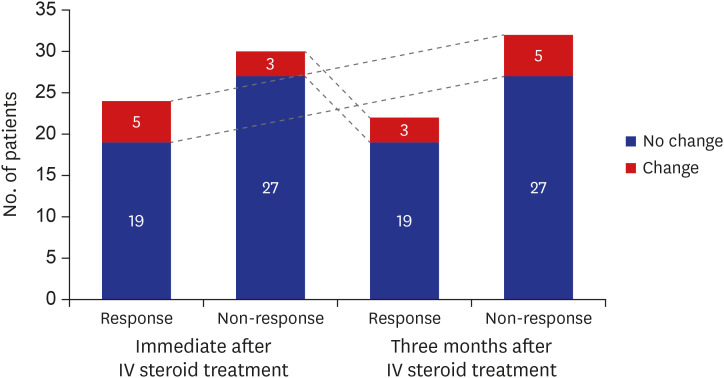
Of the study patients, 24 (44.4%) patients were assigned to the responsive group immediately after IV MPD treatment. Three months after IV MPD treatment, 22 (40.7%) were identified as responders. Five out of 24 responders immediately after IV MPD treatment changed to non-responders at 3 months after IV MPD treatment. On the other hand, 3 out of 30 non-responders immediately after IV MPD treatment were identified as responders at 3 months after IV MPD treatment (Fig. 1).
Fig. 1. Number of patients according to treatment response at immediate and 3 months after 12 weeks intravenous steroid treatment. Five patients who responded immediately after IV steroid treatment changed to non-responders after 3 months, while three patients who did not respond immediately belonged to the responders after 3 months.
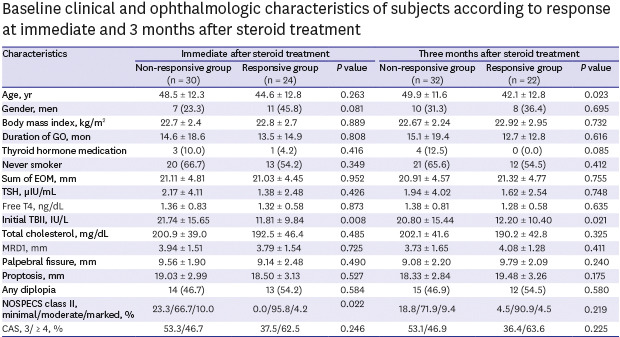
Table 1 showed the baseline characteristics of each group at post-treatment time point. The initial TBII values were found to be statistically low in the response group at both treatment points. In addition, immediately after IV MPD treatment, the responders had more than moderate soft tissue symptoms (100% vs. 76.7%, P = 0.022), and 3 months after IV MPD treatment, the responders were younger (42.1 ± 12.8 years old vs. 49.9 ± 11.6 years old, P = 0.023).
Table 1. Baseline clinical and ophthalmologic characteristics of subjects according to response at immediate and 3 months after steroid treatment.
| Characteristics | Immediate after steroid treatment | Three months after steroid treatment | ||||
|---|---|---|---|---|---|---|
| Non-responsive group (n = 30) | Responsive group (n = 24) | P value | Non-responsive group (n = 32) | Responsive group (n = 22) | P value | |
| Age, yr | 48.5 ± 12.3 | 44.6 ± 12.8 | 0.263 | 49.9 ± 11.6 | 42.1 ± 12.8 | 0.023 |
| Gender, men | 7 (23.3) | 11 (45.8) | 0.081 | 10 (31.3) | 8 (36.4) | 0.695 |
| Body mass index, kg/m2 | 22.7 ± 2.4 | 22.8 ± 2.7 | 0.889 | 22.67 ± 2.24 | 22.92 ± 2.95 | 0.732 |
| Duration of GO, mon | 14.6 ± 18.6 | 13.5 ± 14.9 | 0.808 | 15.1 ± 19.4 | 12.7 ± 12.8 | 0.616 |
| Thyroid hormone medication | 3 (10.0) | 1 (4.2) | 0.416 | 4 (12.5) | 0 (0.0) | 0.085 |
| Never smoker | 20 (66.7) | 13 (54.2) | 0.349 | 21 (65.6) | 12 (54.5) | 0.412 |
| Sum of EOM, mm | 21.11 ± 4.81 | 21.03 ± 4.45 | 0.952 | 20.91 ± 4.57 | 21.32 ± 4.77 | 0.755 |
| TSH, µIU/mL | 2.17 ± 4.11 | 1.38 ± 2.48 | 0.426 | 1.94 ± 4.02 | 1.62 ± 2.54 | 0.748 |
| Free T4, ng/dL | 1.36 ± 0.83 | 1.32 ± 0.58 | 0.873 | 1.38 ± 0.81 | 1.28 ± 0.58 | 0.635 |
| Initial TBII, IU/L | 21.74 ± 15.65 | 11.81 ± 9.84 | 0.008 | 20.80 ± 15.44 | 12.20 ± 10.40 | 0.021 |
| Total cholesterol, mg/dL | 200.9 ± 39.0 | 192.5 ± 46.4 | 0.485 | 202.1 ± 41.6 | 190.2 ± 42.8 | 0.325 |
| MRD1, mm | 3.94 ± 1.51 | 3.79 ± 1.54 | 0.725 | 3.73 ± 1.65 | 4.08 ± 1.28 | 0.411 |
| Palpebral fissure, mm | 9.56 ± 1.90 | 9.14 ± 2.48 | 0.490 | 9.08 ± 2.20 | 9.79 ± 2.09 | 0.240 |
| Proptosis, mm | 19.03 ± 2.99 | 18.50 ± 3.13 | 0.527 | 18.33 ± 2.84 | 19.48 ± 3.26 | 0.175 |
| Any diplopia | 14 (46.7) | 13 (54.2) | 0.584 | 15 (46.9) | 12 (54.5) | 0.580 |
| NOSPECS class II, minimal/moderate/marked, % | 23.3/66.7/10.0 | 0.0/95.8/4.2 | 0.022 | 18.8/71.9/9.4 | 4.5/90.9/4.5 | 0.219 |
| CAS, 3/ ≥ 4, % | 53.3/46.7 | 37.5/62.5 | 0.246 | 53.1/46.9 | 36.4/63.6 | 0.225 |
Data are presented as mean ± standard deviation or number (%).
GO = Graves' ophthalmopathy, EOM = extraocular muscle, TSH = thyroid stimulating hormone, TBII = thyrotropin binding inhibitory immunoglobulin, MRD = margin reflex distance, CAS = clinical activity score.
Response rates of each parameter in the responsive group
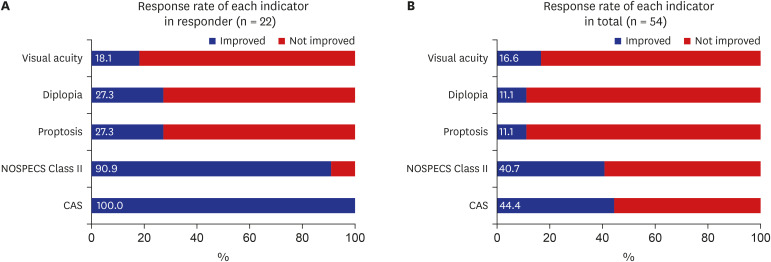
Of the five ophthalmic parameters, all patients in the responsive group (100.0%) showed a decrease in CAS and 90.9% showed less soft tissue involvement after IV steroid treatment. However, only 27.3% of patients in the responsive group showed an improvement in proptosis or diplopia. Only 18.1% of patients in the responsive group showed an improvement in visual acuity (Fig. 2A). Among all study subjects, 44.4% and 40.7% showed an improvement in CAS and soft-tissue involvement, respectively (Fig. 2B).
Fig. 2. Response rate of each indicator at 3 months after IV steroid treatment.
(A) Response rate in responders. In responder, the response rate was 100% in CAS, 90.9% in NOSPECS class II, 27.3% in proptosis and diplopia and 18.1% in visual acuity. (B) Response rate in total subjects. In total subjects, the response rate was 44.4% in CAS, 40.7% in NOSPECS class II, 11.1% in proptosis and diplopia and 16.6% in visual acuity.
CAS = clinical activity score, NOSPECS = N, No Symptoms or signs, O, Only symptoms, S, Soft tissue involvement, P, Proptosis, E, Extraocular muscle involvement, C, Corneal involvement, S, Sight loss due to optic nerve compression.
Predictive factors for response to IV MPD treatment
Multivariate logistic regression analysis was performed to identify the predictive factors for response to IV MPD treatment (Table 2). The odds ratio (OR) of the sum of extraocular muscle diameter was 1.163 (95% confidence interval [CI], 0.973–1.389; P = 0.096); thus, the thicker the diameter of the extraocular muscle, the better the expected therapeutic response. The OR of age was 0.918 (95% CI, 0.856–0.985; P = 0.017) and that of initial TBII was 0.921 (95% CI, 0.864–0.982; P = 0.012). These results suggested that increasing age and TBII values were poor prognostic factors for response to IV MPD treatment. We summarize the prognostic factors associated with IV steroid treatment presented in the present study and other previous studies in Table 3.13,14,15
Table 2. Predictive factors for the response of IV steroid treatment by logistic regression analysis performed using backward stepwise procedures.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 0.947 | 0.902–0.955 | 0.030 | 0.918 | 0.856–0.985 | 0.017 |
| Gender, men | 0.795 | 0.253–2.502 | 0.696 | |||
| Body mass index, kg/m2 | 1.041 | 0.834–1.301 | 0.721 | |||
| Duration of GO, mon | 0.991 | 0.957–1.026 | 0.612 | |||
| Smoking | 1.591 | 0.523–4.873 | 0.413 | |||
| Sum of EOM, mm | 1.020 | 0.905–1.148 | 0.749 | 1.163 | 0.973–1.389 | 0.096 |
| TSH, µIU/mL | 0.972 | 0.820–1.153 | 0.743 | |||
| Free T4, ng/dL | 0.814 | 0.353–1.877 | 0.629 | |||
| Pretreatment TBII, IU/L | 0.952 | 0.908–0.997 | 0.038 | 0.921 | 0.864–0.982 | 0.012 |
| Changes of TBII for 12 wk | 0.959 | 0.901–1.021 | 0.193 | |||
| Total cholesterol, mg/dL | 0.993 | 0.979–1.007 | 0.320 | |||
| MRD1, mm | 1.171 | 0.809–1.694 | 0.404 | |||
| Proptosis, mm | 1.139 | 0.944–1.374 | 0.174 | |||
| Diplopia | 1.360 | 0.458–4.042 | 0.580 | |||
| NOSPECS Class II, ≥ b | 4.846 | 0.540–43.464 | 0.159 | 7.204 | 0.544–95.386 | 0.134 |
| CAS, ≥ 4 | 1.983 | 0.652–6.031 | 0.227 | |||
IV = intravenous, CI = confidence interval, OR = odds ratio, GO = Graves' ophthalmopathy, EOM = extraocular muscle, TSH = thyroid stimulating hormone, TBII = thyrotropin binding inhibitory immunoglobulin, MRD = margin reflex distance, CAS = clinical activity score.
Table 3. Predictive factors for response of IV steroid treatment in active Graves' ophthalmopathy patients.
| Year | Authors | Country | Patient, No. | Response rate of IV steroid Tx, % | Outcomes for IV steroid Tx | Variables | OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| 2015 | Xing et al.13 | China | 92 | 54.3 | No response | Smoking | 13.4 (1.2–128.14) | 0.035 |
| 2018 | Wang et al.14 | China | 90 | 57.8 | Response | Duration of eye symptoms | 0.984 (0.972–0.997) | 0.012 |
| Restoration of euthyroidism | 3.282 (1.062–10.142) | 0.039 | ||||||
| 2019 | Hu et al.15 | China | 302 | 54.0 | Response | Duration of eye symptoms | 0.878 (0.790–0.977) | 0.017 |
| Pretreatment CAS | 3.506 (1.973–6.228) | < 0.001 | ||||||
| Positive pretreatment TRAB, > 9.31 U/L | 0.061 (0.0.18–0.214) | < 0.001 | ||||||
| High pretreatment triglyceride, ≥ 150 mg/dL | 0.090 (0.022–0.372) | 0.001 | ||||||
| Elevated TSH levels during treatment, > 5 µIU/mL | 0.145 (0.037–0.561) | 0.005 | ||||||
| 2020 | Ahn and Lee (present study) | Korea | 55 | 40.7 | Response | Age | 0.918 (0.856–0.985) | 0.017 |
| Pretreatment TBII, IU/L | 0.921 (0.864–0.982) | 0.012 |
IV = intravenous, Tx = treatment, CAS = clinical activity score, TRAB = thyrotropin receptor antibodies, TSH = thyroid-stimulating hormone, OR = odds ratio, CI = confidence interval.
Adverse event during IV steroid treatment
Patients complained of various side effects during IV steroid treatment, but most of them were mild and temporary. Specifically, palpitation (5.6%), weight gain of more than 3 kg (12.9%), digestive system disorders (5.6%), liver function abnormality (9.3%), hyperglycemia (14.8%), sleep disorders (20.4%), facial edema (16.7%), and hot flush (12.9%) were the common side effects.
DISCUSSION
In this retrospective study, we evaluated the response rate for 4.5 g IV MPD treatment in Korean moderate-to-severe active GO patients and attempted to determine the predictive factors for IV MPD treatment response.
Surprisingly, we found that the response rate of IV MPD treatment was less than 50% (44.4% immediately after IV MPD treatment or 40.7% at 3 months after IV MPD treatment). Most patients in the responsive group were found to have improved CAS and soft-tissue involvement; however, improvement in proptosis, diplopia, and visual acuity were not effective with IV MPD treatment. Even if improvement in CAS or soft-tissue involvement is considered the only response criterion, the response rate of IV MPD treatment is less than 50% in our study subjects. Why is the response rate of IV MPD treatment lower in Korean GO patients than that in previous studies?
First, there is a possibility of differences in IV MPD treatment response due to differences in race. Response rates from studies conducted on Chinese GO patients of the same Asian ethnicity were 54.3%–57.8% for IV MPD treatment.13,14,16 In contrast, a randomized study of 35 German GO patients reported that 77% of patients responded to IV MPD treatment.17 In addition, in a randomized study of 52 Turkish GO patients, the response rate of IV MPD treatment was found to be 72% after 3 months of treatment.18 Although only six patients were included, a study in Netherlands showed a high response rate of 83% for IV MPD treatment.6
Second, because each study used different time points to evaluate responses to steroid treatment, the response rates are likely to be different. Our study evaluated the responsive group according to response status immediately and 3 months after the end of steroid treatment; however, the response rate was low regardless of the time of evaluation after IV MPD treatment. Currently, it is unclear when to assess response after IV steroid treatment. However, considering that some patients may show an immediate deterioration in their condition after IV steroid treatment and may consider other therapy, it may be accurate to evaluate the response rate at least 3 months after IV steroid treatment. In a previous study, 12%–13% of patients showed a deterioration in their condition after 12 weeks of IV MPD treatment.19 In our study, 5 (9.3%) patients showed an immediate response to IV MPD treatment; however, their condition subsequently worsened and they were classified as the nonresponsive group.
The thicker the extraocular muscle, the better the response rate to IV MPD treatment. In active GO patients, edema occurs because of an inflammatory response of the orbital fibroblast. Hydrophilic hyaluronan accumulates between the intact extraocular muscle fibers and causes an increase in the muscle volume.1 Our result suggests that IV MPD treatment night be effective for edematous changes. Similarly, another study reported that the inferior rectus/fat ratio on MRI is predictive of the response to glucocorticoid therapy in GO patients.16
The higher the TBII, the lower the response to IV MPD treatment. Expression of the thyrotropin (TSH) receptor in orbital tissue is elevated in active phase GO patients, which suggest an important role of thyrotropin antibodies (TRAbs) in the pathogenesis of GO.20,21 In several previous studies, the TBII titer was strongly associated with clinical activity and severity of GO.22,23 In addition, Eckstein et al.24 reported that the TBII levels remained elevated after steroid treatment in 93% of very active GO patients (non-responder) compared with 42% of inactive GO patients (P = 0.02). Given that steroids can reduce or change the number or function of immune cells and decrease the level of immunoglobulin and cytokines,25 patients with high TBII levels have a more severe immune reaction, and these patients may require stronger immunosuppressive treatment than IV steroid or a higher dose of IV steroid treatment.
The limitation of our study is that, firstly, because this study was conducted in a tertiary hospital, there may be a selection bias in patient selection. It is possible that more severe GO patients were included in our study. Secondly, our study was a retrospective observational study and included a relatively small number of GO patients, which may have influenced the results.
In conclusion, it appears that IV MPD treatment for active moderate-to-severe GO patients is not as effective as reported previously. Parameters associated with CAS and soft-tissue involvement were found to be influenced by IV MPD treatment. An enlarged extraocular muscle, younger age, and TBII were predictive factors for a good response to IV MPD treatment. Therefore, if GO patients were selected by considering these factors before IV MPD treatment, more appropriate treatment would be possible.
Footnotes
Funding: This work was supported by the Korean Thyroid Association Young Investigator Award 2018.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Ahn HY, Lee JK.
- Data curation: Ahn HY.
- Formal analysis: Ahn HY.
- Investigation: Ahn HY.
- Methodology: Ahn HY, Lee JK.
- Software: Ahn HY.
- Validation: Lee JK.
- Writing - original draft: Ahn HY.
- Writing - review & editing: Lee JK.
References
- 1.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362(8):726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002;12(10):855–860. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 3.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves' orbitopathy guidelines for the management of Graves' orbitopathy. Eur Thyroid J. 2016;5(1):9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 1997;47(1):9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 5.Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96(2):320–332. doi: 10.1210/jc.2010-1962. [DOI] [PubMed] [Google Scholar]
- 6.van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves' orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008;158(2):229–237. doi: 10.1530/EJE-07-0558. [DOI] [PubMed] [Google Scholar]
- 7.Macchia PE, Bagattini M, Lupoli G, Vitale M, Vitale G, Fenzi G. High-dose intravenous corticosteroid therapy for Graves' ophthalmopathy. J Endocrinol Invest. 2001;24(3):152–158. doi: 10.1007/BF03343835. [DOI] [PubMed] [Google Scholar]
- 8.Menconi F, Marinò M, Pinchera A, Rocchi R, Mazzi B, Nardi M, et al. Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves' orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab. 2007;92(5):1653–1658. doi: 10.1210/jc.2006-1800. [DOI] [PubMed] [Google Scholar]
- 9.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves' Orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158(3):273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 10.Chun YS, Park HH, Park IK, Moon NJ, Park SJ, Lee JK. Topographic analysis of eyelid position using digital image processing software. Acta Ophthalmol. 2017;95(7):e625–32. doi: 10.1111/aos.13437. [DOI] [PubMed] [Google Scholar]
- 11.Bahn RS, Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid-associated ophthalmopathy. Endocrinol Metab Clin North Am. 1987;16(2):391–407. [PubMed] [Google Scholar]
- 12.Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91(9):3464–3470. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 13.Xing L, Ye L, Zhu W, Shen L, Huang F, Jiao Q, et al. Smoking was associated with poor response to intravenous steroids therapy in Graves' ophthalmopathy. Br J Ophthalmol. 2015;99(12):1686–1691. doi: 10.1136/bjophthalmol-2014-306463. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhang S, Zhang Y, Liu X, Gu H, Zhong S, et al. A single-center retrospective study of factors related to the effects of intravenous glucocorticoid therapy in moderate-to-severe and active thyroid-associated ophthalmopathy. BMC Endocr Disord. 2018;18(1):13. doi: 10.1186/s12902-018-0240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu S, Wang Y, He M, Zhang M, Ding X, Shi B. Factors associated with the efficacy of intravenous methylprednisolone in moderate-to-severe and active thyroid-associated ophthalmopathy: a single-centre retrospective study. Clin Endocrinol (Oxf) 2019;90(1):175–183. doi: 10.1111/cen.13855. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Li L, Xie C, Guan M, Xue Y. Thickness of extraocular muscle and orbital fat in MRI predicts response to glucocorticoid therapy in Graves' ophthalmopathy. Int J Endocrinol. 2017;2017:3196059. doi: 10.1155/2017/3196059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves' orbitopathy. J Clin Endocrinol Metab. 2005;90(9):5234–5240. doi: 10.1210/jc.2005-0148. [DOI] [PubMed] [Google Scholar]
- 18.Aktaran S, Akarsu E, Erbağci I, Araz M, Okumuş S, Kartal M. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves' ophthalmopathy. Int J Clin Pract. 2007;61(1):45–51. doi: 10.1111/j.1742-1241.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 19.Bartalena L, Veronesi G, Krassas GE, Wiersinga WM, Marcocci C, Marinò M, et al. Does early response to intravenous glucocorticoids predict the final outcome in patients with moderate-to-severe and active Graves' orbitopathy? J Endocrinol Invest. 2017;40(5):547–553. doi: 10.1007/s40618-017-0608-z. [DOI] [PubMed] [Google Scholar]
- 20.Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves' ophthalmopathy patients. Clin Endocrinol (Oxf) 2003;58(3):280–287. doi: 10.1046/j.1365-2265.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 21.Iyer S, Bahn R. Immunopathogenesis of Graves' ophthalmopathy: the role of the TSH receptor. Best Pract Res Clin Endocrinol Metab. 2012;26(3):281–289. doi: 10.1016/j.beem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2000;52(3):267–271. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 23.Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves' orbitopathy. J Clin Endocrinol Metab. 2010;95(5):2123–2131. doi: 10.1210/jc.2009-2470. [DOI] [PubMed] [Google Scholar]
- 24.Eckstein AK, Plicht M, Lax H, Hirche H, Quadbeck B, Mann K, et al. Clinical results of anti-inflammatory therapy in Graves' ophthalmopathy and association with thyroidal autoantibodies. Clin Endocrinol (Oxf) 2004;61(5):612–618. doi: 10.1111/j.1365-2265.2004.02143.x. [DOI] [PubMed] [Google Scholar]
- 25.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]