Abstract
Purpose
MYCN amplification (MNA) is associated with poor outcomes in neuroblastoma. Less is known about heterogeneous MNA within a tumor. We compared clinical characteristics, biologic features, and clinical outcomes of patients with heterogeneous MNA to patients with either homogeneous MNA or MYCN wild-type tumors.
Patients and Methods
In this retrospective cohort study, we categorized patients as having tumors with MYCN wild-type, homogeneous MNA (>20% amplified tumor cells) or heterogeneous MNA (≤20% amplified tumor cells). We used chi-squared or Fisher’s exact tests to compare features between groups. We used log-rank tests and Cox models to compare event-free survival (EFS) and overall survival (OS) between groups.
Results
MYCN status and heterogeneity status (if amplified) could be ascertained in diagnostic tumor samples from 5,975 patients, including 57 (1%) with heterogeneous MNA, 981 (16.4%) with homogeneous MNA, and 4,937 (82.6%) with MYCN wild-type tumors. Multiple clinical and biological features differed between patients with heterogeneous vs. homogeneous MNA, including enrichment for thoracic primary sites and paucity of 1p LOH with heterogeneous MNA (p<0.0001). Importantly, EFS and OS were not significantly different between patients with heterogeneous vs. homogeneous MNA. Further, EFS and OS for patients with heterogeneous MNA were significantly inferior to patients with wild-type MYCN.
Conclusion
While neuroblastomas with heterogeneous MNA demonstrate significantly different biological and clinical patterns compared to homogeneous MNA, prognosis is similar between the two groups. These results support current practice that treats patients with heterogeneous MNA similarly to patients with homogeneous MNA.
Keywords: MYCN, heterogeneity, amplification, neuroblastoma, prognosis
Introduction
MYCN amplification (MNA) occurs in 16% of neuroblastomas and is a critical component of risk-stratification systems in this disease.1,2 In most cases, MYCN amplification is seen homogeneously throughout tumor cells, typically with high-level amplification (>4-fold ratio of MYCN signals to reference probe).3 Less commonly, low level copy number increases have been reported.4
With the advent of fluorescent in situ hybridization (FISH) methods to assess MYCN, it has become clear that MNA may be present only in a minority of tumor cells.5 The Children’s Oncology Group (COG) defines heterogeneous MNA as the presence of ≤20% of tumor cells in a sample demonstrating MNA. Investigations revealing heterogeneity of MNA between primary tumors and metastasis (by site) as well as differing MYCN status during the course of disease (by time) have been published, but are not the focus of this analysis.6,7
Heterogeneous MNA within a tumor has been characterized in prior cohorts and case series. The baseline incidence in neuroblastoma patients has been reported as 1.5% and 2.6% in separate cohorts.6,8 These two groups found the majority of patients with heterogeneous MNA were younger than 18 months at diagnosis. In a third cohort, older patients with heterogeneous MNA had associated segmental chromosomal aberrations,9 though some patients with numerical chromosomal aberrations have also been reported.8 In one report, the rare group of patients with both MNA and loss of 11q was enriched for heterogeneous MNA (7 of 19 such cases reported).10
The impact of heterogeneous MNA on clinical outcome has not been clear. This poses an issue with respect to risk stratification, as these patients are currently categorized similarly to patients with homogeneous MNA. Prognostic factors specifically within cohorts of patients with heterogeneous MNA have been described. One group identified older age and segmental chromosomal aberrations as important adverse prognostic factors.11 However, there are a paucity of data regarding outcomes for patients with heterogeneous MNA compared to patients with either homogeneous MNA or MYCN wild-type tumors.
Given the potential clinical impact, we analyzed available data from the COG Neuroblastoma Biology Study (ANBL00B1). We describe the incidence of heterogeneous MNA and compare clinical and biological features of patients with heterogeneous MNA and patients with either wild-type MYCN or homogeneous MNA. Finally, we compare clinical outcomes of patients with heterogeneous MNA to those of patients with wild-type MYCN or homogeneous MNA to evaluate the prognostic impact of heterogeneous MNA in a large cohort of patients with this finding documented in a single central laboratory. We demonstrate that patients with heterogeneous MNA have clinical and biological features that represent an intermediate phenotype between homogeneous MNA and wild-type MYCN, but have outcomes more in line with those seen in patients with homogeneous MNA.
Patients & Methods
Patients
Patients were eligible for inclusion in the analytic cohort if enrolled to the Children’s Oncology Group Neuroblastoma Biology Study ANBL00B1 prior to treatment from 10/1/2007– 6/30/2019, had a confirmed diagnosis of neuroblastoma or ganglioneuroblastoma, had known MYCN status, and known heterogeneity status. Although ANBL00B1 opened prior to 10/1/2007, MYCN heterogeneity data were not collected prior to this date. A total of 7,250 patients were evaluated for potential eligibility, with 1,275 excluded from analyses (35 ineligible for ANBL00B1, 266 with a non-neuroblastoma or non-ganglioneuroblastoma diagnosis, 520 with tissue submitted but unknown MYCN status, 450 with no specimens submitted or MYCN status derived from bone marrow from which MYCN heterogeneity cannot be determined, 3 with MNA but unknown heterogeneity status, and 1 with MNA who had conflicting MYCN heterogeneity results). Consent was obtained at time of enrollment to ANBL00B1.
Primary Predictor Variable
MYCN status was determined by interphase fluorescence in situ hybridization (I-FISH) performed in the COG Neuroblastoma Reference Laboratory at Nationwide Children’s Hospital using frozen or paraffin-embedded tumor tissue as previously described.4 Patients were categorized as MYCN wild-type if the ratio of MYCN signals to chromosome 2 reference probe signals was ≤4-fold and as MNA if >4-fold. Among patients with MNA, patients were dichotomized as homogeneous MNA if >20% of tumor cells demonstrated amplification or heterogeneous MNA if ≤20% of tumor cells demonstrated amplification.
Among patients with MNA, the amplicon structure was recorded as double minutes or homogeneously staining regions12 and MYCN copy number ratio was dichotomized as either >4–10-fold or >10-fold ratio of MYCN signal to reference probe as previously described.4 Among patients with heterogeneous MNA, amplification was further described as focal if amplified cells were clustered or scattered/diffuse if individual amplified cells were seen across a broad area. Cases with either focal or scattered/diffuse heterogeneity were included in this analysis.
Outcome Variables
Clinical variables of interest included sex, age, International Neuroblastoma Staging System (INSS) stage, COG risk group, primary site, LDH level (dichotomized at group median, 662.5 U/mL), and ferritin level (dichotomized at group median, 115 ng/mL). Biological variables of interest determined centrally by the Neuroblastoma Reference Lab and pathologists included ploidy, 1p loss of heterozygosity (LOH), 11q LOH, ALK status, histology, mitosis karyorrhexis index (MKI), and grade. For some markers, testing was only performed on a subset of patients enrolled to specific interventional trials.
Clinical outcomes were event-free survival (EFS) and overall survival (OS). EFS was defined as time from diagnosis to first episode of relapse, progression, death or secondary malignancy. OS was defined as time from diagnosis to death. For both EFS and OS, patients without an event were censored at date of last contact.
Statistical Analyses
Associations between MYCN group and clinical and biologic features were assessed using chi-squared tests or Fisher’s exact tests, depending upon sample size. EFS and OS were estimated by Kaplan-Meier methods and survival compared between groups using log-rank tests. Multivariable Cox proportional hazards models were constructed to further test the prognostic impact of heterogeneous MNA on EFS and OS after controlling for other key prognostic factors (age and INSS stage).
Results
Incidence of Heterogeneous MYCN Amplification
MYCN status and heterogeneity status (if amplified) could be ascertained in 5,975 patients. Of these, 57 patients (1%) had heterogeneous MNA. Heterogeneity was focal in 10 patients, diffuse/scattered in 37 patients, and unknown in 10 patients. Of the remaining patients, 981 (16.4%) had homogeneous MNA and 4,937 (82.6%) had MYCN wild-type tumors.
Clinical Features Differ in Patients with Heterogeneous MYCN Amplification
Clinical features according to MYCN category are shown in Table 1. Compared to patients with homogeneous MNA, patients with heterogeneous MNA were significantly less likely to have stage 4 disease, be considered COG high-risk, or have high LDH, but were more likely to have thoracic primary tumors. There were no significant differences in sex, age, adrenal primary site or ferritin levels between homogeneous MNA and heterogeneous MNA patients.
Table 1.
Clinical and biological features according to MYCN status in patients with neuroblastoma.
| Variable | All Patients N (%) | Heterogeneous MYCN Amplified N (%) | Homogeneous MYCN Amplified N (%) | p-value for Heterogeneous vs. Homogeneous MYCN | MYCN Wild-Type N (%) | p-value for Heterogeneous MYCN vs. Wild- type MYCN |
|---|---|---|---|---|---|---|
| Clinical Features | ||||||
| Male | 3,123 (52.29) | 29 (51.79) | 567 (57.80) | 0.3761 | 2,527 (51.20) | 0.9300 |
| Female | 2,850 (47.71) | 27 (48.21) | 414 (42.20) | 2,409 (48.80) | ||
| Unknown | 2 | 1 | -- | 1 | ||
| Age ≥18 months | 3,260 (54.56) | 35 (61.40) | 657 (66.97) | 0.3859 | 2,568 (52.02) | 0.1583 |
| Age <18 months | 2,715 (45.44) | 22 (38.60) | 324 (33.03) | 2,369 (47.98) | ||
| Unknown | -- | -- | -- | -- | ||
| INSS Stage 4 | 2,419 (40.64) | 33 (57.89) | 762 (77.76) | 0.0006 | 1,624 (33.04) | <0.0001 |
| All other stages | 3,533 (59.36) | 24 (42.11) | 218 (22.24) | 3,291 (66.96) | ||
| Unknown | 23 | -- | 1 | 22 | ||
| High-risk | 2,345 (40.22) | 48 (84.21) | 956 (98.35) | <0.0001* | 1,341 (27.93) | <0.0001 |
| Low/Intermediate-risk | 3,486 (59.78) | 9 (15.79) | 16 (1.65) | 3,461 (72.07) | ||
| Unknown | 144 | -- | 9 | 135 | ||
| Adrenal primary | 1,904 (33.53) | 21 (39.62) | 437 (47.04) | 0.2924 | 1,446 (30.79) | 0.1661 |
| Other primary sites | 3,775 (66.47) | 32 (60.38) | 492 (52.96) | 3,251 (69.21) | ||
| Unknown | 296 | 4 | 52 | 240 | ||
| Thoracic primary | 1,080 (19.02) | 9 (16.98) | 18 (1.94) | <0.0001* | 1,053 (22.42) | 0.3448 |
| Other primary sites | 4,599 (80.98) | 44 (83.02) | 911 (98.06) | 3,644 (77.58) | ||
| Unknown | 296 | 4 | 52 | 240 | ||
| High LDH (≥662.5 U/mL) | 992 (48.65) | 16 (76.19) | 356 (91.05) | 0.0424* | 620 (38.11) | 0.0004 |
| Normal LDH (<662.5 U/mL) | 1,047 (51.35) | 5 (23.81) | 35 (8.95) | 1,007 (61.89) | ||
| Unknown | 3,936 | 36 | 590 | 3,310 | ||
| High ferritin (>115 ng/mL) | 619 (47.84) | 10 (62.50) | 175 (75.11) | 0.2528* | 434 (41.53) | 0.0915 |
| Normal ferritin (<115 ng/mL) | 675 (52.16) | 6 (37.50) | 58 (24.89) | 611 (58.47) | ||
| Unknown | 4,681 | 41 | 748 | 3,892 | ||
| Biologic Features | ||||||
| Diploid | 1,894(36.93) | 22 (45.83) | 503 (61.49) | 0.0309 | 1,369(32.12) | 0.0433 |
| Hyperdiploid | 3,234 (63.07) | 26(54.17) | 315(38.51) | 2,893 (67.88) | ||
| Unknown | 847 | 9 | 163 | 675 | ||
| LOH at 1p | 494(21.41) | 5 (26.32) | 267 (74.37) | <0.0001 | 222(11.51) | 0.0608* |
| No 1pLOH | 1,813(78.59) | 14(73.68) | 92 (25.63) | 1,707 (88.49) | ||
| Unknown | 3,668 | 38 | 622 | 3,008 | ||
| LOH at 11q | 350(15.19) | 2(10.53) | 25 (6.94) | 0.6361* | 323(16.78) | 0.7564* |
| No 11qLOH | 1,954(84.81) | 17(89.47) | 335 (93.06) | 1,602 (83.22) | ||
| Unknown | 3,671 | 38 | 621 | 3,012 | ||
| LOH at 1p and/or 11q | 791 (33.86) | 6(31.58) | 271 (75.49) | <0.0001 | 514(26.25) | 0.6037* |
| No LOH at 1p or 11q | 1,545(66.14) | 13(68.42) | 88(24.51) | 1,444 (73.75) | ||
| Unknown | 3,639 | 38 | 622 | 2,979 | ||
| ALK aberration | 65 (32.34) | --- | 42 (47.73) | 0.0616* | 23(21.30) | 0.5813* |
| No ALK aberration | 136(67.66) | 5(100.0) | 46 (52.27) | 85 (78.70) | ||
| Unknown | 5,774 | 52 | 893 | 4,829 | ||
| Unfavorable histology | 2,604 (46.30) | 32 (62.75) | 856 (92.94) | <0.0001* | 1,716(36.89) | 0.0001 |
| Favorable histology | 3,020 (53.70) | 19(37.25) | 65 (7.06) | 2,936(63.11) | ||
| Unknown | 351 | 6 | 60 | 285 | ||
| High MKI | 943(18.91) | 18(42.86) | 589(68.81) | 0.0005 | 336 (8.22) | <0.0001* |
| Low/Intermediate MKI | 4,043(81.09) | 24(57.14) | 267(31.19) | 3,752 (91.78) | ||
| Unknown | 989 | 15 | 125 | 849 | ||
| Undifferentiated/Poorly Differentiated | 4,753(91.77) | 45 (93.75) | 940(99.16) | 0.0132* | 3,768 (90.08) | 0.6235* |
| Differentiating | 426 (8.23) | 3 (6.25) | 8 (0.84) | 415(9.92) | ||
| Unknown | 796 | 9 | 33 | 754 | ||
Abbreviations: INSS=International Neuroblastoma Staging System; LDH=lactate dehydrogenase; LOH=loss of heterozygosity; MKI=mitosis karyorrhexis
Fisher’s exact test was used in place of chi-squared test
We next compared patients with wild-type MYCN to patients with heterogeneous MNA and found the latter were more likely to have stage 4 disease, be considered COG high-risk, and have high LDH. There were no significant differences in sex, age, adrenal primary site or ferritin levels between patients with wild-type MYCN vs. heterogeneous MNA.
Biological Features Differ in Patients with Heterogeneous MYCN Amplification
Biological features according to MYCN category are shown in Table 1. Compared to patients with homogeneous MNA, patients with heterogeneous MNA were significantly less likely to have unfavorable biological features. ALK and 11q status did not differ between groups, though were only available in a small number of patients.
Compared to patients with wild-type MYCN, patients with heterogeneous MNA were more likely to have diploid tumors, unfavorable histology, and high MKI. Other biological features did not differ between patients with wild-type MYCN and heterogeneous MNA.
We analyzed MYCN copy number ratio, dichotomized as low-level (>4–10x copy number ratio) or high-level (>10x copy number ratio), within our populations of homogeneous vs heterogeneous MNA patients (Table 2). We found that patients with heterogeneous MNA were significantly more likely to have low-level MYCN copy number ratios (5.26% of patients) compared to patients with homogeneous MNA (0.51% of patients; p=0.0072).
Table 2.
MYCN copy number and amplicon structure among patients with MYCN amplification.
| Variable | Heterogeneous MNA N (%) | Homogeneous MNA N (%) | p-value |
|---|---|---|---|
| MYCN Copy Number | |||
| >4–10x | 3 (5.26) | 5 (0.51) | 0.0072* |
| >10x | 54 (94.74) | 976 (99.49) | |
| Unknown | -- | --- | |
| MYCN Amplicon Structure | |||
| Double Minute | 53 (92.98) | 887 (90.70) | 0.5610 |
| Homologous Staining Region | 4 (7.02) | 91 (9.30) 3 | |
| Unknown | -- |
Fisher’s exact test was used in place of chi-squared test
Among patients with MNA, we also compared amplicon structure between patients with homogeneous vs. heterogeneous MNA (Table 2). Double minutes were the predominant amplicon structure, with similar rates between homogeneous and heterogeneous amplified tumors.
Clinical Outcomes for Patients with MYCN Amplification
Kaplan-Meier curves for EFS and OS stratified by MYCN status are provided in Figure 1. There was no statistically significant difference in EFS in patients with heterogeneous MNA compared to patients with homogeneous MNA [5-year EFS 57.5% (95% confidence interval (CI) 39.7–75.3%) vs. 50.7% (95% CI 46.3–55.2%), respectively; Figure 1A; p=0.3537]. Likewise, there was no statistically significant difference in OS in patients with heterogeneous MNA compared to patients with homogeneous MNA [5-year OS 69.6% (95% CI 53.5–85.6%) vs. 56.6% (95% CI 52.2–60.9%), respectively; Figure 1B; p=0.1751]. However, comparing outcomes for patients with heterogeneous MNA vs. wild-type MYCN, EFS and OS were significantly higher for patients with wild-type MYCN [5-year EFS of 78.4% (95% CI 76.6–80.1%) and 5-year OS of 87.9% (95% CI 86.6–89.2%); Figure 1; p<0.0001 for both comparisons].
Figure 1A.
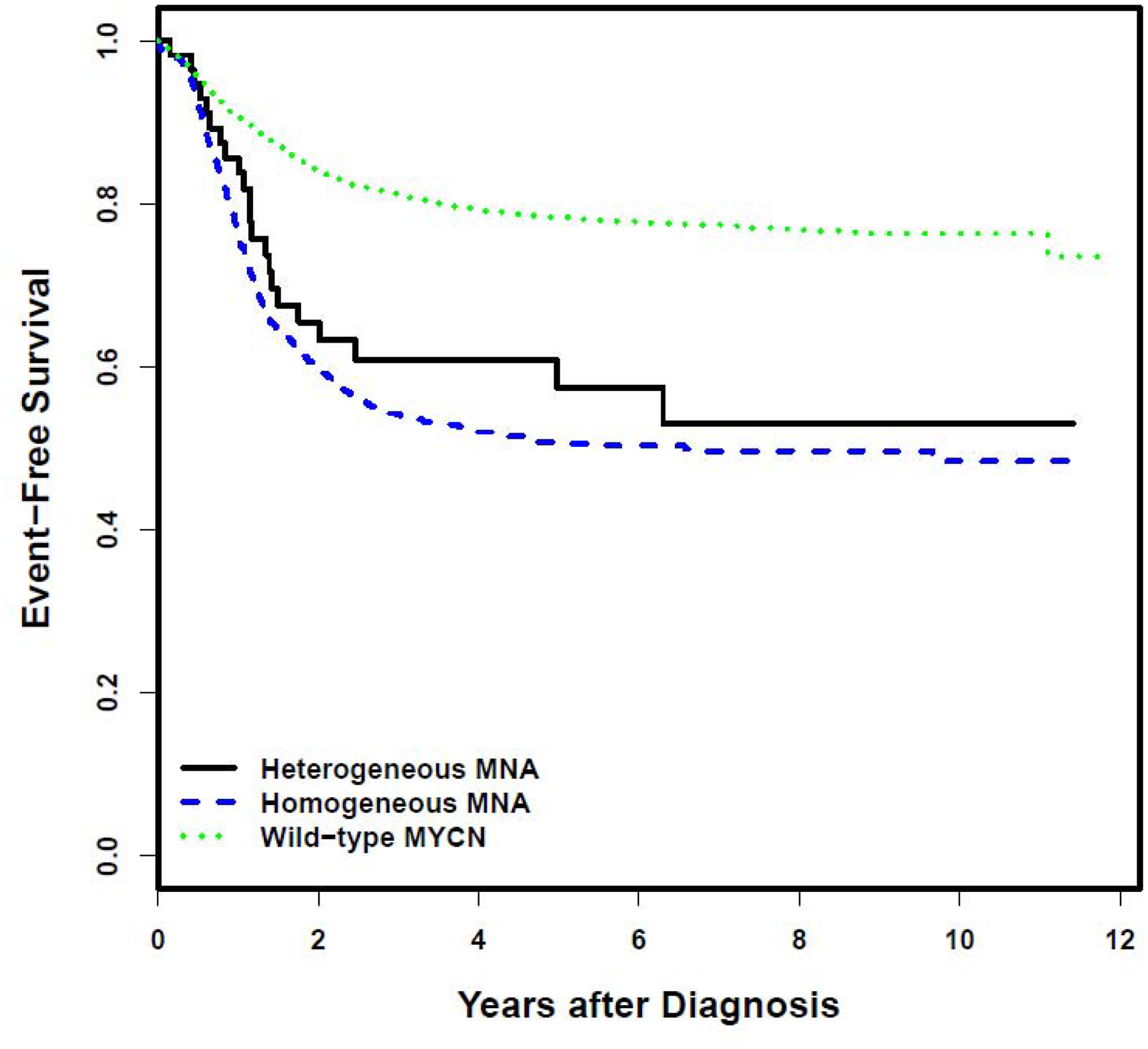
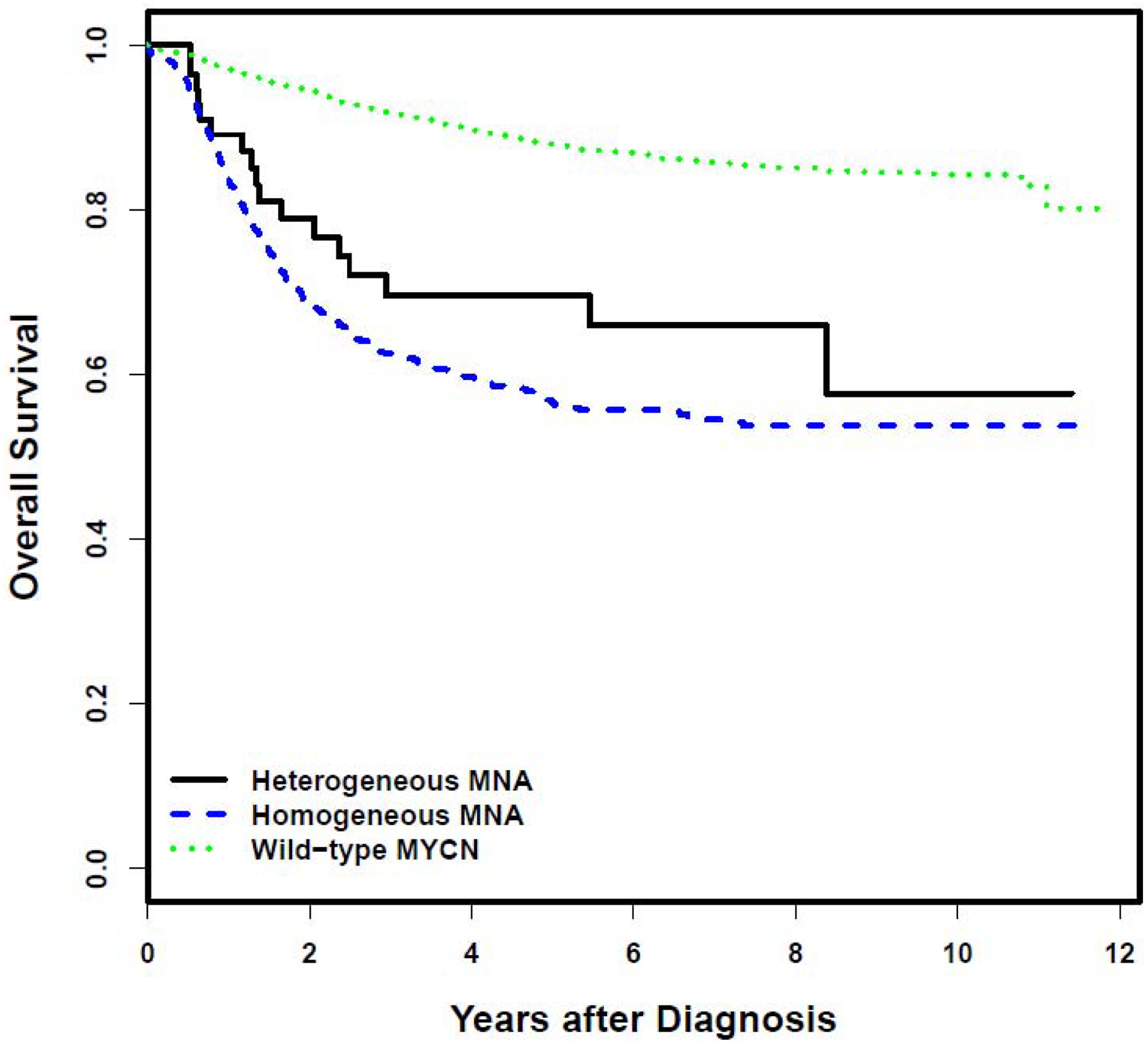
Event-free survival according to MYCN status. B. Overall survival according to MYCN status.
We next evaluated outcomes for patients with MNA without the combination of age ≥18 months and INSS stage 4 disease, as patients with this combination of features are considered high-risk regardless of MYCN status. The 5-year EFS for the 457 patients with homogeneous MNA who lacked this combination of features was 58.7% (95% CI 52.1–65.3%). The 5-year EFS for the 35 patients with heterogeneous MNA who lacked this combination of features was 67.8% (95% CI 46.0–89.6%). In contrast, the 5-year EFS for the 3,812 patients with wild-type MYCN who lacked the combination of age ≥18 months and INSS stage 4 disease was 87.9% (95% CI 86.4–89.5%).
We also evaluated outcomes for patients with the combination of age ≥18 months and INSS stage 4 disease according to MYCN status. The 5-year EFS for the 524 patients in this group with homogeneous MNA was 43.9% (95% CI 38.0–49.7%). The 5-year EFS for the 22 patients in this group with heterogeneous MNA was 43.2% (95% CI 14.7–71.7%). The 5-year EFS for the 1,125 patients in this group with wild-type MYCN was 45.6% (95% CI 41.4–49.7%).
To further understand the impact of heterogeneous MNA on EFS and OS, we used Cox proportional hazard models controlling for other key prognostic factors - age and INSS stage (Table 3). In Cox proportional hazards models for EFS and OS that included heterogeneous MNA compared to homogeneous MNA as the MYCN variable of interest, the only significant predictor of adverse outcome was INSS stage 4 disease. There was no difference in EFS or OS for patients with heterogeneous MNA compared to patients with homogeneous MNA in these models. In Cox proportional hazards models for EFS and OS including heterogeneous MNA vs. wild-type MYCN as the MYCN variable of interest, MYCN status, age, and INSS stage were all significantly associated with EFS and OS. Even after controlling for any differences in age and stage, patients with heterogeneous MNA had EFS and OS hazard ratios of 1.6 and 1.9 compared to patients in the reference group with wild-type MYCN.
Table 3.
Cox proportional hazard models of event-free (EFS) and overall survival (EFS) in patients with neuroblastoma.
| Heterogeneous vs. Homogeneous MNA (n=1,037) | |||||
|---|---|---|---|---|---|
| Variable | Reference Group | EFS | OS | ||
| Hazard Ratio | p-value | Hazard Ratio | p-value | ||
| Category of MNA (Heterogeneous vs. Homogeneous) | Homogeneous | 0.958 | 0.8453 | 0.837 | 0.4735 |
| Age | <18 months | 0.933 | 0.4961 | 0.907 | 0.3720 |
| INSS Stage | Not Stage 4 | 2.511 | <0.0001 | 2.744 | <0.0001 |
| Heterogeneous MNA vs. Wild-type MYCN (n=4,972) | |||||
| Variable | Reference Group | EFS | OS | ||
| Hazard Ratio | p-value | Hazard Ratio | p-value | ||
| MYCN Status (Heterogeneous MNA vs. Wild-type) | Wild-type | 1.605 | 0.0284 | 1.914 | 0.0086 |
| Age | <18 months | 1.740 | <0.0001 | 3.271 | <0.0001 |
| INSS Stage | Not Stage 4 | 3.583 | <0.0001 | 7.657 | <0.0001 |
Abbreviations: MNA=MYCN amplification
Discussion
The prognosis of a given patient with neuroblastoma varies widely based upon specific clinical and biological characteristics. The presence of MNA is an independent prognostic factor associated with rapid tumor progression and poor prognosis,13,14 highlighting the importance of understanding this key factor. Though heterogeneous MNA has previously been described in other cohorts, our comprehensive, multivariate analysis provides novel insights into the clinical, biological and prognostic associations of this phenomenon. Most importantly, our analysis indicates heterogeneous MNA is an independent negative prognostic factor compared with wild-type MYCN, with outcomes in line with those observed in patients with homogeneous MNA. As such, risk-classification systems should continue to group tumors with heterogeneous MNA with other MYCN amplified tumors, classifying most patients with this finding as high-risk. Other key findings include discordant biological and clinical features between heterogeneous MNA and homogeneous MNA and corroboration of the rarity of heterogeneous MNA.
Current risk classification systems and therefore current treatment paradigms consider heterogeneous MNA to be prognostically equivalent to patients with homogeneous MNA. Although differences exist in clinical and biological features between patients with heterogeneous MNA and homogeneous MNA, EFS and OS were statistically indistinguishable between these two groups. While we were able to control for age and INSS stage, these results need to be considered in the context of treatment administered, which evolved over the treatment period for patients with both high-risk and low/intermediate risk disease. Aside from patients with INSS stage 1 MYCN amplified tumors (either heterogeneous or homogeneous) treated as low risk in the COG, patients with heterogeneous MNA received a COG high-risk classification and it is likely that nearly all of these patients were treated with intensive therapies common to other patients with high-risk disease. Despite that, the 5-year EFS estimate for this group was only 57.5%. Even among patients who would not automatically be considered high-risk due to age ≥18 months and INSS stage 4 disease, the 5-year EFS for patients with heterogeneous MNA was only 67.8%.
The proportions for the majority of the clinical and biological features investigated for heterogeneous MNA were intermediate between proportions found for patients with homogeneous MNA and wild-type MYCN. Hallmarks of aggressive disease (e.g., high LDH, high MKI, stage 4 disease) were less common in patients with heterogeneous MNA compared with homogeneous MNA, though clinical outcomes were ultimately similar. We also found that patients with heterogeneous MNA were significantly more likely to have low levels of MNA compared to patients with homogeneous MNA who nearly all have tumors with > 10-fold increase in MYCN signal. There is a now well-described paucity of MNA in patients with thoracic primary tumors.15,16 In the current analysis, we observed that patients with heterogeneous MNA had a higher proportion of thoracic tumors compared to the very low proportion observed in patients with homogeneous MNA. Likewise, there is a well-established positive association between 1p LOH and MNA,17,18 though we observed a much lower proportion of 1p LOH in tumors with heterogeneous MNA. Given the well-established association of 1p LOH and MNA, we cannot exclude the possibility that 1p LOH is also heterogeneous and therefore considered absent in some of these cases with heterogeneous MNA. Of note, previous groups have reported that clinical behavior in cases of heterogeneous MNA is influenced by other chromosomal alterations.9 Work from the INRG database has previously identified subgroups in which MNA is least and most prognostic.19 In the context of the current analysis focused on the rare subgroup of patients with heterogeneous MNA, we were not able to complete a similar analysis.
Our findings both confirmed and contradicted previous published reports characterizing heterogeneous MNA.6,9 We confirm the low incidence of heterogeneous MNA reported in prior studies. While prior studies reported patients with heterogeneous MNA are younger,6 we observed no difference in age compared with patients with homogeneous MNA or wild-type MYCN. Likewise, prior reports suggested an association between heterogeneous MNA and segmental chromosomal alterations (SCAs), specifically 11q LOH.17,18 Our results do not support this finding, with no significant differences in proportion of 11q LOH between tumors with heterogeneous MNA and wild-type tumors, though limited data were available. Consistent with prior literature, patients with homogeneous MNA had the lowest rates of 11q LOH.20 We likewise demonstrate that a minority of tumors with heterogeneous MNA were diploid, a finding previously reported.11
A major strength of this study was the robust cohort size for such a rare entity. Clinical outcomes were tracked uniformly as part of ANBL00B1. Moreover, all samples were tested using uniform methodology performed in a single national laboratory. We acknowledge that neither the International Neuroblastoma Risk Group (INRG) Biology Committee nor the International Society of Paediatric Oncology, European Neuroblastoma (SIOPEN) group provide exact cutoffs for the percentage of tumor cells to differentiate heterogeneous from homogeneous MYCN amplification, making our results difficult to compare to those of other groups.2,11 Data on MYCN status were missing from a subset of otherwise eligible patients and that heterogeneity status could not, by definition, be determined from patients with only bone marrow material submitted for MYCN testing. While it is unclear if missing data influenced our results, our overall incidence of MNA of 17.4% aligns with historical data.21 Though several clinical and biological features had extensive missing data, we have no reason to expect differential missing data between patients with heterogeneous vs. homogeneous MNA.
In summary, this analysis adds to our understanding of patients with neuroblastoma with heterogeneous MNA and establishes the prognostic impact associated with this phenomenon. Importantly, our results support current practice that risk-stratifies patients with heterogeneous MNA similarly to patients with homogeneous MNA. The poor outcomes for these patients further emphasize the need to develop novel therapeutics targeting tumors with MNA.
Highlights.
MYCN amplification (MNA) is a known adverse prognostic factor in neuroblastoma
Only 1% of neuroblastomas have heterogeneous MNA, with MNA in < 20% of tumor cells
Clinical and biological features are distinct for heterogeneous MNA
Outcomes with heterogenous MNA are worse than with wild-type MYCN
Acknowledgements
The authors would like to acknowledge staff at the COG Neuroblastoma Reference Laboratory in the Biopathology Center at Nationwide Children’s Hospital. Specifically, we acknowledge the clinical directors who review and sign out cases: Caroline Astbury; Robert Pyatt; Shalini Reshmi; Maria Alfaro, and Ruthann Pfau, as well as the pathologists who review samples: Nilsa C Ramirez; Samir Kahwash; and Miriam Conces.
Grant Support: Supported by NIH/NCI Grant U10 CA180899 (Children’s Oncology Group Statistics and Data Center), the COG Foundation, NCTN Operations Center Grant U10CA180886, the St. Baldrick’s Foundation, NIH T32 CA136432-08, and Alex’s Lemonade Stand Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: SGD reports travel expenses from Loxo Oncology, Roche, and Salarius and consulting fee from Loxo Oncology. MSI reports consulting fees from Bayer Canada. AN serves on a data safety monitoring committee for Novartis.
References
- 1.Thompson D, Vo KT, London WB, et al. Identification of patient subgroups with markedly disparate rates of MYCN amplification in neuroblastoma: A report from the International Neuroblastoma Risk Group project. Cancer. 2016;122(6):935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009;100(9):1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twist CJ, Schmidt ML, Naranjo A, et al. Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children’s Oncology Group Study ANBL0531. J Clin Oncol. 2019;37(34):3243–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell K, Gastier-Foster JM, Mann M, et al. Association of MYCN copy number with clinical features, tumor biology, and outcomes in neuroblastoma: A report from the Children’s Oncology Group. Cancer. 2017;123(21):4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squire JA, Thorner P, Marrano P, et al. Identification of MYCN Copy Number Heterogeneity by Direct FISH Analysis of Neuroblastoma Preparations. Mol Diagn. 1996;1(4):281–289. [DOI] [PubMed] [Google Scholar]
- 6.Theissen J, Boensch M, Spitz R, et al. Heterogeneity of the MYCN oncogene in neuroblastoma. Clin Cancer Res. 2009;15(6):2085–2090. [DOI] [PubMed] [Google Scholar]
- 7.Marrano P, Irwin MS, Thorner PS. Heterogeneity of MYCN amplification in neuroblastoma at diagnosis, treatment, relapse, and metastasis. Genes Chromosomes Cancer. 2017;56(1):28–41. [DOI] [PubMed] [Google Scholar]
- 8.Berbegall AP, Villamon E, Piqueras M, et al. Comparative genetic study of intratumoral heterogenous MYCN amplified neuroblastoma versus aggressive genetic profile neuroblastic tumors. Oncogene. 2016;35(11):1423–1432. [DOI] [PubMed] [Google Scholar]
- 9.Bogen D, Brunner C, Walder D, et al. The genetic tumor background is an important determinant for heterogeneous MYCN-amplified neuroblastoma. Int J Cancer. 2016;139(1):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villamon E, Berbegall AP, Piqueras M, et al. Genetic instability and intratumoral heterogeneity in neuroblastoma with MYCN amplification plus 11q deletion. PLoS One. 2013;8(1):e53740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berbegall AP, Bogen D, Potschger U, et al. Heterogeneous MYCN amplification in neuroblastoma: a SIOP Europe Neuroblastoma Study. Br J Cancer. 2018;118(11):1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau LA, McGrady P, London WB, et al. Does MYCN amplification manifested as homogeneously staining regions at diagnosis predict a worse outcome in children with neuroblastoma? A Children’s Oncology Group study. Clin Cancer Res. 2006;12(19):5693–5697. [DOI] [PubMed] [Google Scholar]
- 13.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224(4653):1121–1124. [DOI] [PubMed] [Google Scholar]
- 14.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313(18):1111–1116. [DOI] [PubMed] [Google Scholar]
- 15.Oldridge DA, Truong B, Russ D, et al. Differences in Genomic Profiles and Outcomes between Thoracic and Adrenal Neuroblastoma. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vo KT, Matthay KK, Neuhaus J, et al. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. 2014;32(28):3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schleiermacher G, Mosseri V, London WB, et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br J Cancer. 2012;107(8):1418–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janoueix-Lerosey I, Schleiermacher G, Michels E, et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol. 2009;27(7):1026–1033. [DOI] [PubMed] [Google Scholar]
- 19.Campbell K, Shyr D, Bagatell R, et al. Comprehensive evaluation of context dependence of the prognostic impact of MYCN amplification in neuroblastoma: A report from the International Neuroblastoma Risk Group (INRG) project. Pediatr Blood Cancer. 2019;66(8):e27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353(21):2243–2253. [DOI] [PubMed] [Google Scholar]
- 21.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3(3):203–216. [DOI] [PubMed] [Google Scholar]


