Abstract
Objective.
Fatigue is consistently ranked as one of the most problematic symptoms in systemic sclerosis (SSc), but the impact of fatigue on daily life is not well-characterized. The purpose of this study was to examine fatigue’s contribution to deficits in social participation, functioning, and quality of life.
Methods.
Baseline data from a sample undertaking a clinical trial were utilized (N = 267). Fatigue, pain interference, depressive symptoms, physical function, and social participation were assessed by PROMIS measures. Hierarchical linear regressions were performed to determine fatigue’s unique contribution to social participation, physical function, and quality of life, above and beyond the effects of demographic and clinical variables, pain interference, and depressive symptoms.
Results.
The sample was predominantly female (91%), with average age 53.7 years, average disease duration of 9 years, and mean fatigue T-score of 58.7. Of all outcomes, fatigue was most strongly associated with deficits in social participation, explaining 48% of the variance beyond demographic and clinical factors, which is similar to the amount of variance contributed by pain interference and depressive symptoms combined (49%). Fatigue also accounted for significant amounts of variance in physical function and quality of life (R2 = .27 and .33 respectively) above and beyond the effects of demographic and clinical factors.
Conclusion.
Fatigue is an important clinical problem in SSc and is strongly associated with decreased participation in social roles and activities. Rehabilitation interventions that focus on fatigue management may be necessary to maximize participation.
Systemic sclerosis (SSc) is a rare autoimmune disease associated with vascular damage and tissue fibrosis that affects the skin and internal organs (1–3). In the US, it affects between 13.5 – 39.9 per 100,000 people (4). In addition to the classic skin hardening that restricts movement, a major complaint of people with SSc is the substantial symptom burden. Symptoms such as fatigue, pain, and depressive symptoms are common, and because SSc is diagnosed in early to middle age and has no cure, individuals with SSc face many years of managing the manifestations of a complex and progressive condition (5).
Symptoms in SSc significantly disrupt daily activities and diminish quality of life (6–9). Of the symptoms experienced, fatigue has been consistently ranked as one of the most problematic (6, 7, 10–12). Fatigue in SSc is significantly greater than what is experienced by the general population, similar to other rheumatological conditions and those who are actively in cancer treatment (8,9,12). Fatigue affects many facets of life, diminishing the ability to perform usual tasks (7, 13), engage in meaningful activities (7, 14), perform work duties (15, 16) and fulfill family responsibilities (14, 17, 18). The debilitating nature of fatigue has prompted a call for research to better understand fatigue and its correlates (6, 7, 9, 14) in order to better address this symptom to reduce disability and improve quality of life.
To better understand the contribution of fatigue to functioning and quality of life in SSc, we examined baseline data from a sample of participants who undertook a clinical trial investigating the effectiveness of an internet-based self-management program (19). The purpose of this study was to examine fatigue’s contribution to deficits in social participation, functioning, and quality of life in people with SSc. We hypothesized that fatigue would be the strongest unique contributor to each of these outcomes in multivariable models that included other symptoms (pain interference and depressive symptoms), clinical variables, and demographics.
PATIENTS AND METHODS
Procedure.
Adults with SSc were recruited to participate in a randomized controlled trial designed to evaluate the efficacy of an internet-based chronic disease self-management program (19). People were recruited from two universities (in the Midwest and South Eastern United States) as well as from websites and social media from national SSc foundations. To be included in the trial, people needed to be US residents, report a diagnosis of SSc, be 18 years or older, have basic computer literacy and access to a computer with internet and email capabilities, be able to communicate in English, and be willing to complete the study procedures. All participants provided informed consent. After informed consent was obtained, participants were sent a Qualtrics survey to complete baseline assessments examined in this secondary data analysis. The study was approved by institutional human subjects review boards at the University of New Mexico, University of Michigan, and Medical University of South Carolina.
Measures
Fatigue.
Fatigue was measured using the 4 items from the fatigue subscale of the Patient Reported Outcomes Measurement Information System (PROMIS) 29 version 2.0 (v.2). The PROMIS 29 v.2 contains several scales used in this analysis which have been validated in a large international sample of people with SSc (20). Items are referenced for the past 7 days and rated on a scale from 1 (not at all) to 5 (very much): 1) I feel fatigued; 2) I have trouble starting things because I am tired; 3) How run-down did you feel on average? and 4) How fatigued were you on average? Ratings were converted to a T score metric which standardized the ratings to the US population in which the mean is 50 and standard deviation is 10. A higher score indicates worse fatigue.
Outcomes
Social Participation.
The Ability to Participate in Social Roles and Activities scale was part of the PROMIS 29 and consists of 4 items. On a scale of 5 (Never) to 1 (Always), participants were asked to rate the following: 1) I have trouble doing all of my regular leisure activities with others 2) I have trouble doing all of the family activities that I want to do; 3) I have trouble doing all of my usual work (include work at home) and 4) I have trouble doing all of the activities with friends that I want to do. Scores were converted to T scores for analysis. A higher score indicates better ability.
Physical Function.
The PROMIS 29 v.2 has a physical function scale with 4 items. On a scale of 5 (without any difficulty) to 1 (unable to do), participants were asked to rate the following: 1) Are you able to do chores such as vacuuming or yard work? 2) Are you able to go up and down stairs at a normal pace? 3) Are you able to go for a walk of at least 15 minutes? and 4) Are you able to run errands and shop? A higher score indicates better physical function.
Quality of Life.
The EuroQol 5-domain instrument (EQ-5D-5L) is a generic health-related quality of life assessment commonly used in samples with various chronic conditions (21, 22). It has domains of mobility, self-care, activity, pain, and anxiety. Participants are asked to rate their health state on a scale of no problems, slight problems, moderate problems, severe problems and extreme problems. Responses are then transformed to a metric of health utility using an algorithm in which scores range from 0.0 (death) to 1.0 (full/optimal health).
Demographic and Clinical Characteristics
Demographic information included age, race, ethnicity, sex, education level, marital status, and employment status. Clinical characteristics included scleroderma type (limited/CREST/sine, diffuse, or overlap), and disease duration (measured as the year diagnosed). Self-rated health was ascertained through one question in which participants rated their overall health as excellent, very good, good, fair, or poor.
Other Symptoms
Pain interference and Depressive symptoms.
Both of these symptoms were assessed from the PROMIS scales from the PROMIS 29 v.2. Pain interference was assessed by 4 items. In the past 7 days, participants rated pain interference on a scale of 1 (not at all) to 5 (very much) in the following questions: 1) How much did pain interfere with your day to day activities? 2) How much did pain interfere with work around the home? 3) How much did pain interfere with your ability to participate in social activities? and 4) How much did pain interfere with your household chores? Depressive symptoms were also assessed in the past 7 days. On a scale of 1 (never) to 5 (always), participants rated the following: 1) I felt worthless; 2) I felt helpless; 3) I felt depressed; and 4) I felt hopeless. Higher scores on these scales indicated worse symptoms.
Data Analysis
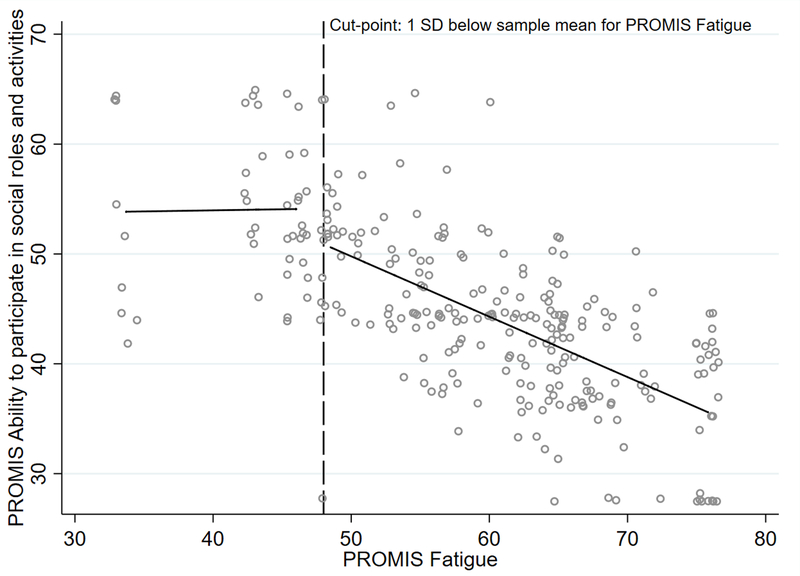
Descriptive statistics were used to characterize the sample. We used frequency and proportion for categorical variables, means and standard deviations for normally distributed continuous data and median and interquartile ranges for non-normally distributed continuous data. The association between fatigue (T score from the PROMIS measure) and three outcome variables was investigated in three separate hierarchical multivariable linear regression analyses with outcome variables: social participation, physical function, and quality of life. For each outcome, 3 models were constructed to examine the relative contributions of fatigue and other symptoms (pain interference and depressive symptoms) above and beyond demographic and clinical variables. This method allowed us to examine the unique contribution of fatigue and the set of other symptoms respectively to the model variance without the influence of each other. It also allowed for comparison across models given the difference in order of entry. In Model 1, demographic and clinical variables (age, gender, race, scleroderma subtype, and years since scleroderma diagnosis) were entered in Block 1 and fatigue was entered in Block 2. In Model 2, demographic and clinical variables were entered in Block 1, fatigue in Block 2, and pain interference and depressive symptoms in Block 3. Model 2 was performed to examine how much the symptom of fatigue explained the variance in each outcome above and beyond clinical factors, and how much unique variance is then explained by pain interference and depressive symptoms. In Model 3 the order of entry of the pain interference and depressive symptoms block and the fatigue block were reversed. Model 3 was performed to examine how much unique variance fatigue adds to the model above and beyond demographic and clinical variables and symptoms of pain interference and depressive symptoms. R2 values indicated the amount of variance in the outcomes attributable to the variable blocks entered into the models. To depict the unadjusted relationship between fatigue and social participation, a scatter plot with overlaid best-fitting lines was constructed, estimated using ordinary least squares piecewise regression. We pre-specified a cut-point of 1SD below the sample fatigue T-score mean.
RESULTS
The characteristics of the sample have been reported in detail elsewhere (19). In brief, the sample was predominantly female (91%), the mean age was 53.7 years (SD 11.7), and consisted of 17% racial/ethnic minorities (non-white). Almost three quarters of the sample (74%) had academic degrees or professional qualifications with a mean of 16 years of education; 64% were married, and 42% reported working part or fulltime. For the scleroderma subtype reported by participants, 45% had limited cutaneous systemic sclerosis or sine scleroderma; 43% had diffuse cutaneous systemic sclerosis; 12% had scleroderma overlapping with another rheumatic disease, and 0.4% (n = 1) did not know the subtype. Time since diagnosis was a median of 9 years with an interquartile range of 5 – 16.
Table 1 shows the values for reported functioning, health, and symptom measures. 43.9% of the sample rated their overall health to be fair or poor. Fatigue was the worst rated symptom (Mean T = 58.7 or 0.87 SD above the US Population) followed by pain interference (Mean T = 58.0). Mean anxiety, sleep disturbance, and depressive symptoms were all within .5 standard deviations of the normative sample mean (T = 50). Using one way analyses of variance or chi-square tests to examine differences across SSc subtype, only fatigue, sleep disturbance, and self-rated health were significantly different (p ≤ 0.05). Participants with overlap SSc had the highest levels of fatigue and sleep disturbance, and the highest proportion of people who rated their health as fair or poor (51.6%). Participants with diffuse SSc also had a high proportion of people who rated their health as fair or poor (50.5%), but their mean fatigue and sleep disturbance levels were similar to those with limited or sine SSc.
Table 1.
Sample Reported Symptoms, Functioning, and Quality of Life (N = 267)
| Measures | Overall Sample | Diffuse SSc N=115 |
Limited SSc N=120 |
Overlap SSc N=31 |
|---|---|---|---|---|
| Fatigue* | 58.7 (10.4) | 57.5 (10.1) | 58.4 (10.4) | 63.7 (10.1) |
| Pain interference | 58.0 (9.3) | 56.9 (9.7) | 58.0 (8.8) | 61.4 (8.9) |
| Pain intensity (0–10 NRS) | 4.2 (2.2) | 3.9 (2.3) | 4.2 (2.1) | 4.9 (2.2) |
| Depressive symptoms | 51.3 (9.8) | 51.3 (10.1) | 51.3 (10.0) | 51.6 (8.7) |
| Anxiety | 54.0 (10.0) | 53.4 (9.9) | 54.4 (10.1) | 54.7 (10.5) |
| Sleep disturbance * | 53.7 (6.5) | 53.9 (6.5) | 52.5 (5.7) | 57.0 (8.2) |
| Social Participation | 45.0 (8.2) | 44.9 (8.0) | 45.8 (8.5) | 43.3 (7.2) |
| Quality of Life EQ-5D-5L | 0.78 (0.08) | 0.78 (0.08) | 0.79 (0.08) | 0.77 (0.07) |
| Self-rated health (n, %)* | ||||
| Excellent | 3 (1.1%) | 3 (2.6%) | 0 (0%) | 0 (0%) |
| Very good | 33 (12.4%) | 16 (13.9%) | 15 (12.5%) | 1 (3.2%) |
| Good | 114 (42.7%) | 38 (33.0%) | 62 (51.7%) | 14 (45.2%) |
| Fair | 100 (37.5%) | 51 (44.4%) | 36 (30.0%) | 13 (41.9%) |
| Poor | 17 (6.4%) | 7 (6.1%) | 7 (5.8%) | 3 (9.7%) |
Note. The PROMIS 29 v.2 was used which comprised scales of Fatigue, Pain Interference, Pain Intensity, Depressive symptoms, Anxiety, Sleep Disturbance, Ability to Participate in Social Roles (Social Participation), and Physical Function. Unless otherwise noted, statistics represented in the table show the mean (standard deviation) for each variable.
p ≤ 0.05 difference among SSc subtypes
Fatigue and Social Participation
Table 2 shows results from hierarchical regression models where fatigue and other variables were examined as predictors of social participation. In Model 1, 50% of the variance in social participation was explained by demographic and clinical factors, which contributed a negligible amount (2%) of variance and by fatigue, which accounted for nearly half (48%) of the variance. Of the demographic and clinical factors, age and the diffuse SSc subtype demonstrated significant independent negative associations with social participation. When pain interference and depressive symptoms were added in a block after fatigue (Model 2), a further increase of 11% of variance in the outcome was explained by these symptoms. In the Model 3, fatigue accounted for a significant amount of variance (9%) above and beyond the effects of pain interference and depressive symptoms combined (49% of variance). Regardless of order of entry, the models accounted for approximately 60% of the variance in social participation.
Table 2.
The Association of Fatigue with Ability to Participate in Social Roles
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Block | B | ΔR2 | Block | B | ΔR2 | Block | B | ΔR2 | |
| Constant | 82.83* | 94.93* | 94.93* | ||||||
| Demographics/ Clinical Factors | 1 | .02 | 1 | .02 | 1 | .02 | |||
| Age | −0.10* | −0.12* | −0.12* | ||||||
| Female | 1.62 | 1.51 | 1.51 | ||||||
| Minority | 1.03 | 1.51 | 1.51 | ||||||
| Diffuse SSc† | −1.49* | −1.65* | −1.65* | ||||||
| Overlap SSc† | 0.10 | −0.19 | −0.19 | ||||||
| Diagnosis year | −0.04 | −0.03 | −0.03 | ||||||
| Fatigue | 2 | −0.56* | .48* | 2 | −0.32* | .48* | 3 | −0.28* | .09* |
| Pain interference | 3 | −0.28* | .11* | 2 | −0.16* | .49* | |||
| Depressive symptoms | −0.16* | 0.32* | |||||||
| Total Model R2 | .50 | .61 | .60 | ||||||
Note. Fatigue, Ability to Participate in Social Roles and Activities, Pain Interference, and Depressive symptoms are scales taken from the PROMIS 29 v.2. Hierarchical regression models were constructed with variable(s) entered in blocks. Beta coefficients included in the table are from full models; ΔR2 is shown for Pain Interference and Depressive symptoms in combination as they were entered together in a block. N=266 in all models (1 participant had missing data for SSc type).
Reference group: Limited or sine scleroderma
p ≤ .05
Figure 1 shows the unadjusted association between fatigue and social participation, with the best-fit line segmented at 1SD below the sample mean (fatigue mean T score 48). In this graph, the negative association between fatigue and social participation is only seen when people have fatigue that is approximately at the mean or greater (T-score of 48 or higher). Fatigue was not associated with social participation for people with low fatigue.
Figure 1. Unadjusted Relationship between Fatigue and Social Participation.
Note. Social participation is measured by the PROMIS Ability to Participate in Social Roles and Activities. Both axes depict T scores. The cut-point used (depicted by the dotted line above) is 1 SD below the sample mean for PROMIS Fatigue (T = 48).
Fatigue and Physical Function
Table 3 shows the results from the hierarchical regression models where physical function was the outcome. In Model 1, 30% of the variance in physical function was explained by demographic and clinical factors (3% combined) and fatigue (27% of the variance). Age and diffuse SSc were significantly negatively associated with physical function and depressive symptoms. In Model 2, fatigue accounted for a significant and substantial amount of variance in physical function (27%); pain and depressive symptoms added a significant amount of variance above and beyond the effect of fatigue on physical function. In Model 3, pain interference and depressive symptoms accounted for a substantial and significant amount of variance in physical function (37%); fatigue added a statistically significant though small amount of variance in physical functioning when added in the third step. The models accounted for 43% of the variance in self-reported physical function.
Table 3.
The Association of Fatigue with Physical Function
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Block | B | ΔR2 | Block | B | ΔR2 | Block | B | ΔR2 | |
| Constant | 66.41* | 76.51* | 76.51* | ||||||
| Demographics/ Clinical Factors | 1 | .03 | 1 | .03 | 1 | .03 | |||
| Age | −0.07* | −0.09* | −0.09* | ||||||
| Female | 0.84 | 1.13 | 1.13 | ||||||
| Minority | 0.47 | 1.00 | 1.00 | ||||||
| Diffuse SSc† | −1.84* | −2.05* | −2.05* | ||||||
| Overlap SSc† | −1.23 | −1.15 | −1.15 | ||||||
| Diagnosis year | −0.07 | −0.06 | −0.06 | ||||||
| Fatigue | 2 | −0.36* | .27* | 2 | −0.16* | .27* | 3 | −0.16* | .03* |
| Pain interference | 3 | −0.35* | .13* | 2 | −0.35* | .37* | |||
| Depressive symptoms | −0.03 | −0.03 | |||||||
| Total Model R2 | .30 | .43 | .43 | ||||||
Note. Fatigue, Physical Function, Pain Interference, and Depressive symptoms are scales taken from the PROMIS 29 v.2. Hierarchical regression models were constructed with variable(s) entered in blocks. Beta coefficients included in the table are from full models; ΔR2 is shown for Pain Interference and Depressive symptoms in combination as they were entered together in a block. N=266 in all models (1 participant had missing data for SSc type).
Reference group: Limited or sine scleroderma
p ≤ .05
Fatigue and Quality of Life
Table 4 shows the results from the hierarchical regression models where quality of life was the outcome. In Model 1, 35% of the variance in quality of life was explained by demographic and clinical factors and fatigue; as in prior models, demographic and clinical variables accounted for very small amounts of the variance in quality of life (2%), whereas fatigue accounted for 33% of the variance. Of the demographic factors, diffuse SSc was significantly associated with lower quality of life. In Model 2, pain interference and depressive symptoms contributed an additional 21% variance in quality of life, above and beyond the effects of fatigue. In contrast, in Model 3, fatigue only contributed an additional 1% variance in quality of life, above the variance explained by pain interference and depressive symptoms, which accounted for 53% of the variance in quality of life. These models explained 56% of the variance in quality of life and depressive symptoms.
Table 4.
The Association of Fatigue with Quality of Life
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Block | B | ΔR2 | Block | B | ΔR2 | Block | B | ΔR2 | |
| Constant | 1.05* | 1.22* | 1.22* | ||||||
| Demographics/ Clinical Factors | 1 | .02 | 1 | .02 | 1 | .02 | |||
| Age | −0.0001 | −0.0004 | −0.0004 | ||||||
| Female | 0.02 | 0.02 | 0.02 | ||||||
| Minority | −0.004 | 0.002 | 0.002 | ||||||
| Diffuse SSc† | −0.01 | −0.02* | −0.02* | ||||||
| Overlap SSc† | −0.004 | −0.008 | −0.008 | ||||||
| Diagnosis year | −0.0004 | −0.0003 | −0.0003 | ||||||
| Fatigue | 2 | −0.005* | .33* | 2 | −0.001* | .33* | 3 | −0.001* | .01* |
| Pain interference | 3 | −0.004* | .21* | 2 | −0.004* | .53* | |||
| Depressive symptoms | −0.002* | −0.002* | |||||||
| Total Model R2 | .35 | .56 | .56 | ||||||
Note. Quality of Life was measured using the EuroQol 5D-5L instrument. Fatigue, Pain Interference, and Depressive symptoms are scales taken from the PROMIS 29 v.2. Hierarchical regression models were constructed with variable(s) entered in blocks. Beta coefficients included in the table are from full models; ΔR2 is shown for Pain Interference and Depressive symptoms in combination as they were entered together in a block. N=266 in all models (1 participant had missing data for SSc type).
Reference group: Limited or sine scleroderma
p ≤ .05
DISCUSSION
Fatigue is a symptom often described in the literature as debilitating by people with SSc (6, 10, 11), but it is not yet clear what aspects of functioning and quality of life are most affected by fatigue and other symptoms. In this study, our objective was to examine fatigue’s contribution to deficits in social participation, functioning, and quality of life. To accomplish this, we examined the relative contributions of fatigue above and beyond demographics and clinical factors and other symptoms (pain interference and depression).
We have three main findings from this study. First, of all outcomes assessed, fatigue was most strongly associated with decreased ability to participate in social roles and activities. Fatigue alone accounted for nearly the same amount of variance in social participation (R2 = .48 in Table 2 Model 2) as pain interference and depressive symptoms combined (R2 = .49 in Table 2 Model 3). Furthermore, the substantial amount of unique variance that fatigue explained over and above symptoms of pain interference and depressive symptoms suggest that fatigue is particularly influential with regard to reduced social participation. These findings are in contrast to those of Sandusky et al. who reported that fatigue was not a significant correlate for social participation after controlling for depressive symptoms (7) and Poole et al. (24) using a single VAS measure for fatigue, who reported no difference in social participation with higher levels of fatigue. However, there are several key differences in the measurement of social participation between the current study and those studies. Sandusky et al. measured social participation via social networks and relationships as opposed to participation in particular activities, and Poole et al. measured social participation by ascertaining frequency of performance of activities, such as gardening, household maintenance, and shopping, and counted higher frequency as better participation. In the current study, social participation was measured using the PROMIS social participation scale which assesses the perception of difficulty in usual social activities and factors in the whether participation is above or below what the individual wants to do. In addition, the PROMIS social participation has been validated and has stronger psychometric properties compared to the instruments used in the prior studies. Lastly, differences in this study’s sample and those studies may also affect the comparisons. For instance, in the Sandusky et al study, a higher proportion of people reported having a high school education or less (32%) in relation to this sample (20%).
One reason why fatigue may have a strong negative association to social participation is because work limitations are included in the social participation measure. In SSc, fatigue is a strong correlate of work disability (25,26), and baseline fatigue severity was a main predictor of work disability in a longitudinal study (27). This study’s findings showing a strong negative association between fatigue and social participation is similar to that of studies in another chronic condition, multiple sclerosis (28,29). In those studies, pain and depressive symptoms are also important factors in decreased physical function and quality of life.
Our findings have implications for both assessment and intervention development. While clinical assessment often includes measures of physical function, it appears important to include measures of social participation when assessing patients with SSc, especially if they report high fatigue. In addition, the assessment used to measure fatigue is an important consideration also as some assessments such as the Multidimensional Fatigue Inventory and Multidimensional Assessment of Fatigue Scale include items asking about the impact of fatigue on participation. Assessment of social participation may reveal areas for intervention that would be appropriate for rehabilitation professionals to address, such as workplace adaptation, and also supports the idea that fatigue management is necessary in this population, similar to others recommendations (6, 7, 9, 12, 14).
Second, although fatigue accounted for about one third of the variance in physical function and quality of life outcomes when entered in the models prior to the addition of pain interference and depressive symptoms, fatigue did not significantly contribute to the variance in physical function and quality of life after these symptoms were included in the models (only 1% and 3% respectively). The findings suggest that interventions to impact physical function and quality of life need to be multifaceted and include strategies to reduce pain and depressive symptoms in addition to fatigue management. Indeed, other studies have confirmed this relationship between fatigue, pain, depressive symptoms and function (7, 12, 30).
Third, this finding extends the understanding of how demographics and clinical factors relate to symptoms, functioning and quality of life in SSc. Neither age nor disease subtype was associated with the outcomes measured. Interestingly, people with SSc all have relatively high symptom severity compared to normative samples and people with the two main subtypes of SSc (diffuse and limited) have somewhat similar fatigue levels (T = 57 and 58). This is similar to a previous study that showed no significant differences in fatigue by subtype (7). Although fatigue severity was similar in these groups, people with diffuse SSc have greater deficits in their ability to participate in social roles and activities suggesting that fatigue management is particularly important in this group. Moreover, lung, gastrointestinal and muscle involvement, more common with diffuse SSc, have been reported to be predictors of fatigue (12).
Limitations
This study utilized cross-sectional data so causality between fatigue and outcomes cannot be assumed. Further, participants were a national sample who self-reported all measures via survey so clinical variables could not be corroborated by medical records. In addition, other measures of health status such as number and types of comorbidities were not collected and this information could have further explained variance in the functioning and quality of life outcomes. Future studies should examine longitudinal associations between fatigue and social participation.
Conclusion
This study showed that fatigue related strongly to deficits in the ability to participate in social roles and activities. Intervention development for fatigue management may be particularly needed to maximize social participation in this population.
SIGNIFICANCE AND INNOVATIONS.
Fatigue is associated with physical function, quality of life, and social participation. People with SSc and higher levels of fatigue had reduced ability to participate in social roles and activities.
Fatigue explains the same amount of variance in social participation as pain and depressive symptoms combined. After pain and depressive symptoms are in the model, fatigue explains an additional 9% of variance in social participation.
Fatigue was a significant predictor of physical function and quality of life, though pain interference and depressive symptoms accounted for more variability, suggesting that different symptoms have variable effects depending on the functional domain.
Acknowledgments
Supported by: The project described was supported by a grant from the Patient Centered Outcomes Research Institute (Poole/Khanna co-PIs) Patient-Centered Outcomes Research Institute Award (CER-1310–08323). The statements presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or its Methodology Committee. Dr. Khanna’s work was supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (K24-AR-063129).
Footnotes
Conflict of Interest: authors have no conflict of interest to disclose.
REFERENCES
- 1.Johnson SR, Chung L, Fransen J, Van den Hoogen FHJ. Evolving concepts of diagnosis and classification. In: Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, editors. Scleroderma: From pathogenesis to comprehensive management. Cham: Springer International; 2017. p. 49–64. [Google Scholar]
- 2.Bolster M, Silver R. Clinical features of systemic sclerosis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 5th ed. Philadelphia: Mosby, Elsevier; 2011. p. 1373–86. [Google Scholar]
- 3.Denton C, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 4.Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epid 2019; 11: 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnitzer M, Hudson M, Baron M, Steele R. Disability in systemic sclerosis-a longitudinal observational study. J Rheumatol. 2010;38:685–92. [DOI] [PubMed] [Google Scholar]
- 6.Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian national survey. Rheumatology. 2011;50:762–7. [DOI] [PubMed] [Google Scholar]
- 7.Sandusky SB, McGuire L, Smith MT, Wigley FM, Haythornthwaite JA. Fatigue: An overlooked determinant of physical function in scleroderma. Rheumatology. 2009;48:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thombs BD, Hudson M, Bassel M, Taillefer SS, Baron M, Markland J, et al. Sociodemographic, disease, and symptom correlates of fatigue in systemic sclerosis: evidence from a sample of 659 canadian scleroderma research group registry patients. Arthritis Care Res. 2009;61:966–73. [DOI] [PubMed] [Google Scholar]
- 9.Thombs BD, Van Lankveld W, Bassel M, Baron M, Buzza R, Haslam S, et al. Psychological health and well-being in systemic sclerosis: State of the science and consensus research agenda. Arthritis Care Res. 2010;62:1181–9. [DOI] [PubMed] [Google Scholar]
- 10.Richards HL, Herrick AL, Griffin K, Gwilliam PDH, Loukes J, Fortune DG. Systemic sclerosis: Patients’ perceptions of their condition. Arthritis Care Res. 2003;49:689–96. [DOI] [PubMed] [Google Scholar]
- 11.van Lankveld WGJM, Vonk MC, Teunissen H, van den Hoogen FHJ. Appearance self-esteem in systemic sclerosis - subjective experience of skin deformity and its relationship with physician-assessed skin involvement, disease status and psychological variables. Rheumatology. 2007;46:872–6. [DOI] [PubMed] [Google Scholar]
- 12.Basta F, Afeltra A, Margiotta D. Fatigue in systemic sclerosis: a systematic review. Clin Exp Rheumatol. 2018;36:150–60. [PubMed] [Google Scholar]
- 13.Peytrignet S, Denton CP, Lunt M, Hesselstrand R, Mouthon L, Silman A, et al. Disability, fatigue, pain and their associates in early diffuse cutaneous systemic sclerosis: the european scleroderma observational study. Rheumatology. 2018;57:370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, et al. Patients’ perspectives and experiences living with systemic sclerosis: A systematic review and thematic synthesis of qualitative studies. J Rheumatol. 2016;43:1363–75. [DOI] [PubMed] [Google Scholar]
- 15.Sandqvist G, Scheja A, Hesselstrand R. Pain, fatigue and hand function closely correlated to work ability and employment status in systemic sclerosis. Rheumatology. 2010;49:1739–46. [DOI] [PubMed] [Google Scholar]
- 16.Sharif R, Mayes MD, Nicassio PM, Gonzalez EB, Draeger H, McNearney TA, et al. Determinants of work disability in patients with systemic sclerosis: a longitudinal study of the GENISOS cohort. Semin Arthritis Rheum. 2011;41:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole JL, Wilier K, Mendelson C. Occupation of motherhood: Challenges for mothers with scleroderma. Am J Occup Ther. 2009;63:214–9. [DOI] [PubMed] [Google Scholar]
- 18.Poole JL, Willer K, Mendelson C, Sanders M, Skipper B. Perceived parenting ability and systemic sclerosis. Musculoskelet Care. 2011;9:32–40. [DOI] [PubMed] [Google Scholar]
- 19.Khanna D, Serrano J, Berrocal VJ, Silver RM, Cuencas P, Newbill SL, et al. Randomized controlled trial to evaluate an internet-based self-management program in systemic sclerosis. Arthritis Care Res. 2019;71:435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwakkenbos L, Thombs BD, Khanna D, Carrier ME, Baron M, Furst DE, et al. Performance of the patient-reported outcomes measurement information system-29 in scleroderma: a scleroderma patient-centered intervention network cohort study. Rheumatology. 2017;56:1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabin R, de Charro F. EQ-SD: a measure of health status from the EuroQol group. Ann Med. 2001;33:337–43. [DOI] [PubMed] [Google Scholar]
- 22.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. [DOI] [PubMed] [Google Scholar]
- 24.Poole JL, Chandrasekaran A, Hildebrand K, Skipper B. Participation in life situations by persons with systemic sclerosis. Disabil Rehabil. 2015;37:842–5. [DOI] [PubMed] [Google Scholar]
- 25.Hudson M, Steele R, Lu Y, Thombs BD, Baron M. Work disability in systemic sclerosis. J Rheumatol 2009;36(11):2481–6. [DOI] [PubMed] [Google Scholar]
- 26.Sandqvist G, Scheja A, Eklund M. Working ability in relation to disease severity, everyday occupations and well-being in women with limited systemic sclerosis. Rheumatology (Oxford) 2008; 47(11):1708–11. [DOI] [PubMed] [Google Scholar]
- 27.Sharif R, Mayes MD, Nicassio PM, Gonzalez EB, Draeger H, McNearney TA, et al. Determinants of work disability in patients with systemic sclerosis: a longitudinal study of the GENISOS cohort. Semin Arthritis Rheum. 2011;41(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kratz AL, Braley TJ, Foxen-Craft E, Scott E, Murphy JF III, Murphy SL. How do pain, fatigue, depressive, and cognitive symptoms relate to well-being and social and physical functioning in the daily lives of individuals with multiple sclerosis? Archives Phys Medicine Rehabil. 2017;98(11):2160–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salter A, Fox RJ, Tyry T, Cutter G, Marrie RA. The association of fatigue and social participation in multiple sclerosis as assessed using two different instruments. Mult Scler Relat Disord. 2019;31:165–72. [DOI] [PubMed] [Google Scholar]
- 30.Yacoub Y, Amine B, Bensabbah R, Hajjaj-Hassouni N. Assessment of fatigue and its relationships with disease-related parameters in patients with systemic sclerosis. Clin Rheumatol. 2012;31:655–60. [DOI] [PubMed] [Google Scholar]