Abstract
Certain food products have been shown to accumulate arsenic (As) and cadmium (Cd) making it critical to monitor individual’s intake, particularly when they live near sources of environmental contamination. After a literature review, a novel dietary assessment was conducted to estimate a child’s potential exposure to these metal(loid)s via consumption of locally grown foods in communities impacted by active or legacy resource extraction activities. Mean ingestion rates of As- and Cd-translocating crops belonging to the Asteraceae, Brassicaceae, Caricaceae, Amaranthaceae, Cucurbitaceae, Liliaceae, Solanaceae, Apiaceae, and Fabaceae plant families were calculated for children: 1 to < 2 years, 2 to < 3 years, and 3 to < 6 years of age. These calculated ingestion rates ranged from 0 to 143,571 mg d−1. Farmer-consumer relationship was the leading motivation for buying locally grown foods, while lack of experience/unfamiliarity was the most frequently reported reason for not buying locally. The median percentages of child's yearly consumption of fruits and vegetables originating from stores (conventionally grown) and from local sources (farmer's market) were 48% and 38%, respectively. Tomato was the crop with the highest intake rate among children 1 to < 2 years and 2 to < 3 years of age and broccoli for children 3 to < 6 years of age. It was concluded that families who are reliant on locally grown food products may be disproportionately exposed to As and Cd, which could cause detrimental health effects.
Keywords: Environmental exposure assessment, Dietary assessment, Arsenic, Cadmium, Homegrown produce, Food frequency questionnaire
1. Introduction
In order to conduct a thorough exposure assessment of heavy metal(loid)s, it is critical to obtain site- and population-specific data to inform the exposure assessment model. In addition to environmental monitoring, dietary assessments are widely used to monitor and record dietary intakes for epidemiological studies. These assessments commonly include a food frequency questionnaire (FFQ), 24-hour recall (24HR), and/or dietary record (DR) administered through a combination of self-reporting and computer-based tools (Shim et al., 2014; Thompson et al., 2010). These assessments are valuable but are commonly critiqued for the lack of specificity of foods relevant to a study’s particular population.
To thoroughly evaluate a child’s consumption patterns of foods, in particular, locally grown foods, a dietary assessment must consider the relevant socioeconomic and cultural factors known to influence consumption patterns. These include proximity to a local farmer’s market, income level, sociodemographic factors (e.g. ethnicity/race, gender), and whether a person or family resides in an urban or rural community (Freedman at al., 2016; Feldman et al., 2015; Grebitus et al., 2013; Racine et al., 2013). A previous study showed that rural residents are also more likely to buy locally than urban and suburban residents (Racine et al., 2013) due to their increased access to local farms and produce (Racine et a., 2013). Rural families with children are more likely to buy fruits and vegetables locally (Racine et al., 2013) and community gardens are known to increase consumption of fruits and vegetables (McCormack et al., 2010).
Food deserts may also impact a rural community’s dependence on locally sourced foods. The U.S. Department of Agriculture (USDA) defines a food desert as a low-income census tract with a substantial number of residents with low access to large grocery stores that sell healthy foods at an affordable price (Ver Ploeg et al., 2011). Food access indicators include, low-income, low-access at 0.5 and/or 1 mi (urban) and 10 and/or 20 mi (rural), low vehicle access, and high (67% of population) group quarters (USDA, 2017). Low-income is classified as a median family income at or below 80% of statewide median family income, while low-access means that at least 33% of residents live more than 1 mi (urban) or 10 mi (rural) from a large grocery store (Rhode et al., 2017). As a result of low-access to nutritional foods at affordable prices, members of communities impacted by food deserts may be more likely to either travel farther for quality food options, not consume nutritiously dense food products, or rely on community- or homegrown foods.
Pollution has ubiquitously impacted the environment, leading to the contamination of air, water, and soil (Landrigan et al., 2018). It is well documented that soil, air, and water quality can be impacted by contamination from mining and other resource extraction activities (e.g. Ramirez-Andreotta et al., 2013a & 2013b; Csavina at al., 2012 & 2011), leading to elevated levels of metal(loid)s in wildlife and humans (Gonzalez & Gonzalez-Chavez, 2006; Castro-Larragoitia et al., 1997). Drinking water from contaminated groundwater is a major source of As exposure (World Health Organization (WHO), 2018). Arsenic is naturally occurring and can be found in sediments, soils, and groundwater and may also be released into the environment via mining, ore smelting, and industrial use of the element (ATSDR, 2015a). Cadmium is naturally occurring and is typically emitted to soil, water, and air by metal mining and refining, manufacture and application of phosphate fertilizers, fossil fuel combustion, and waste incineration and disposal. Generally, cadmium binds strongly to organic matter where it can stay in soil and be taken up by plant life, eventually entering the food supply (ATSDR, 2015b).
Food commodities such as rice, wheat products, fish, meat, and fruit juices have been shown to accumulate As and/or Cd (Yu et al., 2017; Cai et al., 2016; Chunhabundit, 2016; Bian et al., 2015; Newton et al., 2006). Shellfish and some offal such as liver and kidney have been shown to contain increased Cd concentrations due to the organs’ ability to concentrate the metal (Morrow, 2001). As a result of increased As concentrations measured in apple juices, the U.S. Food and Drug Administration established an action level of 10 μg kg−1 for As (Chen et al., 2014). Certain garden crops have also been shown to uptake these metal(loid)s associated with mining operations (e.g. Ramirez-Andreotta et al., 2013a & 2013b; Cobb et al., 2000; Li et al., 2006). Previous studies have shown that leafy vegetables (e.g. lettuce and spinach), root vegetables (e.g. potatoes) grains, peanuts, soybeans, and sunflower seeds may have high levels of Cd (Chunhabundit, 2016; ATSDR, 2012; Morrow, 2001). Thus, if crops are grown near resource extraction waste and other hazardous waste sites with As and Cd contaminants, members of these communities may be at an increased risk of exposure through their diet. This is especially critical because the U.S., the majority of Cd exposures for children is through dietary intake (ATSDR, 2012). Concomitantly, certain food preparation methods may also contribute to As exposure such as boiling foods or irrigation of crops with As contaminated waters (WHO, 2018). Improper cleaning of crops may increase As and Cd exposure if the consumer does not fully remove the soil particles from the surface of the crop.
Evaluating ingestion of food products as a potential exposure pathway to contaminants in rural environmental justice communities is critical due to their dependence on locally grown foods. An understanding of the target population’s demographic and cultural influences can greatly improve dietary assessments. For example, the Hispanic Health and Nutrition Examination Survey (HHANES) provided a well-established list of commonly eaten foods in Hispanic and Latin cultures (CDC, 1984). Careful selection of foods to include in the FFQ is important to estimate the typical intake of garden produce among a targeted population growing local foods and living near a waste site. Furthermore, the influence of demographic and cultural influences are important to consider for serving size estimations. For example, Asian and Latino populations’ perception of a small portion of rice, may be larger than the general population (Thompson & Subar, 2017). This difference in estimated serving size could have a drastic impact on one’s potential risk to adverse health effects associated with an increased consumption of rice that has been shown to accumulate elevated levels in As (Bhattacharya et al., 2010).
The goal of this study was to determine: 1) which crops are known to translocate As and Cd to the edible portion of the plant, and of these crops, determine which are commonly grown locally and culturally relevant, and 2) design a dietary assessment to capture a child’s intake of these crops in rural, vulnerable communities.
2. Materials and Methods
2.1. Literature review on produce that translocate As and Cd
A literature search was conducted to identify specific foods and plant families that have been shown to translocate As and Cd. Two online databases were used to search for peer-reviewed publications: Web of Science and Google Scholar. Combinations of keywords such as “arsenic”, “cadmium”, “food”, “diet”, “garden”, and “accumulating” were used to search for articles from September 1999 to July 2017. Further publications were identified from the reference list of several of these articles. The inclusion criteria included articles that describe accumulation and/or translocation of As and/or Cd in the edible portion of the crop. Of approximately 75 articles reviewed, 15 provided crops relevant for the target population shown in Table 1. Crops were categorized based on their bioconcentration factor (BCF), which is defined as the ratio of metal(loid) concentration (dry weight) in the plant to the concentration in the soil the plant was grown, expressed as BCF = Cplant/Csoil (Ramirez-Andreotta et al, 2013a; Ávila et al., 2017; Alam et al., 2003). Using the BCF, the following categories were used define metal accumulators and hyperaccumulators: BCF < 1 – no metal(loid) accumulation; 1 ≤ BCF < 10 – metal(loid) accumulation; and BCF ≥ 10 – metal(loid) hyperaccumulation (Ávila et al., 2017).
Table 1.
Common garden crops that translocate As and Cd in the edible portions of the plant.
Potential hyperaccumulator of Cd (Ávila et al., 2017)
Potential accumulator of Cd (Manjon et al., 2019)
Potential hyperaccumulator of As (Manjon et al., 2019)
Potential accumulator of Cd (Manjon et al., 2019)
Includes Chinese cabbage (Bian et al., 2015; Li et al., 2006)
2.2. Site description
The dietary assessment was designed for and administered at preschools (N = 4, referred to as S1-S4) throughout the rural cities of Nevada City (95959) and Grass Valley (95945) in Nevada County. CA. According to the 2010 Census, Grass Valley and Nevada City had approximately 12,860 and less than 5,000 residents, respectively (U.S. Census Bureau, 2010). Grass Valley meets the food desert indicator of low-income and low-access at 10 and 20 mi, while Nevada City only meets the low-access at 10 and 20 mi criteria (USDA, 2017). Nevada County was historically impacted by the California Gold Rush, which has caused persistent metal(loid) contamination in the region (Alpers, 2017). Each preschool has a vegetable garden and playground surrounding areas in which children frequently interact in throughout the school year. Each site also had a site administrator that provided information regarding the garden’s use and history.
2.3. Gardening behavior assessment
A gardening assessment tool was created to characterize the preschool’s environment and gardening practices. The assessment was administered using the online Qualtrics software (Qualtrics, Provo, UT, 2018). Each preschool’s administrator completed the online survey without an interviewer. The assessment included questions regarding the garden and preschool site, gardening activities, and preschool children behavior (Supplemental Material A). All respondents who completed the questionnaire and dietary assessment (described below) were consented under the University of California, San Francisco (UCSF) Institutional Review Board (IRB) rule as an approved project.
2.4. Dietary assessment
A dietary assessment was designed to determine child daily intake rates of various foods and evaluate consumption patterns of locally grown crops. The considerations detailed above (Section 2.1) guided the selection of foods included in the dietary assessment to prevent the underestimation of a child’s usual intake of locally grown crops. For this study, the dietary assessment was administered to parents and comprised of a 24HR and an FFQ to collect dietary information.
2.4.1. Food frequency questionnaire (FFQ) design
A modified FFQs was designed based on national surveys: National Health and Nutrition Examination Survey (NHANES) (CDC, 2016) and Diet History Questionnaires (DHQ-II) (National Cancer Institute, 2010). Since these national surveys do not adequately represent the average diet of a child in Nevada County, CA, a modified FFQ was designed to integrate the rurality, demographic and socioeconomic status influences of the preschool families (Thompson & Subar, 2017; Shim et al., 2014; Garcia et al., 2000; Baxter, 1997). Furthermore, this novel FFQ accounted for the potential exposure to As and Cd by including common garden crops that have been shown to translocate As and Cd (Table 1).
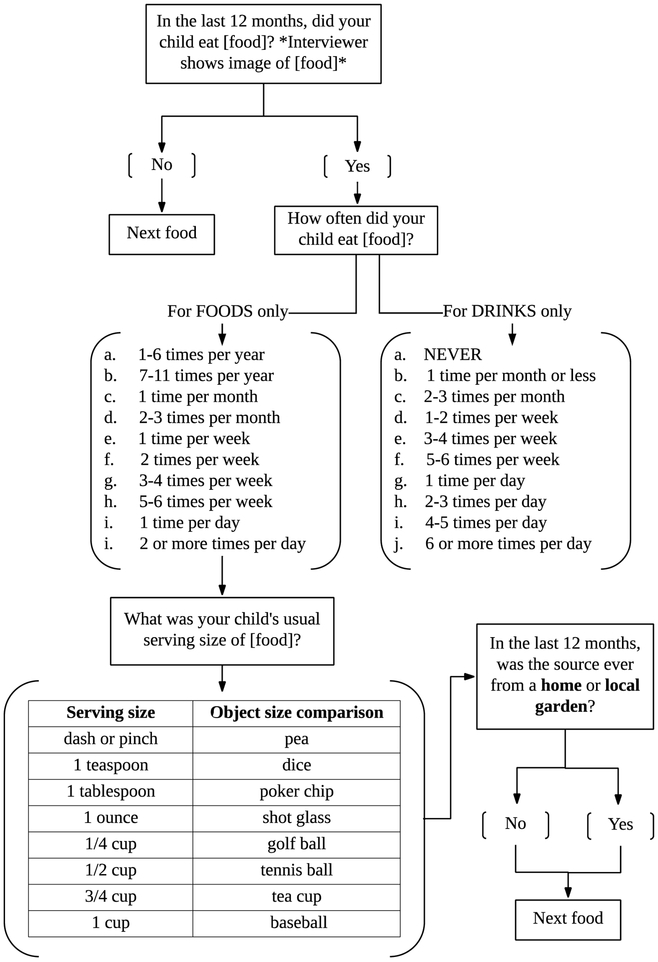
Community partners were trained to administer the FFQ by face-to-face and phone interviews in English and Spanish. Food images and physical serving size props were used during face-to-face interviews for participants to better estimate food portions (full assessment available in Supplemental Material B). Descriptive icons and/or an “object size comparison” was used for the question, “What was your child's usual serving size of [food]?” to reduce this possible recall bias for phone and self-administered interviews (Figure 1). An example of the schematic of the flow of FFQ questions for this study is depicted in Figure 1.
Figure 1.
Schematic of FFQ questions for each food.
The FFQ was also designed and administered using Qualtrics software (Qualtrics, Provo, UT, 2018). Skip-logic features were used to reduce participant burden and fatigue and reduce the amount of time needed to complete the survey by omitting non-applicable questions. Further, it reduced user error by preventing users from accidently answering those non-applicable questions. The force-response feature on the survey ensured completion of the survey in comparison to paper-administrated FFQs not administered by interview.
Recent studies have characterized interest in locally grown crops based on factors including proximity, quality of product, price, health-related exposures, and overall being more aware of environmental consequences (Ramirez-Andreotta et al., 2019; Freedman et al., 2016; Brown, 2003). Therefore, in the FFQ, participants were also asked to select any and all applicable factors that they believe drive their motivation for buying or not buying locally (Freedman et al. 2016) (Supplemental Material B).
2.4.2. 24-hour recall (24HR) design
The 24HR asks participants to provide a detailed list of foods consumed over a 24-hour time period. The 24HRs were completed by the parents on their own time at home without an interviewer. Participants recorded their child’s food and beverage intake for three nonconsecutive days to limit reactivity (change in diet as a result of having to record) (Thompson et al., 2015; Walton, 2015). When filled out correctly, the open-ended questions provide a detailed description regarding food and beverage ingredients, serving size, and preparation for that recorded day (Shim et al., 2014). Modifications were made for this study in order to obtain more detail than typically reported in traditional 24HRs. Participants were asked to report the following details about their child’s consumed foods and beverages (Supplemental Material C):
Meal/snack time
Food/beverage ingredient
Food type/brand
Serving size (or dimension)
Mode of preparation
Food source
While the self-reporting approach provides little to no burden to the investigator, the subjective survey relied on the respondents’ ability to recall the details of their meals that are often overlooked (i.e. seasoning) as well as estimated portion size (Walton, 2015; Shim et al., 2014; Thompson, 2010). Therefore, participants were instructed to fill out the 24HR to the best of their ability within an hour of each of their child’s meal to limit recall bias (Thompson, 2010; Baxter, 1997).
2.5. Intake rate calculation
A child’s intake rate (IR) can vary by age. For ingestion of food, the intake rate is the amount (mass) of the food consumed over a single day expressed by IR (mg d−1) = massfood/day. Average intake rates for crops grown at the preschools were calculated using the consumption frequency and usual serving size data reported in the FFQ (described in Supplemental Material B). For comparison, the U.S. Environmental Protection Agency (U.S. EPA) Exposure Handbook (U.S. EPA, 2011) estimations for carrots, cabbage, romaine lettuce, and kale were used. Due to the low response rate, the results of 24-hour recall were not used for intake rate calculation.
3. Results
3.1. Literature review
Twenty-five crops that translocate or accumulate/hyperaccumulate As and/or Cd in the edible portion of the plant (Table 1) were identified from the preliminary literature review. Cabbage, lettuce, and kale were identified as potential hyperaccumulators of As or Cd (bioconcentration factor ≥10) (Manjón et al., 2019; Ávila et al., 2017). Based on their capacity to uptake and translocate As and Cd, these crops were included in the FFQ in addition to foods previously included in traditional NHANES and DHQ-II FFQs.
3.2. Participant socio-demographics
A total of 10 parents completed the dietary assessment on behalf of their child(ren). The children represented in this study ranged from 9 months to 4.5 years of age. Four parents completed the FFQ on behalf of two or more children, so the average age of the children in the household was used for those FFQ responses. Five children identified as white, Caucasian, and/or European American; two identified as Latino or Hispanic and white, Caucasian, and/or European American; and one identified as an African American, black, and white, Caucasian, European American. One child identified as Latino or Hispanic, and another as American Indian, Native American, or Alaskan Native. Each parent reported English as their primary language, with one reporting Spanish as their second language.
When asked about the highest level of education, four parents reported some college (less than four years), three completed a Bachelor’s degree, two completed high/secondary school, and one received a post-graduate degree (Masters/PhD, MD, JD, etc). The parents also reported having 2 to 8+ people within their households, with a median total household income of $31,035 (participant range: $17,400 – $76,600).
3.3. Gardening behavior assessment
A total of 4 site administrators (one from each preschool) completed the online gardening behavior assessment on their own (not administered by interview) regarding garden history and practices. Each preschool reported growing leafy vegetables regularly along with a variation of other garden produce such as fruiting plants, herbs, and/or roots. Site administers reported using less than 25% of their garden produce for school meals as often as once per day to 1-6 times per year. Further, each site reported that aside from students; families, teachers, and staff also consume the preschool-grown crops.
3.4. Dietary Assessment
Ten parents from 3 of the 4 preschools (n = 1 from S1; n = 5 from S2; n = 4 from S3) completed the dietary assessment between October to December 2018. Of the ten participants, all completed the FFQ with an interviewer, but only 4 completed and returned their 24HR that was instructed to be completed at home. Unfortunately, each of the four was completed incorrectly. Thus, we were unable to validate each participant’s responses in the FFQ.
In the FFQ, parents reported the percentage of each child’s yearly consumption of various food groups and sources: homegrown, locally sourced, or store-bought (organically or conventionally grown) (Table 2). The highest median percentage of child's yearly consumption of fruits and vegetables originated from a store (conventionally grown) and locally grown/farmer's markets. Whereas, fruits and vegetables originating from a home garden had the lowest median value. Lastly, the highest median percentage of child's yearly consumption of meats (fish, poultry, beef, pork, etc.) and corn, grains, oats, and rice originated from a store (conventionally grown).
Table 2.
Median (min-max) percentage of child’s yearly consumption of food groups from different sources (N = 10 children).
| Fruits and vegetables |
Corn, grains, oats, and rice |
Meat (fish, poultry, beef, pork, etc.) |
|
|---|---|---|---|
| Home grown | 1.5% (0 – 50%) | 0% (0 – 20%) | 0% (0 – 25%) |
| Locally grown/farmer’s market | 38% (0 – 50%) | 0% (0– 50%) | 0% (0 – 50%) |
| Store (organically grown) | 5.0% (0 – 50%) | 2.5% (0 – 100%) | 0% (0 – 100%) |
| Store (conventionally grown) | 48% (0 – 100%) | 78% (0 – 100%) | 65% (0 – 100%) |
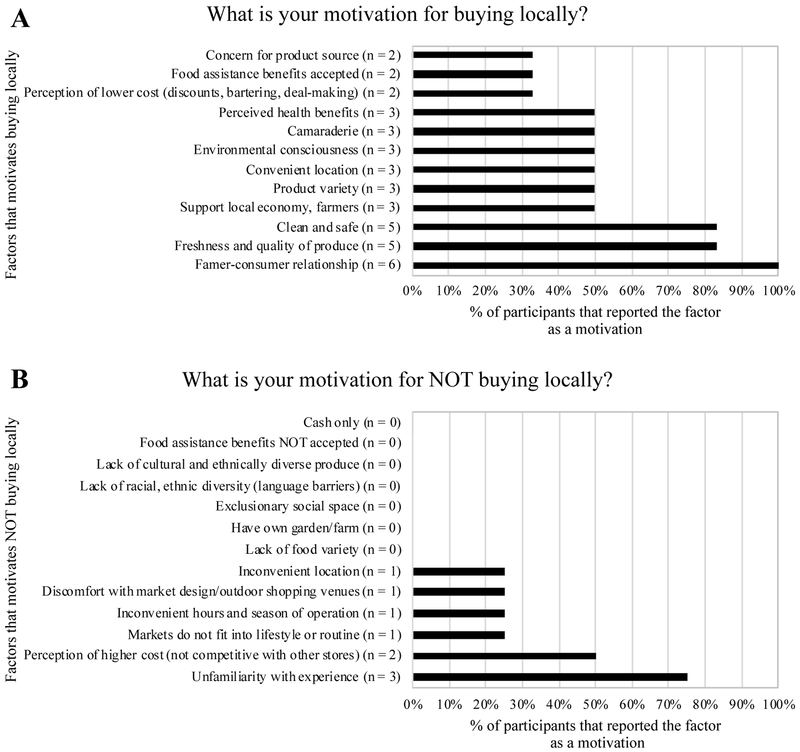
Participants were asked to select any and all applicable motivations for buying or not buying foods locally. Of those that indicated that they prefer to buy foods from local sources, all respondents reported their farmer-consumer relationship (n = 6) and half reported “environmental consciousness” and “perceived health benefits” as motivations (Figure 2). All other motivations for buying locally were reported by at least 2 participants (Figure 2). “Unfamiliarity with experience” was the most reported motivation for not buying locally among the four participants whom do not prefer to buy foods from local sources. No participant reported that having his/her own garden or farm as a motivation to not buy locally.
Figure 2.
Reported motivations for buying locally (A) or not buying locally (B) and the percentage of participants that reported that motivation.
The intake rates for common As- and Cd-translocating crops are shown in Table 3. In general, crop consumption decreased with increasing age. The highest estimated intake rate for children 1 to < 2 years and 2 to < 3 years of age was for tomatoes (7,165 and 143,571 mg d−1, respectively) and broccoli (11,375 mg d−1) for the 3 to < 6-year-old child. Radishes were the least consumed crop of all age groups.
Table 3.
Age-specific mean intake rate (IR) (mg d−1) of children that reported crop consumption over the past 12 months.
| 1 to < 2 yrs (N = 6) |
2 to < 3 yrs (N = 3) |
3 to < 6 yrs (N=1) |
|||||
|---|---|---|---|---|---|---|---|
| Plant Family | Crop | IR (mg d−1) | n | IR (mg d−1) | n | IR (mg d−1) | n |
| Asteraceae | Lettuce | 947 | 5 | 6,960 | 3 | 78 | 1 |
| Brassicaceae | Radish | 556 | 1 | – | 0 | – | 0 |
| Kale | 336 | 3 | 168 | 2 | – | 0 | |
| Broccoli | 2,830 | 5 | 8,161 | 3 | 11,375 | 1 | |
| Cabbage | 607 | 3 | 340 | 1 | 268 | 1 | |
| Turnip | 2,167 | 1 | – | 0 | – | 0 | |
| Cauliflower | 1,509 | 5 | 9,554 | 2 | 2,229 | 1 | |
| Caricaceae | Papaya | 6,042 | 1 | – | 0 | – | 0 |
| Amaranthaceae | Spinach | 430 | 4 | 23,571 | 1 | 250 | 1 |
| Swiss chard | 762 | 2 | – | 0 | – | 0 | |
| Cucurbitaceae | Pumpkin | 241 | 4 | 29,000 | 1 | 278 | 1 |
| Squash | 2,550 | 5 | 8,071 | 2 | 1,883 | 1 | |
| Cucumber | 1,228 | 5 | 10,071 | 3 | – | 0 | |
| Liliaceae | Garlic | 1,239 | 6 | 1,414 | 1 | 707 | 1 |
| Onions | 5,887 | 5 | 10,000 | 1 | – | 0 | |
| Solanaceae | Potato | 3,635 | 6 | 6,994 | 3 | 360 | 1 |
| Tomato | 7,165 | 4 | 143,571 | 3 | 6,429 | 1 | |
| Eggplant | 205 | 2 | 98 | 1 | – | 0 | |
| Pepper | 1,072 | 6 | 879 | 3 | – | 0 | |
| Apiaceae | Carrot | 3,372 | 5 | 15,389 | 3 | 2,265 | 1 |
| Cilantro | 229 | 4 | 31 | 2 | 3 | 1 | |
| Celery | 83 | 1 | 3,200 | 3 | – | 0 | |
| Fabaceae | Kidney bean | 1,059 | 4 | 441 | 1 | 1,533 | 1 |
| Green bean | 3,183 | 6 | 7,440 | 3 | 833 | 1 | |
N = number of children within that exposure age group
n = number of children within exposure age group who reportedly consumed the crop over the past 12 months
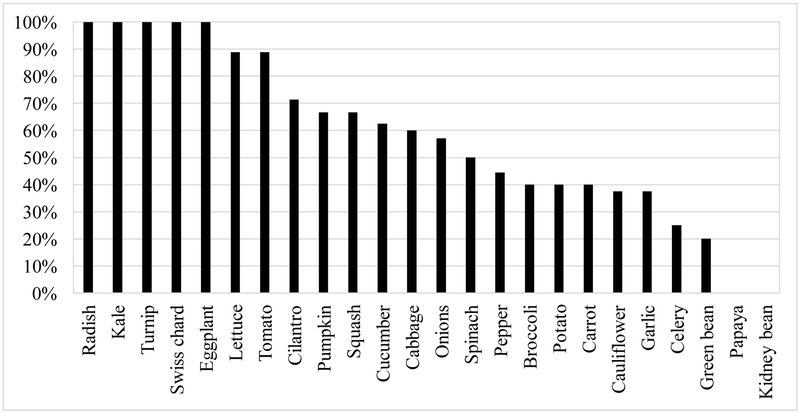
The percentage of respondents whom reported that the crop their child consumed over the past 12 months was from a home or local garden is shown in Figure 3. The majority (14 out of 24) were (≥ 50%) locally sourced.
Figure 3.
Percentage of ‘yes’ responses to the question, “Was the source ever from a home or local garden?” for each crop.
4. Discussion
4.1. Age-specific child intake rates
This study presents a novel dietary assessment designed for preschool-aged children in rural Nevada County, CA. Our literature review identified 25 crops that have been shown to translocate As and/or Cd in the edible portions of the plant. By integrating this list into our novel FFQ, the research group determined mean ingestion rates specific to the children at these preschools.
Data reported in the dietary assessment by participants was used to determine age-specific child intake rates (Table 3). The intake rates of cabbage (268–607 mg d;−1), and kale (0–336 mg d−1) were similar to the Brassica vegetables intake rate of 400 mg d−1 (children 2–7 years-old) reported by Liu et al. (2010) whom conducted a comparable study. Similarly, the intake rate of leafy vegetables (1,400 mg d−1) and potato (1,900 mg d−1) reported by Liu et al. (2010) were also within the intake rate range for lettuce (78–6,960 mg d−1) and potato (360–6,994 mg d−1) from this study. In contrast, the intake rate of green beans (833–7,440 mg d−1) and tomato (6,429–143,571 mg d−1) in this study were greater than the intake rates (200 and 2,700 mg d−1, respectively) of these vegetables (Liu et al., 2010). It is important to recall however, that the age of children in this study ranged from 9 months to 4.5 years, while those in the study by Liu et al. (2010) ranged from 2 to 7 years of age.
The U.S. EPA Exposure Factors Handbook (U.S. EPA, 2011) provides a summary of recommended intake rates (normalized to body weight) based on rates reported in the peer-reviewed literature and national surveys such as NHANES (U.S. EPA, 2018). The intake rates calculated from this study were reported as mg d−1. To make a direct comparison to those reported in the U.S. EPA Exposure Handbook for various child age groups, we recalculated this study’s intake rates to consider body weight (g kg d−1). Since not all of the study’s selected crops were individually reported in the U.S. EPA Exposure Handbook, the estimated intake rates were compared to general food groups. These groups included root and tuber vegetables, leafy vegetables (brassica and non-brassica), tropical fruits, cucurbit vegetables, bulb vegetables, fruiting vegetables, and beans (U.S. EPA, 2018). In general, the mean intake (g kg d−1) of radish, kale, papaya, turnip, cauliflower, spinach, swiss chard, squash, garlic, potato, tomato, eggplant, pepper, carrot, cilantro, celery, kidney bean, and green bean for children 1-6 years of age were lower than the national average (U.S. EPA, 2018).
The intake rates reported above were used as part of a child’s cumulative exposure assessment (water, incidental soil ingestion, homegrown produce, and dust inhalation) to As and Cd (Manjón et al., 2019). The results of Manjón et al. (2019) indicated that the As and Cd accumulating and hyperaccumulating crops identified in this study did indeed pose an increased exposure to preschool-aged children. In addition to dietary exposures, incidental soil ingestion was also a major contributor to preschool-aged children’s As and Cd exposure. Furthermore, lettuce, carrot, and cabbage grown in the preschool gardens accumulated a higher concentration of metal(loid) than those store-bought nation-wide. Consumption of preschool-grown cilantro, lettuce, and kale accounted for the highest percent contribution to the cumulative exposure to As for children aged 1 to < 2 years, 2 to < 3 years, and 3 to < 6 years, respectively, whereas carrot contributed the most to a child’s Cd cumulative exposure for each aforementioned age group (Manjón et al., 2019). Thus, each site was advised that special precaution should be taken when growing these crops in As- and Cd-contaminated garden soil. For example, even though cilantro and kale plants had a high contribution to a child’s As exposure, more caution should be taken with carrot and lettuce due to their higher ingestion rates (Manjón et al., 2019).
4.2. Motivation for buying locally
All children (N = 10) in this study consumed broccoli, potato, carrot, and green beans. Only 40% (n = 4) of children that ate broccoli, potato, and carrot over the past 12 months report them ever being sourced locally, while only 20% of children (n = 2) consumed green beans from a local source (Figure 3). When parents were asked “Do you prefer to buy your foods from local sources?”, 60% of the parents prefer buying their food from local sources (survey question in Supplemental Material B). However, the median percentage reported by parents of their child’s consumption of fruits and vegetables from locally-grown sources and home-grown was 37.5% and 1.5 %, respectively (Table 2). A study done in North Carolina observed that families with children under the age of five who ate relatively high amounts of fruits and vegetables a day, were more likely to buy their produce from a local vendor (Racine et al., 2013). Further, the authors also reported that families living in rural areas, bought from local vendors more frequently than those residing in suburban regions. In this study, 50% of the parents reported “convenient location” as a motivation for buying locally, while only 25% of those that did not prefer buying locally reported “inconvenient location” as a discouraging factor. Thus, this study supports the finding by Grebitus et al. (2013) that families are more likely to buy locally grown foods if they are in relatively close proximity.
All participants reported that the farmer-consumer relationship was a motivation for buying locally grown foods. Additionally, 50% report “camaraderie” and “support of local economy and farmers” as another motivation. Similarly, a previous study analyzing the extrinsic and intrinsic motivation for community gardening also observed that building relationships among other gardeners and the community was a prominent motivation (Ramirez-Andreotta et al., 2019). These findings provide evidence for and support the need to evaluate potential dietary exposures from local grown produce in at-risk communities.
4.3. Socioeconomic factors’ impact on fruits and vegetable consumption
Socioeconomic barriers have been identified as a factor that impacts the likelihood of consuming locally grown foods (Racine et al., 2013). The median household income of participants in this study was $31,035, almost half of the median household income of residents in Nevada County, CA ($60,610) and statewide ($67,169) (U.S. Census Bureau, 2017). In this study, children from families with an annual income of $25,100 or below (n = 3) generally consumed less carrot, cabbage, kale, lettuce, and cilantro than others of the same age groups from higher-income families. This is contrary to national trends reported by the “What We Eat in America”, which reports that low-income children ate more fruits and vegetables than children of the same age from higher-income families (USDA, 2018). Furthermore, in a North Caroline study, low-income families also bought more local produce than those of higher income (Racine et al., 2013). This discrepancy may be due to the present study’s confinement to rural communities and small sample size.
4.4. Limitations
The dietary assessment in the present study was subject to limitations, primarily due to lack of community outreach and participation. The small sample size (N = 10 parents reporting on the consumption patterns of 10 children) may have affected the accuracy of ingestion rates that may not fully representing children of the area. When prompted to report the age of their preschool child in the FFQ, a number of participants listed the ages or gave an age range for all preschool children in the home, and then completed one FFQ on behalf of all of them. Thus, the average age for those FFQ reports were taken to address the reporting error due to the open-ended question design. Since FFQs are prone to recall biases such as under- and over reporting (Hu et al., 1999), a 24HR was administered to the participants to validate the accuracy of the usual intake reported in the FFQ. The 24HRs provide a less-biased validation tool when unbiased methods, such as measured biomarkers are unavailable (Thompson & Subar, 2017). Unfortunately, due to the low response rate, the 24HR were not used and the FFQ was not validated.
Motivation was a limiting factor in receiving completed 24HRs. Each participant that completed an FFQ was also given a paper copy of the 24HR to complete on behalf of their child at home. Only 40% of participants returned their 24HR, and all were incomplete suggesting respondent fatigue (Thompson & Subar, 2017). However, of the participants whom completed the FFQ with an interviewer, 70% stated that the FFQ was extremely easy to complete and are likely to participate in future dietary assessments. Perhaps, filling out the 24HRs was more demanding to the participant than the online FFQ. Efforts to improve to dietary assessment via web-based programs are becoming more common and can reduce time commitments as well as administrative costs (Thompson et al., 2010). A study using the self-reporting, web-based tool, DietAdvice suggests that participants are more likely to accurately report food intake with limited face-to-face interviewing (Probst & Tapsell, 2007). Nevertheless, removing the role of the interviewer requires the participants to be highly motivated and have a high literacy level to complete the assessment properly (Thompson & Subar, 2017). In this study, all ten completed FFQs were administered by an interviewer from the research team. Future considerations for dietary assessments include gathering preliminary information of the target population’s usual diets and preferred administration methods using focus groups as recommended by Johnson-kozlow et al. (2011). This will allow the researchers to understand what foods are commonly eaten and how they are prepared within the study group, as well as how to modify the FFQ and 24HR to improve response rates and limit recall bias and participant fatigue. This study highlights the need for participant support and engagement throughout the research efforts to increase participant recruitment and response rates. Future studies with dietary assessments are encouraged to maintain ongoing communication with participants completing at-home components of the dietary assessment (e.g. 24HRs) through regular reminders via the participants’ preferred communication mode.
Further, when necessary, the estimated intake rates from this study were compared to general food groups since not all of these specific crops were reported in the NHANES. These groups included root and tuber vegetables, leafy vegetables (brassica and non-brassica), tropical fruits, cucurbit vegetables, bulb vegetables, fruiting vegetables, and beans (U.S. EPA, 2018). Lastly, this study focused on a dietary assessment tool to estimate As and Cd exposure via locally grown produce. However, incidental soil ingestion is another important exposure pathway to consider, along with expanding the scope of metal(loid)s (Manjón et al., 2019). Future studies should include: an adult dietary assessment tool to capture other age groups whom interact in these gardens and a wider range of produce regularly grown and shown to accumulate and/or hyperaccumulate deleterious contaminants.
5. Conclusion
Dietary assessments can provide valuable information regarding an individual’s or population’s intake rates, which directly informs exposure science studies. The comprehensive list of crops commonly consumed and grown in gardens that translocate As and Cd at noteworthy levels will help community and home gardeners make informed decisions. Families need to be aware of growing these crops in contaminated soils, and if necessary, limit their consumption of these crops from those sources. When combined with site-specific environmental data, dietary assessments reduce the uncertainty associated with the extrapolation of intake rates from the literature. Furthermore, these assessments can also inform mitigation strategies that address specific dietary behaviors and needs of a community. Food products, particularly, locally grown food products are known sources of As and Cd. These contaminants can cause detrimental health effects and are commonly associated with legacy and active resource extraction activities. By developing a novel dietary assessment, this study provides the basis for future investigations to assess the dietary patterns, gardening behaviors, and ultimately, exposure to metal(loid)s through consumption of metal(loid)-translocating and/or accumulating crops in other environmentally vulnerable communities.
Supplementary Material
Acknowledgments
This work was funded by the California Breast Cancer Research Program (Grant 23AB-1301A) and the National Institute of Environmental Health Sciences Superfund Research Program (P42ES04940). The authors are grateful to the families and site administrators that completed the dietary assessment for this study, as well as the entire Gardenroots: The Nevada County, CA Garden Project research team including our colleagues, Joanne Hild, M. Katy Janes, Dr. Peggy Reynolds, Annika Alexander-Ozinskas, and Allison Hacker. We would also like to thank and Dr. A. Eduardo Sáez, Dr. Robert A. Root, and Barbara Moore from the University of Arizona for their help in preparation of this manuscript.
Footnotes
Declaration of interest: None
Supplementary Material
Supplemental Materials Include: Gardening Description Survey (Supplemental Material A), Food Frequency Questionnaire (Supplemental Material B), and the 24-Hour Recall (Supplemental Material C). Below is digital access to Supplemental Materials (A-B). All Supplemental Materials (A-C) were also submitted with manuscript.
Digital access to Supplemental Material A (English): https://uarizona.co1.qualtrics.com/jfe/form/SV_e2nO9QPspLLo68t
Digital access to Supplemental Material B (English/Spanish): https://uarizona.co1.qualtrics.com/jfe/form/SV_8HdFSBF4OMVpw3z
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Agency for Toxic Substances and Disease Registry (ATSDR). (2015a). ToxFAQsTM for Arsenic. Last Updated on March 12, 2015 https://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=19&tid=3
- ATSDR. (2015b). ToxFAQsTM for Cadmium. Last Updated on March 12, 2015 https://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=47&tid=15
- ATSDR. (2012). Toxicological profile for Cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- Alam M, et al. (2003). “Arsenic and Heavy Metal Contamination of Vegetables Grown in Samta Village, Bangladesh.” Science of The Total Environment, vol. 308, no. 1-3, pp. 83–96., doi: 10.1016/s0048-9697(02)00651-4. [DOI] [PubMed] [Google Scholar]
- Alpers CN (2017). Arsenic and mercury contamination related to historical gold mining in the Sierra Nevada, California. Geochemistry: Exploration, Environment, Analysis, 17(2), 92–100. 10.1144/geochem2016-018 [DOI] [Google Scholar]
- Ávila PF, Ferreira da Silva E, & Candeias C (2017). Health risk assessment through consumption of vegetables rich in heavy metals: the case study of the surrounding villages from Panasqueira mine, Central Portugal. Environmental Geochemistry and Health, 39(3), 565–589. 10.1007/s10653-016-9834-0 [DOI] [PubMed] [Google Scholar]
- Bian B, Zhou LJ, Li L, Lv L, & Fan YM (2015). Risk assessment of heavy metals in air, water, vegetables, grains, and related soils irrigated with biogas slurry in Taihu Basin, China. Environmental Science and Pollution Research, 10.1007/s11356-015-4292-2 [DOI] [PubMed] [Google Scholar]
- Baxter SD, Thompson WO, Davis HC, Johnson MH, (1997). Impact of Gender, Ethnicity, Meal Component, and Time Interval Between Eating and Reporting on Accuracy of Fourth-Grader s’ Self-Reports of School Lunch. J. Am. Diet. Assoc 97, 1293–1298. doi: 10.1016/S0002-8223(97)00309-X [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, et al. (2010). “Arsenic Contamination in Rice, Wheat, Pulses, and Vegetables: A Study in an Arsenic Affected Area of West Bengal, India” SpringerLink, Springer; Netherlands, 9 March 2010, link.springer.com/article/10.1007/s11270-010-03619. [Google Scholar]
- Brown C (2003). Consumers’ preferences for locally produced food: A study in southeast Missouri. American Journal of Alternative Agriculture, 18, 213–224. 10.1079/AJAA200353 [DOI] [Google Scholar]
- Bunzl K, et al. (2001). “Availability of Arsenic, Copper, Lead, Thallium, and Zinc to Various Vegetables Grown in Slag-Contaminated Soils.” Journal of Environment Quality, vol. 30, no. 3, 2001, p. 934., doi: 10.2134/jeq2001.303934x. [DOI] [PubMed] [Google Scholar]
- Cai M, McBride MB, & Li K (2016). Bioaccessibility of Ba, Cu, Pb, and Zn in urban garden and orchard soils. Environmental Pollution, 208, 145–152. 10.1016/j.envpol.2015.09.050 [DOI] [PubMed] [Google Scholar]
- Castro-Larragoitia J, Kramar U, Puchelt H (1997). 200 years of mining activities at La Paz, San Luis Potosi, Mexico - consequences for environment and geochemical exploration. J Geochem Explor, 58:81–91. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (1984). National Center for Health Statistics (NCHS) Hispanic Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2016). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2015 [Google Scholar]
- Chen Y, Reddy RM, McLaughlin MA, Taher FA, Yettella RR, Uhlig S, & Blaul C (2014). Method validation and proficiency testing for determination of total arsenic in apple juice by inductively coupled plasma/ mass spectrometry. Journal of AOAC International, 97(4), 1143+. Retrieved from http://link.galegroup.com.ezproxy3.Library.arizona.edu/apps/doc/A382086459/AONE?u=uarizona_main&sid=AONE&xid=d20e3c63 [DOI] [PubMed] [Google Scholar]
- Chunhabundit R (2016). Cadmium exposure and potential health risk from foods in Contaminated area, Thailand. Toxicological Research, 32(1), 65–72. 10.5487/TR.2016.32.1.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb GP, Sands K, Waters M, Wixson BG, & Dorward-King E (2000). Accumulation of Heavy Metals by Vegetables Grown in Mine Wastes. Environmental Toxicology and Chemistry, 19(3), 600–607. [Google Scholar]
- Csavina J, Field J, Taylor MP, Gao S, Landázuri A, Betterton EA, & Sáez AE (2012). A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Science of the Total Environment, 58–73. 10.1016/j.scitotenv.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csavina J, Landázuri A, Wonaschütz A, Rine K, Rheinheimer P, Barbaris B, … Betterton EA (2011). Metal and Metalloid Contaminants in Atmospheric Aerosols from Mining Operations. Water, Air, & Soil Pollution, 221(1–4), 145–157. 10.1007/s11270-011-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/Hhanes/Default.aspx
- Feldmann C, & Hamm U (2015). Consumers’ perceptions and preferences for local food: A review. Food Quality and Preference, 40(PA), 152–164. 10.1016/j.foodqual.2014.09.014 [DOI] [Google Scholar]
- Ferri R, Hashim D, Smith DR, Guazzetti S, Donna F, Ferretti E, … Lucchini RG (2015). Metal contamination of home garden soils and cultivated vegetables in the province of Brescia, Italy: Implications for human exposure. Science of the Total Environment, 518–519, 507–517. 10.1016/j.scitotenv.2015.02.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DA, Vaudrin N, Schneider C, Trapl E, Ohri-vachaspati P, Taggart M, … Flocke S (2016). Systematic Review of Factors Influencing Farmers’ Market Use Overall and among Low-Income Populations. Journal of the Academy of Nutrition and Dietetics, 116(7), 1136–1155. 10.1016/j.jand.2016.02.010 [DOI] [PubMed] [Google Scholar]
- Garcia RA, Taren D, Teufel NI, (2000). Factors associated with the reproducibility of specific food items from the southwest food frequency questionnaire. Ecol. Food Nutr 38, 549–561. doi: 10.1080/03670244.2000.9991596 [DOI] [Google Scholar]
- Gaw SK, et al. (2008). “Uptake of ΣDDT, Arsenic, Cadmium, Copper, and Lead by Lettuce and Radish Grown in Contaminated Horticultural Soils.” Journal of Agricultural and Food Chemistry, vol. 56, no. 15, pp. 6584–6593., doi: 10.1021/jf073327t. [DOI] [PubMed] [Google Scholar]
- Gonzalez RC, Gonzalez-Chavez MCA (2006). Metal accumulation in wild plants surrounding mining wastes: Soil and Sediment Remediation. Environ Pollut, 144:84–92. [DOI] [PubMed] [Google Scholar]
- Grebitus C, Lusk JL, & Nayga RM (2013). Effect of distance of transportation on willingness to pay for food. Ecological Economics, 88, 67–75. 10.1016/j.ecolecon.2013.01.006 [DOI] [Google Scholar]
- Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC (1999). Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr 69, 243–9. [DOI] [PubMed] [Google Scholar]
- Johnson-kozlow M, Matt GE, Rock CL, Rosa R, De, Conway TL, Romero RA, (2011). Assessment of Dietary Intakes of Filipino-Americans: Implications for Food Frequency Questionnaire Design. J. Nutr. Educ. Behav 43, 505–510. doi: 10.1016/j.jneb.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, et al. 2018. “The Lancet Commission on Pollution and Health.” Lancet 391(10119): 462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Lee YZ, Suzuki S, Kawada T, Wang J, Koyama H, Rivai IF, & Herawati N (1999). Content of cadmium in carrots compared with rice in Japan. Bulletin of Environmental Contamination and Toxicology, 10.1007/s001289901038 [DOI] [PubMed] [Google Scholar]
- Li Yu, et al. (2006). “Risk Assessment of Heavy Metals in Soils and Vegetables around Non-Ferrous Metals Mining and Smelting Sites, Baiyin, China.” Journal of Environmental Sciences, vol. 18, no. 6, pp. 1124–1134., doi: 10.1016/s1001-0742(06)60050-8. [DOI] [PubMed] [Google Scholar]
- Liu P, Wang C, Song X, & Wu Y (2010). Dietary intake of lead and cadmium by children and adults – Result calculated from dietary recall and available lead/cadmium level in food in comparison to result from food duplicate diet method. International Journal of Hygiene and Environmental Health, 213(6), 450–457. 10.1016/j.ijheh.2010.07.002 [DOI] [PubMed] [Google Scholar]
- Manjón I, Ramirez-Andreotta MD, Saez AE, Root RA, Hild J, Janes K, Alexander-Ozinskas A Ingestion and Inhalation of Metal(loid)s Through Preschool Gardening: An Exposure and Risk Assessment in Legacy Mining Communities. In press, Science of the Total Environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack LA, Laska MN, Larson NI, & Story M (2010). Review of the Nutritional Implications of Farmers’ Markets and Community Gardens: A Call for Evaluation and Research Efforts. Journal of the American Dietetic Association, 110(3), 399–408. 10.1016/j.jada.2009.11.023 [DOI] [PubMed] [Google Scholar]
- Morrow H 2001. Cadmium and cadmium alloys In: Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons, Inc., 471–507.http://www.mrw.interscience.wiley.com/emrw/9780471238966/kirk/article/cadmcarr.a01/current/pdf?hd=All%2Ccadmium. April 29, 2008. [Google Scholar]
- National Cancer Institute. (2010). National Institute of Health, Epidemiology and Genomics Research Program. Diet History Questionnaire, Version 2.0. [Google Scholar]
- Newton K, Amarasiriwardena D, & Xing B (2006). Distribution of soil arsenic species, lead and arsenic bound to humic acid molar mass fractions in a contaminated apple orchard. Environmental Pollution, 143(2), 197–205. [DOI] [PubMed] [Google Scholar]
- Probst Y, Tapsell L, (2007). Over- and underreporting of energy intake by patients with metabolic syndrome using an automated dietary assessment website. Nutr. Diet 64, 280–284. doi: 10.1111/j.1747-0080.2007.00220.x. [DOI] [Google Scholar]
- Racine EF, Mumford EA, Laditka SB, & Lowe AE (2013). Understanding Characteristics of Families Who Buy Local Produce. Journal of Nutrition Education and Behavior, 45(1), 30–38. 10.1016/j.jneb.2012.04.011 [DOI] [PubMed] [Google Scholar]; Thompson Frances E., & Subar Amy F. (2001). Dietary Assessment Methodology Chapter 1. In Nutrition in the Prevention and Treatment of Disease (pp. 3–30). [Google Scholar]
- Ramirez-Andreotta MD, Brusseau ML, Artiola JF, & Maier RM (2013a). A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Science of the Total Environment, 443, 299–306. 10.1016/j.scitotenv.2012.10.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Andreotta MD, Brusseau ML, Beamer P, & Maier RM (2013b). Home gardening near a mining site in an arsenic-endemic region of Arizona: Assessing arsenic exposure dose and risk via ingestion of home garden vegetables, soils, and water. Science of the Total Environment, 454–455, 373–382. 10.1016/j.scitotenv.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Andreotta MD, Tapper A, Clough D, Carrera JS, & Sandhaus S (2019). Understanding the Intrinsic and Extrinsic Motivations Associated with Community Gardening to Improve Environmental Public Health Prevention and Intervention. International Journal of Environmental Research and Public Health, 16(3), 494 10.3390/ijerph16030494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhone A, Ver Ploeg M, Dicken C, Williams R, & Breneman V (2017). Low-Income and Low-Supermarket-Access Census Tracts, 2010-2015, EIB-165, U.S. Department of Agriculture, Economic Research Service. [Google Scholar]
- Shim J-S, Oh K, Kim HC, (2014). Dietary assessment methods in epidemiologic studies. Epidemiol. Health e2014009. doi: 10.4178/epih/e2014009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Juhasz AL, & Weber J (2009). Arsenic uptake and speciation in vegetables grown under greenhouse conditions. Environmental Geochemistry and Health. 10.1007/s10653-008-9242-1 [DOI] [PubMed] [Google Scholar]
- Thompson FE, Dixit-Joshi S, Potischman N, Dodd KW, Kirkpatrick SI, Kushi LH, … Subar AF (2015). Comparison of Interviewer-Administered and Automated Self-Administered 24-Hour Dietary Recalls in 3 Diverse Integrated Health Systems. American Journal of Epidemiology, 181(12), 970–978. 10.1093/aje/kwu467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FE, & Subar AF (2017). Dietary Assessment Methodology Chapter 1. In Nutrition in the Prevention and Treatment of Disease (pp. 3–30). [Google Scholar]
- Thompson FE, Subar AF, Loria CM, Reedy JL, Baranowski T, (2010). Need for Technological Innovation in Dietary Assessment. J. Am. Diet. Assoc 110, 48–51. doi: 10.1016/j.jada.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau, Population Division. (2010). QuickFacts. Nevada County, California; Grass Valley city, California: Retrieved from: https://www.census.gov/quickfacts/fact/table/nevadacountycalifornia,grassvalleycitycalifornia/PST045218 [Google Scholar]
- U.S. Census Bureau. (2017). U.S. Census Bureau QuickFacts: Nevada County, California; California: Retrieved April 11, 2019, from https://www.census.gov/quickfacts/fact/table/nevadacountycalifornia,ca/PST045218. [Google Scholar]
- U.S. Department of Agriculture (USDA), Economic Research Service (ERS). (Updated 2017). Food Access Research Atlas, https://www.ers.usda.gov/data-products/food-access-research-atlas/
- U.S. Department of Agriculture (USDA) (2018). What We Eat in America NHANES 2015-2016, individuals 2 years and over (excluding breast-fed children), day 1 dietary intake data, weighted. Food Patterns Equivalents Database (FPED) 2015-2016; Available at: www.ars.usda.gov/nea/bhnrc/fsrg. [Google Scholar]
- U.S. EPA. (2011). Exposure Factors Handbook 2011 Edition (Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F, 2011. [Google Scholar]
- U.S. EPA. (2018). Exposure Factors Handbook Chapter 9 (Update): Intake of Fruits and Vegetables. U.S. EPA Office of Research and Development, Washington, DC, EPA/600/R-18/098F. [Google Scholar]
- Ver Ploeg M, Nulph D, & Williams R (2011). USDA ERS - Mapping Food Deserts in the United States. Retrieved June 18, 2019, from https://www.ers.usda.gov/amber-waves/2011/december/data-feature-mapping-food-deserts-in-the-us/ [Google Scholar]
- Walton J, (2015). Dietary Assessment Methodology for Nutritional Assessment. Top. Clin. Nutr 30, 33–46. doi: 10.1097/TIN.0000000000000018 [DOI] [Google Scholar]
- Warren GP, Alloway BJ, Lepp NW, Singh B, Bochereau FJM, & Penny C (2003). Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides. Science of the Total Environment, 311(1–3), 19–33. 10.1016/S0048-9697(03)00096-2 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2018). Arsenic. Retrieved June 18, 2019, from https://www.who.int/news-room/fact-sheets/detail/arsenic
- Yu G, Zheng W, Wang W, Dai F, Zhang Z, Yuan Y, & Wang Q (2017). Health risk assessment of Chinese consumers to Cadmium via dietary intake. Journal of Trace Elements in Medicine and Biology. 10.1016/j.jtemb.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang Y, Zhang Z, Wang D, Luo C, Xu F (2015). Health Risk Assessment of Heavy Metals and As in Vegetable and Soil System in Chongqing, Southwest of China. Journal of Residuals Science and Technology, 12(4), 231–240. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.