Abstract
Background:
Tumor-associated glycoprotein (TAG)-72 is a pancarcinoma antigen that is overexpressed in greater than 80% of colorectal adenocarcinomas. CC49 is a TAG-72-specific antibody. The aim of the present study was to demonstrate selective imaging of colon tumors and metastases with humanized TAG-72 antibody (anti-huCC49) conjugated to a near-infrared fluorophore in orthotopic mouse models.
Methods:
Anti-huCC49 was conjugated to near-infrared dye IR800CW. Mouse imaging was performed with the Pearl Trilogy Small Animal and FLARE Imaging Systems. Subcutaneous mouse models of colon cancer cell line LS174T were used to determine the dose of administration and timing of imaging. Orthotopic mouse models of LS174T were established by surgical orthotopic implantation of LS174T tumors onto the serosa of the cecum. Peritoneal carcinomatosis models were established by injection of LS174T cells into the peritoneum of nude mice. Mice were administered anti-huCC49-IR800 via tail vein injection. Mice were euthanized 72 hours later and imaged after laparotomy.
Results:
Subcutaneous LS174T xenografts demonstrated optimal tumor detection 72 hours after administration with 50 μg of anti-huCC49-IR800CW. Tumors were visualized with fluorescence imaging with a mean tumor to liver ratio of 7.39 (SD 2.76). In the orthotopic model, metastases smaller than 1 mm were fluorescently visualized that were invisible with bright light.
Conclusion:
Anti-huCC49-IR800CW provides sensitive and specific imaging of colon cancer and metastases at sub-millimeter resolution in metastatic nude mice models. This provides a promising near-infrared probe for the imaging of colon cancer and metastases for pre-operative diagnosis and fluorescence-guided surgery.
Keywords: Humanized Anti-TAG-72, Fluorescence Imaging, Near-Infrared Imaging, Colon Cancer, Metastases
INTRODUCTION
Colorectal cancer is the third most common cancer diagnosed and the third leading cause of cancer related death in men and women in the United States.1 While surgery remains the mainstay treatment for most patients with colorectal cancers, those who develop local recurrence after surgical resection have a very poor prognosis.2 The most common causes of recurrence are positive margin status and previously undetected metastases at the time of surgery. Therefore, it is imperative to identify methods to decrease the rate of positive margins after surgical resection and detect micro-metastases.2
Antibody-mediated fluorescence-guided surgery (FGS) can improve detection of tumor margins and detect sub-millimeter metastases. The use of antibodies targeted to tumor-specific surface antigens allows for specific fluorescence intraoperative tumor and metastases imaging. Prior studies have identified antibodies that are effective at specific detection of colon cancer in mouse models. An early study detected tumors in a patient derived orthotopic (PDOX) mouse model with an anti-carcinoembryonic antigen (CEA) conjugated to a dye emitting near-infrared wavelengths.3 Another studied utilized a claudin-1 antibody conjugated to an 800 nm fluorescent dye and was able to detect colon cancer as well as regional metastases in orthotopic mouse models.4 Another study used a humanized monoclonal antibody specific for CEA conjugated to a 700 nm fluorophore for detection of orthotopic mouse models of colon cancer and demonstrated adequate intravital imaging of tumors.5 Although the prior studies have validated the use of tumor specific antibodies conjugated to near-infrared fluorophores for visualization of colon tumors and metastases, the use of FGS for colorectal cancer remains an undeveloped field in the clinical setting. Identification of further surface antigens that can be utilized for fluorescence imaging can assist in the future advancement of FGS for colorectal cancers.
Tumor-associated glycoprotein (TAG)-72 is a pancarcinoma antigen that has been shown to have overexpression in greater than 80% of colorectal adenocarcinomas.6 Normal colon does not generally express TAG-72, which makes this an ideal antigen to target for cancer-specific imaging.7 CC49 is a monoclonal antibody that specifically binds to TAG-72.8 HuCC49 is a humanized version of this antibody, which has been shown to target colon cancer cell lines and human colon cancer xenografts in mouse models with good affinity.8 A variant of HuCC49 has been extensively studied in radiolabeling of colon cancer cell lines in mouse models8,9 as well as human clinical trials and has been shown to be effective at radiometrically localizing colon adenocarcinoma with no reported adverse effects.10,11,12
Since huCC49 has been effective at specific labeling of colorectal cancer and has a demonstrated safety profile in clinical trials, it is an ideal candidate for near-infrared fluorescence detection of tumors. A prior study assessed the efficacy of huCC49 conjugated with near infrared dye cyanine7, which demonstrated specific tumor labeling in murine models of human cell-line colon adenocarcinoma.13 The aim of the present study is to validate huCC49 conjugated to the near infrared fluorophore IRDye800 for imaging of colorectal tumors in mouse models.
MATERIALS AND METHODS
Animals
All studies were approved by the San Diego Veterans Administration Medical Center Institutional Animal Care and Use Committee (IACUC, animal use protocol A17-020). Nude (nu/nu) mice purchased from Jackson Lab (Bar Harbor, ME) were used for this study. The animals were fed an autoclaved laboratory diet. All surgical procedures were performed with anesthesia by intra-peritoneal injection of ketamine and xylazine reconstituted in phosphate-buffered saline (PBS). Mice were treated with subcutaneous buprenorphine for pain control after surgical procedures. At the conclusion of the study, mice were euthanized with CO2 inhalation, which was confirmed with cervical dislocation.
Antibody Conjugation
An Amicon 3 mL stirred cell (Millipore, Burlington, MA) was assembled using a 30 kDa Ultracel Ultrafiltration disc (Millipore, Burlington, MA), placed on a stir table and attached to a flow through collection reservoir connected to a vacuum pump. One mL of plasma grade water (Fisher Scientific, Waltham, MA) was added to the stirred cell. Fifteen mL of plasma water was added to the supply reservoir and attached to the stirred cell inlet. With the impeller stirring, the plasma water was allowed to flow through the chamber using a light vacuum to maintain a consistent chamber fluid level. Once the supply reservoir and chamber were empty, 5 mg of the humanized anti-TAG-72 CC49 monoclonal antibody was added to the chamber. The suspension was dialyzed with 10 diavolumes of conjugation buffer (20 mM sodium phosphate at pH 8.5). The IRDye-800CW-NHS (LI-COR Biosciences, Lincoln, NE) was dissolved in conjugation buffer to a concentration of 10 mg/mL and added at a 10:1 molar ratio. The stirred cell was protected from light and the dye/antibody reaction stirred for one hour at room temperature. Post conjugation dialysis was performed by flowing 10 diavolumes of post conjugation buffer (1X PBS at pH 7.2). The dialyzed mAb-dye conjugate (1mL) was removed and filtered through a sterile low protein binding 0.2 μm syringe filter (Pall Corporation, Port Washington, NY) into a sterile 2 mL amber glass vial (Fisher Scientific, Waltham, MA). Protein concentration and degree of labeling was determined using spectrophotometry at 280 nm and 780 nm as per dye manufacturer’s protocol. Antibody-dye conjugate purity was assessed by HPLC size exclusion chromatography (Superdex200) (GE Healthcare Life Sciences, Chicago, IL) monitored at 280 nm and 774 nm.
Western Blotting
Tumor lysates were created using normal colon and colon cancer cell line LS174T (ATCC, Manassas, VA) xenografts. Human normal colon was obtained under standard sterile conditions in the operating room at UCSD Thornton hospital under UCSD IRB protocol 140046 with informed patient consent. Western blotting procedures were carried out at room temperature. Protein samples were isolated and transferred to Trans Blot turbo Mini-size nitrocellulose membranes (Bio-Rad, Cat. 1704270) using the Bio-Rad Trans-Blot Turbo Transfer System. The membrane was then incubated with antibody conjugated with IRDye800CW at 4°C overnight. The membrane was scanned with a LI-COR Odyssey Infrared Imaging System model 9120 (LI-COR, Lincoln, NE) and detection and quantification of band intensities was conducted using Image Studio Lite Version 5.2 software (LI-COR, Lincoln, NE). Bands were normalized to total protein by dividing the intensity of the band by the intensity of the total protein from the same sample on the same blot. β-actin was utilized as a standard.
Establishment of Orthotopic Colon Cancer Mouse Models
The human colon cancer cell line LS174T was utilized for establishment of subcutaneous and orthotopic tumors. Mice were anesthetized and the skin was sterilized with a 70% ethanol solution. LS174T cells (1x106) reconstituted in Matrigel Matrix (Corning Life Sciences, Corning, NY) were injected into the bilateral flanks of nude mice. Tumors grew until they reached a volume of 50 mm3. Tumors were resected and sectioned into 1 mm fragments. Subcutaneous models for imaging were established by surgical implantation of 1 mm LS174T tumor fragment into the bilateral flank of nude mice (n=3). A small incision was made on the back of the mice and tumor fragment was directly implanted in the bilateral flank. The skin was closed with 6-0 nylon suture (Ethicon Inc., Sommerville. NJ). Tumors were allowed to grow until 5 mm in diameter. Orthotopic models of LS174T were then established with nude mice (n=7). After intraperitoneal anesthesia, the abdomen was sterilized with the 70% ethanol solution. A midline abdominal incision was made and the cecum was carefully exposed. A 1 mm tumor fragment was implanted onto the serosa of the cecum using 8-0 nylon suture (Ethicon Inc., Somerville, NJ). The cecum was then carefully returned to the peritoneal cavity and the abdominal wall was closed with 6-0 nylon suture (Ethicon Inc., Sommerville, NJ). Orthotopic implants grew until the tumor was palpable and approximately 5 mm in diameter.14 Peritoneal metastases models were created by direct injection of LS174T cells (1x106) reconstituted in PBS into the peritoneum of nude mice (n=3). Tumors were allowed to grow for 4 weeks, when intra-peritoneal tumors became palpable.
Animal Imaging
Subcutaneous LS174T mouse models were utilized for dose and time-response imaging. Mice were administered 25 μg, 50 μg or 72 μg of anti-huCC49-IR800CW via tail vein injection. Each mouse was imaged 24, 48 and 72 hours after administration to determine the tumor-to-background ratio (TBR). Orthotopic models were then imaged 72 hours after tail vein administration of anti-huCC49-IR800CW on the Pearl Trilogy Small Animal Imaging System (LI-COR, Lincoln, NE) and the FLARE Imaging System (Curadel, Natick, MA). Mice were euthanized and laparotomy was performed for imaging of intra-abdominal organs. Unconjugated IR800 dye was administered to one mouse with bilateral subcutaneous LS174T flank tumors as a non-specific control.
Statistical Analysis
Imaging analysis was performed using Image Studio Software Small Animal Imaging Analysis (LI-COR, Lincoln, NE). Statistical analysis was performed using SPSS version 24. Maximum fluorescence signal was determined for tumor and liver for each mouse. TBR was determined for various time points to determine optimal timing of imaging. Tumor to liver ratio (TLR) was calculated using the maximum tumor and maximum liver signal in each orthotopic mouse. Maximum tumor signal was compared to maximum liver signal using the student’s t-test with 2-tailed significance and equal variances assumed. Significance cut-off was pre-determined at p value < 0.001.
RESULTS
Western Blot
Western blotting was performed for normal colon and colon cancer cell line LS174T which demonstrated expression of TAG-72 protein (Figure 1). Normal colon did not have expression of TAG-72 protein.
Figure 1:
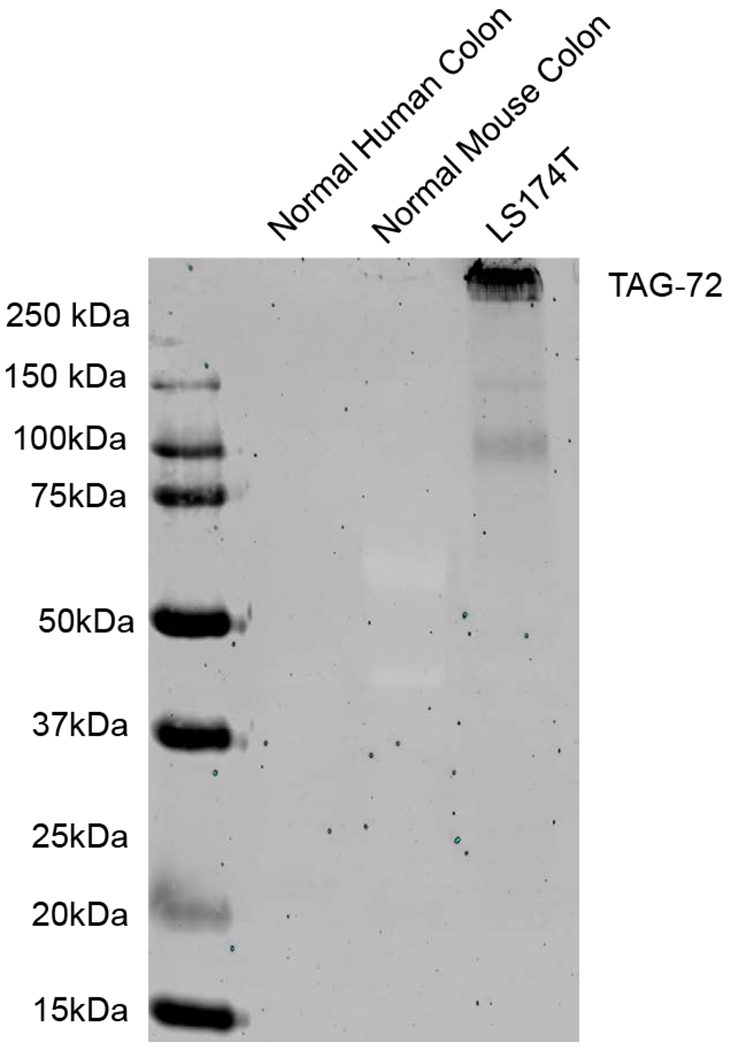
Western blot for TAG-72 expression. LS174T demonstrated expression of TAG-72 protein while normal colon did not. β-actin was used as a standard.
Imaging of Subcutaneous Tumors
Three subcutaneous mouse models were used for dose and time response imaging. The mice were given either 25, 50 or 75 μg of antibody-fluorophore conjugate. Non-invasive imaging was performed on the mice 24 hours, 48 hours and 72 hours after tail vein administration of anti-huCC49-IR800CW. The mouse that received 25 μg had minimal tumor signal at all time points. After administration of 75 μg of anti-huCC49-IR800CW, tumor signal was high at all time points. At 72 hours, intra-vital imaging demonstrated minimal liver signal. Further imaging was obtained 72 hours after administration of 50 μg of anti-huCC49-IR800CW. Tumor to background ratios (TBR) at 24, 48 and 72 hours were 2.83, 4.84 and 5.60, respectively. Therefore, optimal timepoint for imaging after administration of 50 μg of APC was 72 hours, which can be seen in Figure 2. Imaging of a control mouse with bilateral flank subcutaneous LS174T tumors after administration of unconjugated IR800 dye demonstrated non-specific fluorescence of the mouse without sequestration in the tumors.
Figure 2:
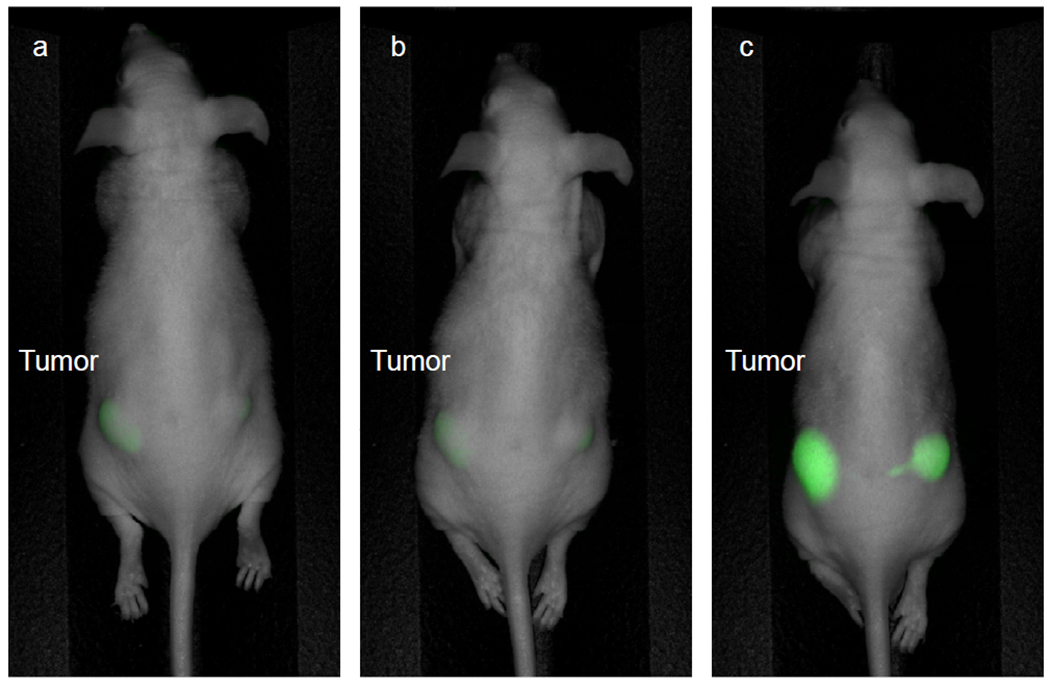
Subcutaneous LS174T tumor time response imaging. Imaging on the Pearl Trilogy Small Animal Imaging System was performed after administration of 50 μg of anti-huCC49-IR800CW. (a) 24 hours (TBR 2.83) (b) 48 hours (TBR 4.84) (c) 72 hours (TBR 5.60). Highest fluorescence signal was seen when imaging was performed after 72 hours.
Imaging of Orthotopic Tumors and Metastases
Intravital imaging was performed 72 hours after tail vein administration of anti-huCC49-IR800CW. After mice were euthanized, laparotomy was performed and imaging confirmed distinct tumor margins with minimal background signal. Analysis of all tumors images at 72 hours yielded a mean TLR of 7.39 (SD ± 2.76). Mean tumor signal at 72 hours was significantly higher than mean liver signal at this time point with mean values 1.249 and 0.175, respectively (p < 0.001, CI = 0.59, 1.56). Multiple orthotopic models developed small regional metastases that were detected with fluorescence imaging (Figure 3). The smallest metastases were less than 1 mm in diameter and invisible under bright light. Inspection of intra-abdominal organs demonstrated no gross toxicity.
Figure 3:
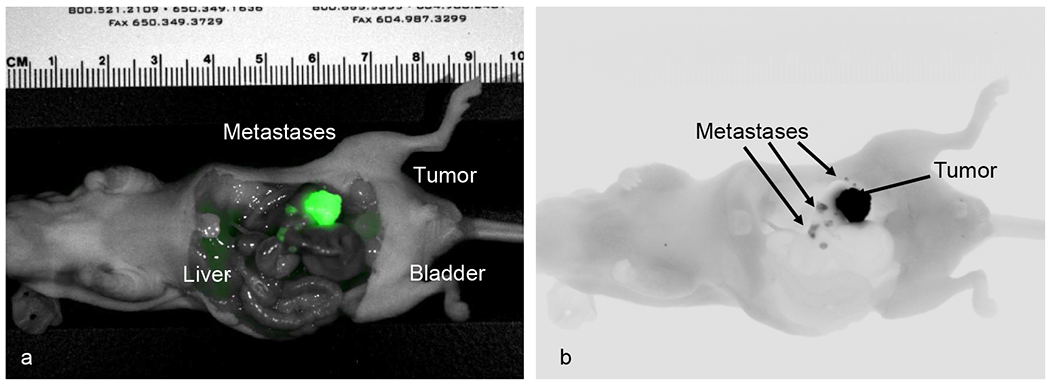
Intravital imaging of orthotopic LS174T model with multiple local metastases on the Pearl Trilogy Small Animal Imaging System with fluorescent TAG-72 antibody. (a) Near-infrared 800 nm color imaging. (b) Near-infrared 800 nm grey scale imaging.
Peritoneal Metastases Imaging
Four weeks after tumor growth, mice were euthanized and laparotomy was performed. Mice were imaged on the LI-COR Pearl Trilogy and the Curadel FLARE Imaging System for comparison. While the Pearl Trilogy is a closed-space imaging system, the FLARE provides dynamic fluorescence imaging, which improves detection of small metastases that are otherwise invisible. FLARE imaging system enables fluorescence channel overlay on bright-light images for easy distinction of tumor margins during FGS. Any area that demonstrated fluorescence signal was carefully inspected after imaging to confirm the presence of tumor. The distinction between tumor and surrounding tissue was readily visualized on merged overlay imaging (Figure 4).
Figure 4:
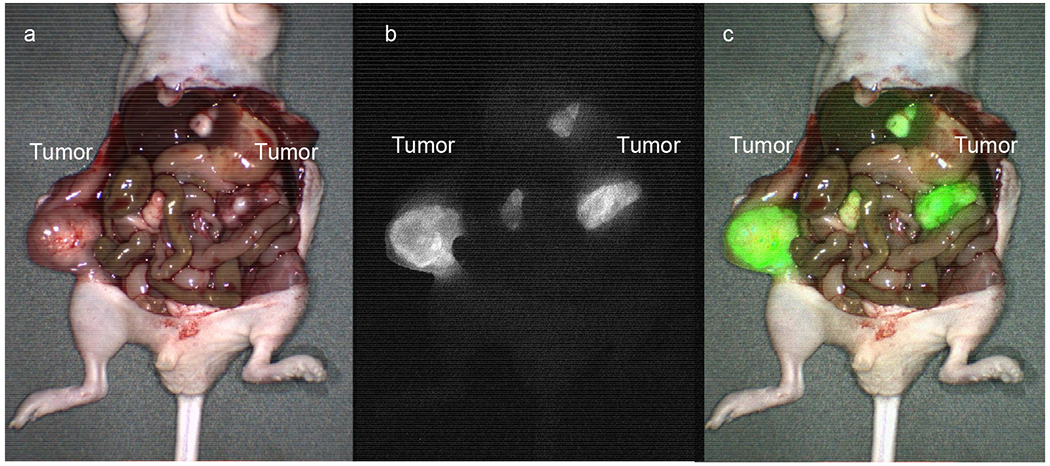
Intravital imaging of peritoneal carcinomatosis models on the FLARE Imaging System with fluorescent TAG-72 antibody. (a) Bright-light imaging of peritoneal metastases. (b) Fluorescence imaging of peritoneal metastases. (c) Merged image with 800 nm channel overlay on bright-light image.
DISCUSSION
The present study validates the use of humanized TAG-72 monoclonal antibody conjugated with IR800 dye for sensitive and specific fluorescence imaging of colorectal cancer and metastases at sub-millimeter in orthotopic mouse models. Dose and time response imaging demonstrated high tumor-to-background ratio 72 hours after administration of 50 μg of antibody-fluorophore conjugate. Orthotopic mouse models confirmed distinct tumor margins with absent background signal and minimal liver signal, with a mean tumor to liver ratio of 7.39 at 72 hours after fluorescence antibody injection. Small regional metastases were readily visualized with fluorescence imaging and peritoneal metastases were also imaged in orthotopic mouse models. These small tumors were otherwise invisible prior to imaging.
Although occurring infrequently, positive margins after resection of primary colon cancer can lead to poor outcomes. Previous studies have noted that radial margin positivity, “defined as primary disease involvement at the cut edge of the mesentery or nonserosalized portions of the colon,” is significantly associated with increased rate of recurrence, increased mortality and worse overall survival.15 Improved detection of radial margin positivity will lead to improved outcomes. The sensitivity of anti-huCC49-IR800CW to visualize tumor nodules less than 1 mm in size makes this a promising probe for intraoperative imaging to detect positive radial margins after resection.
With the introduction of minimally invasive surgical techniques, many operations for colorectal cancer are now performed laparoscopically or robotically.16 While minimally invasive approaches offer many advantages, one disadvantage is the loss of tactile function of the surgeon as well as decreased visibility of the operative field. This becomes a challenge in procedures that require adequate margins for a complete oncologic resection. Achieving negative margins becomes even more challenging in cases of rectal cancer given the small operative field created by the pelvis. Prior studies have demonstrated a circumferential resection margin rate of 17.2%, which leads to worsened long-term outcomes.17
Improved resection and subsequent decreases in the rate of recurrence can significantly improve outcomes. Prior mouse studies have also demonstrated effectiveness of huCC49 antibody conjugated to cyanine7 probe in subcutaneous mouse xenografts of colon cancer cell line tumors.13 In the present study, we utilize IRDye800, which can be imaged on current robotic and laparoscopic platforms commonly used in the operating room, which makes the use of this probe easily translatable to current surgical techniques, in contrast to Cyanine7 which is not readily visualized on currently minimally invasive platforms. In addition, the present study utilized orthotopic mouse models, which provides greater clinical translatability.
In the present study, we demonstrate that anti-huCC49-IR800CW specifically labels colorectal tumor and provides distinct visualization of tumor margins. Sensitive detection was achieved with fluorescent visualization of tumor nodules less than 1 mm in diameter. Future mouse studies will provide insight to further applications for the anti-huCC49-IR800CW probe in various presentations of colorectal cancer, including regional and hepatic metastases. In addition, future studies involving fluorescence-guided surgery in mouse models after administration of anti-huCC49-IR800CW can demonstrate effectiveness at reducing recurrence and rate of positive margins after resection of colorectal tumors.
CONCLUSIONS
Humanized anti-huCC49-IR800CW provides sensitive and specific imaging of orthotopic colon cancer and metastases in orthotopic nude mice models. This provides a promising near-infrared probe for the imaging of colon cancer and metastases for pre-operative diagnosis and fluorescence-guided surgery.
Funding Acknowledgements
This study was funded by VA Merit Review grant number 1 I01 BX003856-01A1 (MB) and NIH/NCI T32CA121938 (HH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Presentation: This study will be presented at the Annual Academic Surgical Congress Meeting February 4-6, 2020.
REFERENCES
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014; 64(2): 104–17 [DOI] [PubMed] [Google Scholar]
- 2.Abulafi AM, Williams NS. Local recurrence of colorectal cancer: the problem, mechanisms, management and adjuvant therapy. Br J Surg. 1994; 81(1): 7–19. DOI: 10.1002/bjs.1800810106. [DOI] [PubMed] [Google Scholar]
- 3.Hiroshima Y, Maawy A, Meltildi CA, et al. Successful Fluorescence-Guided Surgery on Human Colon Cancer Patient-Derived Orthotopic Xenograft Mouse Models Using a Fluorophore-Conjugated Anti-CEA Antibody and a Portable Imaging System. J Lap & Advan Surg Tech. 2014; 24(4): 241–247. doi.org/10.1089/lap.2013.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollandsworth HM, Lwin TM, Amirfakhri S, et al. Anti-Claudin-1 Conjugated to a Near-Infrared Fluorophore Targets Colon Cancer in PDOX Mouse Models. J Surg Res. 2019;242:145–150. doi: 10.1016/j.jss.2019.04.048.ChimericCEA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLong JC, Murakami T, Yazaki PJ, et al. Near-infrared-conjugated humanized anti-carcinoembryonic antigen antibody targets colon cancer in an orthotopic nude-mouse model. J Surg Res. 2017;218:139–143. doi: 10.1016/j.jss.2017.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stramignoni D, Bowen R, Atkinson BF, et al. Differential reactivity of monoclonal antibodies with human colon adenocarcinomas and adenomas. Int. J. Cancer, 31: 543–552, 1983 [DOI] [PubMed] [Google Scholar]
- 7.Yoon SO, Lee TS, Kim SJ, et al. Contraction, affinity maturation and biologic characterization of an anti-tumor associated glycoprotein-72 humanized antibody. J Biol Chem March 17 2006; 281(11). 6985–92. [DOI] [PubMed] [Google Scholar]
- 8.Kashmiri SV, Shu L, Padlan EA, et al. Generation, characterization, and in vivo studies of humanized anticarcinoma antibody CC49. Hydridoma October 1995; 14(5): 461–73 [DOI] [PubMed] [Google Scholar]
- 9.Slavin-Chiorini DC, Kashmiri SV, Lee HS, et al. A CDR-grafted (humanized) domain-deleted antitumor antibody. Cancer Biother Radiopharm. 1997;12(5):305–16 [DOI] [PubMed] [Google Scholar]
- 10.Agnese DM, Abdessalam SF, Burak WE, et al. Pilot study using a humanized CC49 monoclonal antibody (HuCC49DeltaCH2) to localize recurrent colorectal carcinoma. Ann Surg Oncol. 2004;11 (2): 197–202 [DOI] [PubMed] [Google Scholar]
- 11.Forero A, Meredith RF, Khazaeli MB, Carpenter DM, et al. A novel monoclonal antibody design for radioimmunotherapy. Cancer Biother Radiopharm. 2003;18(5):751–9 [DOI] [PubMed] [Google Scholar]
- 12.Xiao J, Horst S, Hinkle G, et al. Pharmacokinetics and clinical evaluation of 125I-radiolabeled humanized CC49 monoclonal antibody (HuCC49deltaC(H)2) in recurrent and metastatic colorectal cancer patients. Cancer Biother Radiopharm. 2005;20(1): 16–26. [DOI] [PubMed] [Google Scholar]
- 13.Zou P, Xu S, Povoski SP, et al. Near-infrared fluorescence labeled anti-TAG-72 monoclonal antibodies for tumor imaging in colorectal cancer xenograft mice. Mol Pharm. 2009;6 (2:428–440). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu XY, Besterman JM, Monosov A, and Hoffman RM. PNAS. 1991; 88 (20) 9345–9349; 10.1073/pnas.88.20.9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amri R, Bordeianou LG, Sylla P, Berger DL. Association of Radial Margin Positivity with Colon Cancer. JAMA Surg. 2015; 150(9): 890–8. Doi: 10.1001/jamasurg.2015.1525. [DOI] [PubMed] [Google Scholar]
- 16.D’Annibale A, Morpurgo E, Fiscon V, et al. Robotic and Laparoscopic Surgery for Treatment of Colorectal Diseases. Dis Colon Rectum. 2004; 47(12): 2162–2168. [DOI] [PubMed] [Google Scholar]
- 17.Rickles AS, Dietz DW, Chang GJ, et al. High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann Surg. 2015; 262(6): 891–898. doi: 10.1097/SLA.0000000000001391. [DOI] [PMC free article] [PubMed] [Google Scholar]


