Abstract
Background
Indications for adjuvant radiation therapy (XRT) in breast cancer have expanded. Although highly effective, XRT damages surrounding tissues and vasculature, often resulting in delayed or compromised breast reconstruction. Thus, effective, yet safe methods of radiation injury prophylaxis would be desirable. Amifostine is a FDA-approved radio-protectant, however, concerns about its potential to also protect cancer remain. The purpose of this study was to evaluate the oncologic safety of Amifostine in vitro and determine its effect on human breast cancer cells in the setting of XRT.
Methods
One ER+/PR+/Her2- (MCF-7) and two ER-/PR-/Her2- (MDA-MB-231,MDA-MB-468) breast cancer cell lines were investigated. Female Fibroblasts (FF) were utilized as controls. Cells were treated with WR-1065, the active metabolite of Amifostine, 20 minutes before 0Gy, 10Gy, or 20Gy XRT. Live and dead cells were quantified; percent cell death was calculated.
Results
WR-1065 treatment significantly preserved viability and reduced healthy FF death after XRT compared to untreated controls. All three breast cancer cells lines exhibited radio-sensitivity with substantial cell death. Cancer cells retained their radio-sensitivity despite WR-1065 pretreatment, achieving the same degree of cell death as untreated controls.
Conclusions
This study demonstrated the proficiency of Amifostine to selectively protect healthy cells from XRT, while breast cancer cells continued to remain radiosensitive. These results support the oncologic safety of Amifostine in breast cancer in vitro. Further investigation is now warranted in vivo to ascertain the translational potential of using Amifostine as a radio-protectant for breast reconstruction after radiation treatment.
Introduction
Breast cancer is the most common cancer diagnosed in the United States, with approximately 253,000 new cases of invasive breast cancer diagnosed in 2017.1,2 Amidst new screening protocols, earlier detection, and improved treatment options, the majority of patients diagnosed with breast cancer are treated with curative intent.3 As such, breast cancer survivors are often forced to live with the aftermath of treatment, which is a reality faced by a growing number of breast cancer survivors, estimated to be approximately 3.1 million women in the United States alone.4 While surgical resection remains the primary treatment for breast cancer; there are increasing indications for adjuvant XRT after breast conserving surgery and post-mastectomy,5–7 as it is a highly effective treatment shown to reduce disease recurrence.8
Despite advancements in radiotherapy, acute and long term sequelae associated with XRT persist after treatment concludes.9–11 Radiotherapy has profoundly destructive short and long-term effects on skin, soft tissue, and surrounding vasculature. XRT-associated pathologic injury to healthy tissue not only prolongs patients’ road to recovery, but also poses major obstacles to achieving a timely and satisfactory breast reconstruction outcome.12–14 Autologous breast reconstruction is often delayed until months after radiotherapy completion, while implant-based approaches bear higher rates of complications and failure in the setting of XRT, thereby limiting the reconstructive options available to both breast cancer patients and their surgeons. Plastic and reconstructive surgeons continue to work towards solutions to overcome the unique clinical challenges faced in the aftermath of radiation therapy.
Additionally, in certain cases, some XRT associated sequelae are so severe and poorly tolerated that they limit the dose of radiation patients can receive, which can compromise local tumor control and significantly impact oncologic treatment outcomes. 10,15 Considering that healthy tissue tolerance limits radiotherapy dosing, there is increasing clinical utility for radio-protective agents that selectively reduce XRT-induced injury in healthy tissue without decreasing the tumoricidal efficacy of XRT.16,17 Such agents would not only reduce insult to healthy tissue and improve reconstructive options available to patients and plastic surgeons, but would also allow for higher doses of XRT to be administered and better tolerated by cancer patients.
Currently, no therapeutics exist to prevent radiation injury in breast cancer patients. Previous studies in our laboratory have identified Amifostine (AMF) as a potential therapeutic solution to this unaddressed clinical problem. While AMF is one of only a few FDA approved prophylactic radio-protectants, indicated to prevent xerostomia in patients with head and neck cancer receiving radiotherapy, it has not been investigated for use in breast cancer patients receiving XRT. One major barrier to utilizing AMF as a radio-protectant in breast cancer patients is remaining uncertainty whether AMF may potentially protect breast cancer cells from XRT in addition to protecting healthy cells. While the oncologic safety of AMF in the setting of head and neck cancer has been well studied,18–20 it has yet to be investigated in the setting of breast cancer. Therefore, the objective of this study was to evaluate the oncologic safety of Amifostine and determine its effect on human breast cancer cells in the setting of XRT. Our hypothesis is that Amifostine will afford radio-protection to healthy cells, but will not extend protection to breast cancer cells. Determining the oncologic safety of AMF in breast cancer in vitro is a critical preliminary step in ascertaining the translational potential of utilizing AMF as a radioprotectant in breast cancer and breast reconstruction patients receiving radiation therapy.
Methods
Study Cell Line Selection
Validated triple negative (ER-/PR-/Her2-) breast cancer cell lines, MDA-MB-231 and MDA-MB-468, and ER+/PR+/Her2- breast cancer cell line, MCF-7, were investigated (Table 1). Female Fibroblasts (FF) served as a healthy control cell line in this study. All cells were grown in two-dimensional culture in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Antibiotic Antimycotic in a humidified C02 incubator.
Table 1:
Immunohistochemistry Characterization of Breast Cancer Cell Lines under Investigation
| Breast Cancer Cell Line | Immunohistochemistry Markers | ||
|---|---|---|---|
| ER+ | PR+ | Her2+ | |
| MDA-MB-231 | No | No | No |
| MDA-MB-468 | No | No | No |
| MCF-7 | Yes | Yes | No |
Breast cancer cell lines investigated in this study, characterized by immunohistochemistry markers: Estrogen Receptor (ER), Progesterone Receptor (PR), and Human Epidermal Growth Factor Receptor 2 (Her2).
Standardization Study to Determine Experimental Conditions
To identify appropriate doses of XRT and AMF, breast cancer cell lines were treated with varying doses of XRT (0, 5, 10, 20 Gy) and WR-1065 (0, 0.125, 0.25, 0.5, 1.0, 2.5, 5.0 mM). Each experimental condition was evaluated in triplicate. Cells grown to 70% confluence were trypsinized and counted using hemocytometer. Approximately 25,000 MDA-MB-231, MDA-MB-468, and MCF-7 cells and 50,000 FF cells were suspended in 450 μl of media and seeded in 24-well plates. 24 hours following plating, the cells were treated with the above concentrations of WR-1065, the active metabolite of AMF. Cells were exposed to WR-1065 for 20 minutes, then washed and the media was replaced. Immediately following pharmacologic treatment, the cells underwent XRT. After 48 hours, trypan blue counting was utilized to calculate cell viability of each treatment group. Based on this dose response, the concentration of WR-1065 and the dosing of XRT was selected for the evaluation of cancer cell response at radio-protective treatment conditions.
Evaluating the Effect of Radiation Therapy after Amifostine Pretreatment
The cells were plated as previously described. 24 hours after plating, the cells were treated with a final concentration of 0.25 mM WR-1065 for 20 minutes, while untreated cells served as a control. Following this exposure, cells were washed with sterile PBS and fresh media was added. Immediately thereafter, a single dose of 0, 10, or 20 Gy XRT was administered. Breast cancer cell response to treatment was evaluated 48 hours following XRT.
Prior to analysis, images of the cells were captured utilizing light microscopy. Then, the media from each well containing the detached dead cells was collected. Wells were washed with 50 μl of PBS, which was then collected. 200 μl of trypsin was added to the remaining cells attached to the plate. Cells were incubated at 37°C with trypsin for 3 minutes, and subsequently neutralized with 400 μl of media with FBS. The volume isolated from each of these steps (media removal, wash, neutralization) was collected in a microcentrifuge tube for each well. The isolate was then evaluated utilizing a trypan blue staining assay. Live and dead cells were quantified.
Data Analysis
Average values for each well were calculated based on three sample counts. Overall average for each cell line and treatment condition was then calculated based on the average value of each well. Percent cell death and percent cell viability was calculated. Two sample t-test was performed to compare values between groups and two-tail p-values were determined, where p<0.05 was taken to be significant.
Results
Breast Cancer Cells Remain Radio-Sensitive after WR-1065 Treatment
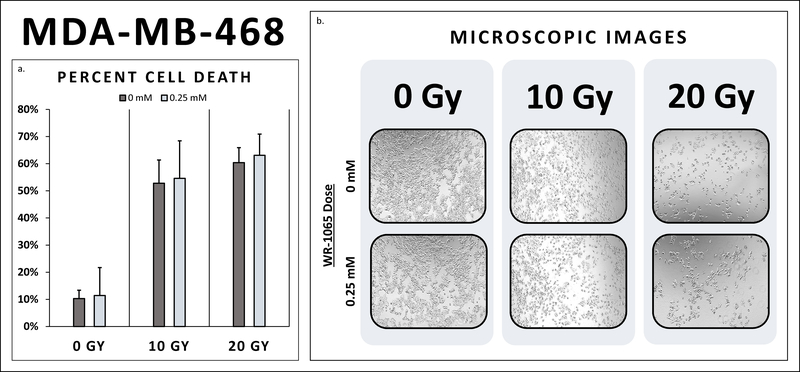
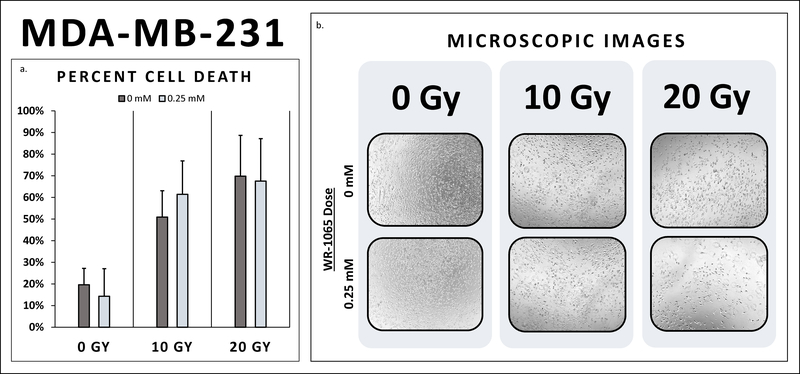
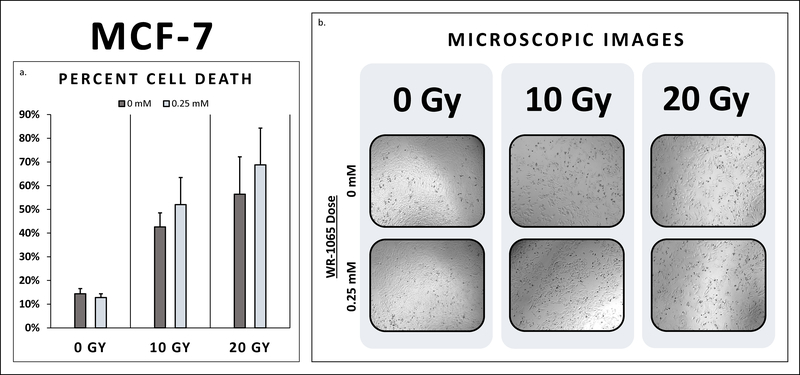
Both triple negative cancer cell lines, MDA-MB-468 and MDA-MB-231, exhibited radio-sensitivity with substantial cell death at 10 and 20 Gy (Table 2). In fact, cell death did not differ between triple negative breast cancer cells, MDA-MB-468 and MDA-MB-231, pretreated with WR-1065 and their untreated controls at 10 Gy and 20 Gy (Figures 1–2). Additionally, the ER+/PR+/Her2- breast cancer cell line, MCF-7, also demonstrated radio-sensitivity at both 10 Gy and 20 Gy (Figure 3). Cell death did not differ between MCF-7 pretreated with WR-1065 and the untreated control. Also, of note, higher levels of cell death were seen at 20 Gy for all three breast cancer cells lines as expected (Figure 2).
Table 2:
Percent Cell Death According to Treatment Condition
| Cell Line | Cell Death at 0 Gy |
Cell Death at 10 Gy |
Cell Death at 20 Gy |
||||||
|---|---|---|---|---|---|---|---|---|---|
| WR-1065 Dose |
P-value | WR-1065 Dose |
P-value | WR-1065 Dose |
P-value | ||||
| 0 mM | 0.25 mM | 0 mM | 0.25 mM | 0 mM | 0.25 mM | ||||
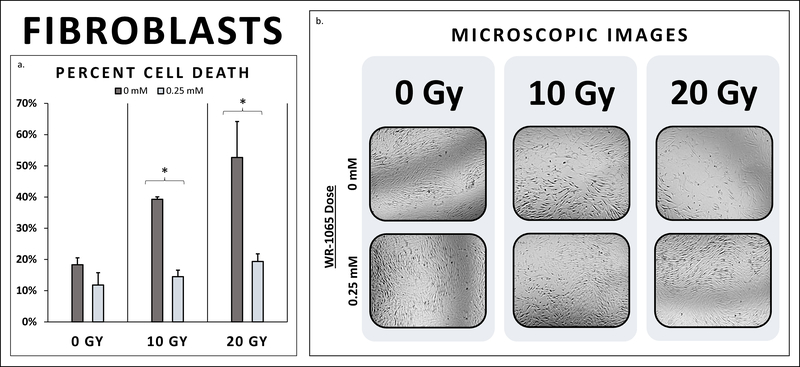
| FF | 18.3% | 11.8% | 0.15 | 39.3% | 14.5% | 0.000 | 52.7% | 19.4% | 0.01 |
| MDA-MB-468 | 10.3% | 11.4% | 0.39 | 52.8% | 54.6% | 0.895 | 60.4% | 63.1% | 0.66 |
| MDA-MB-231 | 19.6% | 14.3% | 0.77 | 50.9% | 61.4% | 0.408 | 69.8% | 67.5% | 0.89 |
| MCF-7 | 14.4% | 12.8% | 0.05 | 42.6% | 52.0% | 0.102 | 56.4% | 68.8% | 0.55 |
There was no significant difference in cell death between breast cancer cells (MDA-MB-468, MDA-MB-231, MCF-7) treated with 0.25 mM WR-1065 prior to XRT compared to their untreated controls at 0 Gy, 10 Gy, and 20 Gy. This same dose of WR-1065 demonstrated a significant decrease in FF cell death compared to untreated FF controls at 10 Gy and 20 Gy.
Figure 1:
1a. MDA-MB-468 Percent Cell Death. Cell death did not differ between MDA-MB-468 breast cancer cells pretreated with WR-1065 and their untreated controls at 10 Gy and 20 Gy. Breast cancer cells receiving radio-protective pretreatment reached the same degree of cell death as untreated breast cancer cells. (10 Gy: 54.6% vs. 52.8%, p>0.05; 20 Gy: 63.1% vs. 60.4%, p>0.05) 1b. MDA-MB-468 Microscopic Images. There was decreased breast cancer cell density at 10 Gy compared to 0 Gy. There was even further reduction in cells at 20 Gy compared to 10 Gy. No remarkable difference in cell density was observed between breast cancer cells pre-treated with WR-1065 compared to the controls at 0 Gy, 10 Gy, and 20 Gy, respectively.
Figure 2:
2a. MDA-MB-231 Percent Cell Death. Cell death did not differ between MDA-MB-231 breast cancer cells pretreated with WR-1065 and their untreated controls at 10 Gy and 20 Gy. Breast cancer cells pretreated with radio-protective doses of WR-1065 achieved the same degree of cell death as untreated breast cancer cells. (10 Gy: 61.4% vs. 50.9%, p>0.05; 20 Gy: 67.5% vs. 69.8%, p>0.05) 2b. MDA-MB-231 Microscopic Images. There was decreased breast cancer cell density and notable changes in cell morphology at 10 Gy compared to 0 Gy. There was even further reduction in cells at 20 Gy compared to 10 Gy. There was no notable difference in cell density between breast cancer cells pre-treated with WR-1065 compared to the controls at 0 Gy, 10 Gy, and 20 Gy, respectively.
Figure 3:
3a. MCF-7 Percent Cell Death. Cell death did not differ between MCF-7 breast cancer cells pretreated with WR-1065 and their untreated controls at 10 Gy and 20 Gy. Breast cancer cells pretreated with radio-protective doses of WR-1065 attained the same level of cell death as untreated breast cancer cells. (10 Gy: 52.0% vs. 42.6%, p>0.05; 20 Gy: 68.8% vs. 56.4%, p>0.05) 3b. MCF-7 Microscopic Images. There was decreased breast cancer cell density and notable changes in cell morphology at 10 Gy compared to 0 Gy, with an even further reduction in cells at 20 Gy compared to 10 Gy. There was no notable difference in cell density between breast cancer cells pre-treated with WR-1065 compared to the controls at 0 Gy, 10 Gy, and 20 Gy, respectively.
WR-1065 Mitigates Cell Death in Female Fibroblasts Receiving XRT
WR-1065 treatment of fibroblasts significantly preserved FF cell viability and conversely reduced healthy FF cell death after XRT compared to untreated controls at 10 Gy and 20 Gy. At 10 Gy, untreated fibroblasts exhibited 39.3% cell death 48 hours after irradiation, while fibroblasts pre-treated with WR-1065 experienced 14.5% cell death (p<0.001). Additionally, at 20 Gy, the cell death of untreated fibroblasts was 52.7%, whereas WR-1065 pre-treated fibroblasts demonstrated 19.4% cell death (p=0.008) (Figure 4). Notably, there was no significant difference in cell death between irradiated fibroblasts receiving radio-protective treatment at 10 Gy and 20 Gy and the non-irradiated controls. Therefore, WR-1065 treatment demonstrated a highly significant radio-protective effect in healthy female fibroblasts at both 10 Gy and 20 Gy.
Figure 4:
4a. Fibroblast Cell Death. Healthy fibroblast cells pre-treated with WR-1065 demonstrated a significant reduction in cell death compared to untreated controls at 10 Gy (14.5% vs. 39.3%, p<0.001). WR-1065 pre-treatment also preserved healthy FF cell viability compared to untreated controls at 20 Gy (19.4% vs. 52.7%, p=0.008). 4b. Fibroblast Microscopic Images. Untreated fibroblasts at 10 Gy show decreased cell density compared to untreated controls at 0 Gy. There is even further reduction in cell density for untreated FF cells at 20 Gy. FF cells pre-treated with WR-1065, however, demonstrate marked preservation of FF cell density. There is marked retention in cells compared to untreated FF at both 10 Gy and 20 Gy. WR-1065 pre-treatment preserves FF cell density, to levels similar to non-irradiated (0 Gy) controls.
Microscopic Images Uphold Cell Death Findings
To supplement and support these quantified findings, microscopic images were taken of each of the wells 24 hour after plating, which we denote as t=0, and 48 hours after XRT. As shown, these images demonstrate the marked reduction in the viable number of cells, the change in cell morphology, and the presence of rounded cells, denoting dead or dying cells. There is a marked decrease in the number of living cells on the plate comparing the plates at 48 hours post-XRT. For all three breast cancer cell lines, 0 Gy serves as a baseline, showing marked proliferation, while there is a marked decrease in cell density at 10 Gy, and even further reduction in cells at 20 Gy, regardless of WR-1065 pre-treatment (Figures 1–3). This same extent of reduction in cell number was exhibited by untreated FF cells, but was not seen for the FF cells pretreated with WR-1065 at 10 Gy and 20 Gy, which demonstrated similar plate densities between irradiated and non-irradiated groups (Figure 4).
Discussion
This study highlights important investigational findings validating the preliminary oncologic safety of AMF in breast cancer in vitro. Both ER+/PR+/Her2- (MCF-7) and ER-/PR-/Her2- (MDA-MB-231, MDA-MB-468) breast cancer cells remained radiosensitive despite prophylactic treatment with radio-protective doses of WR-1065, the active metabolite of AMF. In fact, there was no significant difference in cell death between breast cancer cells receiving WR-1065 pretreatment and untreated controls at both 10 Gy and 20 Gy. Therefore, not only do breast cancer cells remain radio-sensitive when treated with WR-1065, but they maintain the same level of radio-sensitivity as the untreated controls. WR-1065 pretreatment of control female fibroblast cells, however, did in fact produce a profoundly protective effect. Prophylactic WR-1065 preserved cell viability of healthy female fibroblasts receiving radiation, as fibroblast viability was increased by 24.8% at 10 Gy and by 33.3% at 20 Gy in cells receiving radio-protective pre-treatment compared to untreated controls. Therefore, WR-1065 afforded protection to the female fibroblast cells but did not offer any protection to the three breast cancer cell lines investigated. The results of this study demonstrated that Amifostine is an effective radio-protective agent that selectively reduces XRT-induced injury in healthy FF cells without decreasing the tumoricidal efficacy of XRT in both ER+/PR+/Her2- and ER-/PR-/Her2- breast cancer.
We selected two triple negative breast cancer cell lines (MDA-MB-231 and MDA-MB-468) to investigate in this study, as 10–20% of breast cancer patients present with this type of breast cancer.21 Triple negative breast cancers do not express the three most common receptors that many therapeutics target: ER, PR, and Her2. Thus, these patients typically have poor prognoses due to the aggressive nature of this cancer and its lack of response to hormone therapy and many chemotherapeutic agents. XRT is the most common and most effective treatment modality for patients with triple negative breast cancer after surgical resection. As such, this patient population would likely benefit from the use of a radio-protective agent like AMF. Therefore, we found it of utmost clinical importance to evaluate the oncologic safety of utilizing AMF in triple negative breast cancer.
In addition to triple negative breast cancer cell lines, the ER+/PR+/Her2- cell line, MCF-7, was studied to evaluate the effect of AMF in receptor positive breast cancer. This type of breast cancer is often treated with chemotherapy and/or radiotherapy in addition to surgical resection, depending on the staging and extent of disease.22 Furthermore, unlike majority of the triple negative cancer patients who have mutant P53 expression, MCF-7 breast cancers do not have P53 mutations; however, it is well established that P53 plays a major role in DNA damage due to XRT.23–25 Therefore, it was important to evaluate the effect of AMF on receptor positive MCF-7 breast cancer in the setting of XRT.
Given the high volume of ongoing research on breast cancer treatment protocols, there are expanding indications for adjuvant radiation therapy in the setting of both breast conserving surgery and mastectomy.5,6 Although XRT is a highly effective component to breast cancer treatment shown to improve survival,26 it has profoundly destructive short and long-term effects on the skin, soft tissue, and surrounding vasculature. This can limit reconstructive options and negatively impact the aesthetic outcome in these patients. XRT can delay or compromise autologous tissue flap reconstruction, as it decimates local tissue vascularity, which is critical to flap success.27 Immediate autologous breast reconstruction with subsequent radiotherapy can lead to skin and flap atrophy, distortion, fibrosis, and fat necrosis.6,7,28 While some recent studies have demonstrated success with immediate autologous reconstruction,29 the aforementioned onerous complications often deter surgeons from this approach, opting for delayed approaches instead, which postpones autologous reconstruction months after the conclusion of radiotherapy.
Radiotherapy also complicates implant-based breast reconstruction, resulting in overall higher failure rates and increased incidence of complications, including infection and capsular contracture.7,30 While some have found that the use of tissue extracellular matrix formulations reduces the incidence of capsular contracture,31–33 it does not eliminate this burdensome complication, which requires additional surgical procedures for these patients. Despite the potential complications associated with implant-based reconstruction in the setting of XRT, it still remains the most common reconstructive option pursued by women undergoing postmastectomy radiotherapy.6 Evidenced by the extensive body of literature on this topic, breast reconstruction in the setting of XRT remains a major clinical challenge, with ongoing debate over best practice techniques and timing of interventions. Regardless of the reconstructive approach, there is extraordinary clinical utility for an effective, yet safe method of radiation injury prophylaxis in the setting of breast reconstruction after breast cancer treatment, as it has the potential to increase reconstructive options available to both breast cancer patients and plastic surgeons.
Previous in vivo studies in our laboratory have investigated the use of AMF in irradiated tissue expander based breast reconstruction in a murine model.34–37 Animals exposed to XRT developed a fibrotic capsule around the tissue expander, extensive fibrosis of the surrounding local soft tissue, increased collagen disorganization, ulceration of local skin, and a reduction of skin vascularity.14,34–38 AMF was shown to mitigate many of these pathologic effects in vivo,34–37 therefore, we sought to evaluate the safety of such an efficacious therapy in vitro in order to ascertain the translational potential of our findings to the clinical setting.
Satisfactory breast reconstruction is essential to the physical, psychological, and emotional well-being and recovery of breast cancer patients.30,39,40 As such, oncologic surgical planning has become increasingly collaborative in recent years, where breast surgeons and plastic surgeons partner to deliver the most optimal results from both oncologic and aesthetic perspectives. With broadening indications for XRT in breast cancer, there is a growing need to minimize the destructive effects of XRT on healthy tissue, without decreasing the tumoricidal efficacy of radiation therapy. While some may posit radio-protective agents are only beneficial from a reconstructive perspective, this rationale is inherently flawed, as the use of radio-protectants like AMF also offers the ability to deliver higher tolerable doses of XRT, which can result in more effective disease control and treatment. This is a highly important consideration for breast cancers requiring radiation to the internal mammary lymph nodes and thoracic cavity, where healthy tissue toxicity can limit XRT dosing, and ultimately, disease control. Therefore, AMF could offer tremendous clinical utility in lymph node positive disease and cancers that utilize XRT as a primary treatment modality, including triple negative breast cancer, which we investigated in this study.
Limitations and Future Implications
While this study evaluated the oncologic safety of AMF in both receptor positive and receptor negative breast cancer in vitro, future studies in our laboratory will evaluate its oncologic safety in vivo. Addressing the oncologic safety of AMF in applications other than head and neck cancer, however, is not the only obstacle to AMF use in the clinical setting. Admittedly, the side effect profile of AMF has also hindered its clinical adoption. The current FDA approved formulations of AMF are intravenous and subcutaneous. Administered IV and SC, AMF reaches peak levels within seconds to minutes of administration, which can result in nausea, vomiting, and dizziness in a subset of patients. Our laboratory however, has been developing and investigating oral formulations of AMF to reduce peak drug exposure levels and time to peak levels, to ultimately mitigate these side effects.41 By developing improved methods for AMF administration, reducing its side effect profile, and addressing the oncologic safety of AMF, it is our hope to increase the clinical feasibility of AMF, and therefore, its clinical utility. If AMF is found to be safe and efficacious in shielding the skin, soft tissue, and vasculature of the breast from radiation induced injury, further studies can then be performed to ascertain the translational potential of utilizing AMF as a radio-protectant in breast cancer patients receiving radiation therapy.
Conclusion
Based on the results of this in vitro study, prophylactic treatment with AMF mitigates healthy cell death after XRT, but does not impact the tumoricidal efficacy of XRT on breast cancer cells. Radio-protective agents, like Amifostine, provide major advantages in the setting of breast cancer from both oncologic and reconstructive perspectives. AMF offers the ability to deliver higher doses of XRT, which can result in more effective disease control and treatment and improve breast reconstructive outcomes and quality of life for breast cancer survivors. Therefore, AMF is particularly important to consider and evaluate in cancers that utilize XRT as a primary treatment modality, including triple negative breast cancer, which we investigated in this study. While AMF is FDA approved for prophylaxis of XRT in the setting of head and neck cancer, we make the case, based on the results of this study and previous work in our laboratory, that AMF offers tremendous potential clinical utility in the setting of breast cancer and reconstruction. This study demonstrated the proficiency of Amifostine to selectively protect healthy fibroblast cells from XRT, while breast cancer cells remained radiosensitive. These results support the oncologic safety of Amifostine in breast cancer in vitro, however, further investigation is now warranted to validate these findings in breast cancer tumors in vivo.
Acknowledgments
Funding: This work was supported by National Institute of Health grant NIH-R01 CA 125187–06 awarded to Steven R. Buchman.
Footnotes
Financial Disclosures: The authors have no personal financial disclosures or conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Cancer Trends and Progress Report. Breast Cancer. Available at: https://progressreport.cancer.gov/treatment/breast_cancer. Accessed: December 3, 2018. [Google Scholar]
- 3.Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nature Reviews Drug Discovery. 2013;12:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Survivors: A Growing Population. Available at: http://pressroom.cancer.org/SurvivorshipStats2016. Accessed: December 3, 2018.
- 5.Billig J, Jagsi R, Qi J, et al. Should immediate autologous breast reconstruction be considered in women who require post-mastectomy radiation therapy? A prospective analysis of outcomes.” Plastic and Reconstructive Surgery. 2017;139:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho AY, Hu ZI, Mehrara BJ, Wilkins EG. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. The Lancet Oncology. 2017;18:e742–e753. [DOI] [PubMed] [Google Scholar]
- 7.Ricci JA, Epstein S, Momoh AO, Lin SJ, Singhal D, Lee BT. A meta-analysis of implant-based breast reconstruction and timing of adjuvant radiation therapy. Journal of Surgical Research. 2017;218:108–116. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group, Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garibaldi C, Jereczek-Fossa BA, Marvaso G, et al. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. New England Journal of Medicine. 2009;360:63–70. [DOI] [PubMed] [Google Scholar]
- 11.Harper JL, Franklin LE, Jenrette JM, Aguero EG. Skin toxicity during breast irradiation: pathophysiology and management. Southern Medical Journal. 2004;97:989–994. [DOI] [PubMed] [Google Scholar]
- 12.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: a claims-based analysis. Ann Surg. 2016;263:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiation Oncol Biol Phys. 1995;31:1171–1185. [DOI] [PubMed] [Google Scholar]
- 14.Snider AE, Lynn JV, Urlaub KM, et al. Topical deferoxamine alleviates skin injury and normalizes atomic force microscopy patterns following radiation in a murine breast reconstruction model. Annals of plastic surgery. 2018;81:604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nature Reviews Cancer. 2009;9:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan JL, Krishnan S, Movsas B, et al. Decreasing the adverse effects of cancer therapy: an NCI Workshop on the preclinical development of radiation injury mitigators/protectors. Radiation research. 2011;176:688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCumber LM. The potential influence of cell protectors for dose escalation in cancer therapy: an analysis of amifostine. Medical Dosimetry. 2004;29:139–143. [DOI] [PubMed] [Google Scholar]
- 18.Andreassen CN, Grau C, Lindegaard JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy In Seminars in radiation oncology, vol. 13, no. 1, pp. 62–72. WB Saunders, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. Journal of Clinical Oncology. 2000;18:3339–3345. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman TH, Brizel DM, Henke M, Monnier A, Eschwege F, Sauer R, Strnad V. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and-neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. International Journal of Radiation Oncology Biology Physics. 2005;63:985–990. [DOI] [PubMed] [Google Scholar]
- 21.Breastcancer.org “Triple-Negative Breast Cancer.” Available at: https://www.breastcancer.org/symptoms/diagnosis/trip_neg. Accessed on: December 11, 2018.
- 22.Anders CK, Zagar TM, Carey LA. The management of early-stage and metastatic triple-negative breast cancer: a review. Hematology/Oncology Clinics. 2013;27:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang D and Peng Y. Roles of p53 and p16 in triple-negative breast cancer. Breast Cancer Management. 2013;2:537–544. [Google Scholar]
- 24.Lim LY, Vidnovic N, Ellisen LW, and Leong CO. Mutant p53 mediates survival of breast cancer cells. British Journal of Cancer 2009;101:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudkov AV and Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nature Reviews Cancer. 2003;3:117. [DOI] [PubMed] [Google Scholar]
- 26.Shirvani SM, Pan IW, Buchholz, et al. Impact of evidence-based clinical guidelines on adoption of postmastectomy radiation in older women. Cancer. 2011;117:4595–605. [DOI] [PubMed] [Google Scholar]
- 27.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plastic Reconstr Surg. 2002;109:1919–24. [DOI] [PubMed] [Google Scholar]
- 28.Kelley BP, Ahmed R, Kidwell KM, Kozlow JH, Chung KC, Momoh AO. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Annals of Surgical Oncology. 2014;21:1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang EI, Liu TS, Festekjian JH, Da Lio AL, Crisera CA. Effects of radiation therapy for breast cancer based on type of free flap reconstruction. Plastic Reconstr Surg. 2013;131;1e–8e. [DOI] [PubMed] [Google Scholar]
- 30.Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plastic Reconstr Surg. 2014;134:588–595. [DOI] [PubMed] [Google Scholar]
- 31.Mowlds DS, Salibian AA, Scholz T, Paydar KZ, Wirth GA. Capsular contracture in implant-based breast reconstruction: Examining the role of acellular dermal matrix fenestrations. Plastic Reconstr Surg. 2015;136:629–635. [DOI] [PubMed] [Google Scholar]
- 32.Lardi AM, Ho-Asjoe M, Junge K, Farhadi J. Capsular contracture in implant based breast reconstruction—the effect of porcine acellular dermal matrix. Gland Surg. 2017;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leong MC, Basu B, Hicks MJ. Further evidence that human acellular dermal matrix decreases inflammatory markers of capsule formation in implant-based breast reconstruction. Aesthetic Surg Journal. 2015;35:40–47. [DOI] [PubMed] [Google Scholar]
- 34.Felice PA, Nelson NS, Page EE, et al. Amifostine reduces radiation-induced complications in a murine model of expander-based breast reconstruction. Plastic Reconstr Surg. 2014;134:551e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polyatskaya Y, Nelson NS, Rodriguez JJ, et al. Prophylactic amifostine prevents a pathologic vascular response in a murine model of expander-based breast reconstruction. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2016;69:234–240. [DOI] [PubMed] [Google Scholar]
- 36.Polyatskaya Y, Nelson NS, Rodriguez JJ, et al. Amifostine prophylaxis ameliorates radiation-induced injury in a murine model of expander-based breast reconstruction. Plastic Reconstr Surg. 2015;135:74. [Google Scholar]
- 37.Snider AE, Lynn JV, Urlaub KM, Nelson NS, Donneys A, Buchman SR. Mitigation of radiation induced type I collagen dermal disorganization utilizing amifostine in a murine breast reconstruction model. Plastic Reconstr Surg Global Open. 2018;6. [Google Scholar]
- 38.Rodriguez JJ, Kung T, Wang Y, et al. Changes in skin vascularity in a murine model for post-mastectomy radiation. Annals of plastic surgery. 2016;76:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plastic Reconstr Surg. 2011;127:2154. [DOI] [PubMed] [Google Scholar]
- 40.Harcourt DM, Rumsey NJ, Ambler NR, et al. The psychological effect of mastectomy with or without breast reconstruction: a prospective, multicenter study. Plastic Reconstr Surg. 2003;111:1060–8. [DOI] [PubMed] [Google Scholar]
- 41.Ranganathan K, Simon E, Lynn J, et al. Novel formulation strategy to improve the feasibility of amifostine administration. Pharmaceutical research. 2018;35:99. [DOI] [PubMed] [Google Scholar]