Two Medicaid health plans' models and motivations for improving colorectal cancer screening rates.
Keywords: Cancer screening, Health plans, Program design, Implementation, Direct-mail FIT outreach, Qualitative
Abstract
Screening rates for colorectal cancer (CRC) remain low, especially among certain populations. Mailed fecal immunochemical testing (FIT) outreach initiated by U.S. health plans could reach underserved individuals, while solving CRC screening data and implementation challenges faced by health clinics. We report the models and motivations of two health insurance plans implementing a mailed FIT program for age-eligible U.S. Medicaid and Medicare populations. One health plan operates in a single state with ~220,000 enrollees; the other operates in multiple states with ~2 million enrollees. We conducted in-depth qualitative interviews with key stakeholders and observed leadership and clinic staff planning during program development and implementation. Interviews were transcribed and coded using a content analysis approach; coded interview reports and meeting minutes were iteratively reviewed and summarized for themes. Between June and September 2016, nine participants were identified, and all agreed to the interview. Interviews revealed that organizational context was important to both organizations and helped shape program design. Both organizations were hoping this program would address barriers to their prior CRC screening improvement efforts and saw CRC screening as a priority. Despite similar motivations to participate in a mailed FIT intervention, contextual features of the health plans led them to develop distinct implementation models: a collaborative model using some health clinic staffing versus a centralized model operationalizing outreach primarily at the health plan. Data are not yet available on the models’ effectiveness. Our findings might help inform the design of programs to deliver mailed FIT outreach.
Implications
Practice: We present two different models for health plans to consider if they want to implement a mailed fecal immunochemical testing (FIT) intervention for their populations, and we describe ways to adapt the program for different organizational contexts and available resources.
Policy: We found that US Medicaid and Medicare policy motivated health plans implementing cancer screening outreach in those populations and outreach could be implemented in different organizational contexts.
Research: We add to the implementation literature a study of what enables two different health insurance organizations to institutionalize a real-world mailed FIT intervention.
INTRODUCTION
While screening to detect colorectal cancer (CRC) is strongly recommended by the U.S. Preventive Services Task Force (USPSTF), increasing uptake remains a persistent and complicated problem [1, 2]. This screening gap is even more pronounced among certain subgroups; screening rates among low-income (47%), recent immigrant (36%), African American (59%), and Hispanic (47%) populations [2, 3], and those served by U.S. Federally Qualified Health Centers (FQHCs) (39%) are lower than the 63% screening rate in the United States overall [3–5]. However, screening disparities can be decreased through outreach strategies [6–8].
CRC screening outreach varies by the target population and funding source. For example, encouraging fecal immunochemical testing (FIT) might be especially important among disadvantaged populations (e.g., non-White, low income, elderly, and disabled). A low-cost, system-based approach to CRC screening is key to overcoming the wide variety of patient and clinic barriers in health care settings with traditionally underserved populations [5, 9–12]. Several EU and other countries have CRC screening programs [13, 14], many of which use fecal testing approaches, but the lack of a national healthcare system in the United States complicates screening outreach efforts [15–17]. Even with this challenge, U.S. studies have shown that mailed FIT programs can improve rates of CRC screening compared to offering colonoscopy alone [6, 18].
One pragmatic study, the Strategies and Opportunity to STOP Colon Cancer in Priority Populations (STOP CRC), attempted to increase CRC screening rates in 26 FQHCs by having clinic staff identify and mail FITs to people overdue for screening [19]. Providers and patients had positive reactions to the mailed FIT program and in general to using FIT [14, 18, 20–22]. However, community-based clinics face a variety of challenges implementing such a program, including turnover in key staff, competing time pressures, electronic health records (EHRs) that are incomplete or not designed to support population-based screening, challenges with staffing a centralized mailing program, and colonoscopy access [11, 20, 21, 23].
No in-person visit is needed for direct-mail FIT programs, therefore a mail-based outreach initiated by U.S. health insurance plans offers a possible solution to some of these clinic-specific challenges. Health plans can offer infrastructure, more reliable staffing for mailed programs, broad reach, and claims data that may better capture prior screening tests, such as colonoscopies. Also, patients have no unanticipated costs since the insurance pays for the testing. On the other hand, health plans may face challenges interfacing with primary care providers to ensure follow-up to FIT is being provided, such as arranging colonoscopies for patients with positive FIT tests. While health plans may be engaged in CRC screening outreach [24, 25], few previous evaluations have explored the widespread practice of CRC screening outreach interventions implemented by health insurance plans, especially plans that serve U.S. Medicaid populations [26, 27].
In response to this gap, BeneFIT is a 4-year study that supports two health plans in implementing a mailed FIT outreach program to U.S. Medicaid and Medicare populations. As a core component of the intervention, the health plans mail FITs to the homes of age-eligible health plan members overdue for CRC screening. However, in accordance with translation research that allows for local adaptations [28–32], each health plan had flexibility in how they would organize implementation of the mailed FIT program. Throughout the study, the research team is conducting qualitative interviews and observing planning meetings with health plan leadership and clinic staff at each stage of development and implementation. This paper describes the development and design of the two health plan models for implementing the mailed FIT program. We utilize data gathered from early in-depth interviews with health plan leaders and staff, along with planning meeting minutes, to describe the contextual factors that motivated the health plans to initiate a mailed FIT program, and the unique implementation models each health plan initially developed.
METHODS
Setting
This study included two U.S. health plans covering enrollees in both the Medicaid and Medicare insurance programs in Oregon and Washington states. In the United States, Medicaid provides health coverage for people who have low income; it is funded both by the federal government and state governments. Medicare is the U.S. federal health insurance program for adults aged 65 and older or younger people with disabilities. The Medicaid population is the largest line of business for both health plans that took part in this study, and most study participants had Medicaid or dual Medicaid–Medicare insurance. Oregon’s and Washington state’s Medicaid programs provide full coverage for CRC screening and follow-up testing with no out-of-pocket costs.
One of the two health plans in this study (which we will call Health Plan Oregon) is a nonprofit organization that operates in a single state with about 220,000 enrollees (called plan members). Health Plan Oregon provides insurance for Medicaid, Medicare (with most Medicare patients dually eligible for Medicaid), and dental coverage. Health Plan Oregon contracts with hundreds of primary care providers ranging from large to small practices as well as hospitals and specialists. Oregon’s Medicaid population is divided among networks of health care providers, called Coordinated Care Organizations (CCOs), which are responsible for primary and secondary care, addictions and mental health, and dental care [33]. Health Plan Oregon works with four of these CCOs.
The other health plan in the study (which we will call Health Plan Washington) is a for-profit organization that operates in multiple states and that has approximately 2 million members overall. It is a government-contracted health plan that covers Medicaid, Medicare, Marketplace, and dual-eligible members. The Marketplace segment refers to plans that have been developed as part of the Affordable Care Act, which extended federal and state health coverage benefits in the United States.
Recruitment and program design
The research team recruited the health plans into the study through existing contacts with health plans in Oregon and Washington state that served a Medicaid and Medicare population. The research investigators held discussions with health plan leadership and quality improvement administrative leaders about their past efforts and whether they would be willing to take part in the BeneFIT study. The health plans agreed to implement a mailed FIT program and provide data for the research teams to evaluate the implementation. Health Plan Oregon identified and invited health systems within their plan that might be interested in taking part in the mailed FIT program. Health Plan Washington announced the mailed FIT program to the provider groups within their health plan and allowed providers to opt out. Both programs were offered in addition to any currently existing CRC screening efforts happening at the health system level.
The research team members worked with each health plan, supporting their development of an implementation model for a mailed FIT program. The health plans developed mailed FIT program protocols, created new data reports, created mailed materials, and designed a program workflow, calling on the research team’s expertise as needed. The mailed FIT program was based in-part on the mailed intervention conducted in the STOP CRC study [34] and the Washington plan’s experience offering the program to Medicare enrollees previously. The research team met regularly with the health plan representatives, received program workflows, and developed an evaluation plan.
Qualitative interviews
An open-ended, semi-structured interview guide was developed based on review of prior meeting minutes, research staff expertise, and concepts drawn from the Consolidated Framework for Implementation Research (CFIR) [35]. The guide explored the following domain areas: prior and current CRC screening strategies used by the health plans, challenges in previous efforts aimed at CRC screening, current quality and population-based initiatives, and factors shaping participation in the BeneFIT study and influencing design of the mailed FIT program models. Health plans were asked to identify leadership staff involved with the design and implementation activities of the mailed FIT programs. From this list, two members of the research team (J.L. Schneider and J.S. Rivelli) trained in qualitative methods conducted in-depth, 60-min interviews with key stakeholders from each health plan. These research team members were separate from those who were supporting development of the implementation models. Depending on interviewee preferences, the interviews were conducted by phone or in-person and were either one-on-one or in small groups. Interviews were audio-recorded and transcribed for content analysis [36–38]. A coding dictionary was developed following review of a subset of interview transcripts. Aided by a qualitative software program, Atlas.ti [39], the qualitative staff applied codes to interview transcripts, marking sections of text with as many codes as appropriate reflecting the content of the passage. The two coders (J.L. Schneider and J.S. Rivelli) met regularly to discuss any discrepancies in application of codes, refine the codebook, and come to consensus on interpretation. Next, the qualitative staff generated reports of coded text using the query function of Atlas.ti and reviewed the reports multiple times to summarize content and generate topical themes. Summaries were shared and discussed with the overall research team, resulting in refined themes. The Human Subjects Division of the University of Washington reviewed and approved all interview procedures and materials. The design and early implementation phase of BeneFIT’s mailed FIT programs spanned from January to September of 2016, with interviews occurring after the main design period and during early implementation phase. We report our qualitative findings first, followed by a description of the models generated by each health plan.
RESULTS
Interviews
Between June and September 2016, nine health plan leaders were identified and approached; all agreed to the interview. For Health Plan Oregon, we completed a total of five interviews with health plan staff, including the chief medical director, clinical quality improvement manager, senior manager of primary care projects, quality improvement consultant, and population supervisor. For the Washington health plan, four interviews were completed with the national medical director, state chief medical officer, vice president of quality, and director of interventions. Based on our content analysis, findings from the interviews are organized into three areas: organizational context and prior CRC screening activities; prior challenges to CRC screening efforts; and motivating factors to participation in the BeneFIT mailed program.
Organizational context and prior CRC screening activities
The qualitative interviews asked about existing initiatives addressing health care quality in general and specifically to increase CRC screening rates. Both health plans had formal systems in place to address quality improvement efforts and measurement goals, such as the Medicare STARS measures, Healthcare Effectiveness Data and Information Set (HEDIS) measures, and Oregon’s CCO quality metric targets. Medicare and Medicaid quality reporting metrics directly influenced organizational initiative choices. For CRC screening, the Oregon plan had previously focused on increasing opportunistic screening among its provider network. It had conducted a small mailed FIT pilot in three clinics, but had not yet implemented a central, system-wide mailed FIT approach. The other plan, Health Plan Washington, had piloted a mailed FIT program for its Medicare members, but not its Medicaid members.
Organizational structure and context were important determinants of planning the program within each health plan. In Health Plan Oregon, a central team provided each health center with clinical quality dashboards showing key quality metrics, including CRC screening, for the Health Plan Oregon members getting care at that health system. The team also provided a list of members with care gaps for these quality measures. In addition, Health Plan Oregon had innovation specialists, called “practice coaches,” who visited health centers to help them improve their capacity to work on preventive care measures. For example, practice coaches helped identify and address workflow challenges or designed workflows to address clinic goals. Finally, Health Plan Oregon had a team of “panel managers” embedded at the larger clinics to help with quality goals. For CRC screening in particular, the panel managers regularly reviewed the patients’ charts, identifying members who were scheduled for a visit that day and were due for CRC screening. Then they alerted providers and medical assistants to offer a FIT test during their clinic visit.
Health Plan Washington focused on initiatives that could “benefit the greatest number of members” so they could improve care and performance. In the past, some monetary incentives were tied to provider performance on measures at the state level, such as chronic disease, pediatric well child visits, immunizations, and asthma adherence. For CRC screening, they had used phone outreach (live calls from the Member Services team) to members who appeared to be due for CRC screening, encouraging them to visit their provider to complete screening. Health Plan Washington also had a standing relationship with a lab that provided lab processing of many types of tests, including FIT tests, for their member population.
Prior CRC screening challenges
When asked about prior organizational challenges to increasing CRC screening, we found similar barriers that both health plans were hoping this program might address. Both organizations experienced barriers to colonoscopy access for their health plan members; wait times for colonoscopies varied depending on the particular regional area in which a clinic was located.
There’s just such a huge demand and lack of access [for colonoscopy]. – Oregon
Providers [Gastroenterologists] often reserve only a certain number of colonoscopies a month for our population, so it can be hard to get them.—Washington
As described by both health plans, efforts to meet other state-based and national metrics can take energy, focus, and resources away from meeting CRC screening goals. Additionally, one health plan noted challenges pertaining to clinic providers being less supportive of FIT, compared to colonoscopy as a CRC screening option, thus hindering population-based CRC screening efforts. The other health plan described challenges with lab vendor requirements regarding type of FIT being offered and the provision of FIT kits from the vendor, which impeded timely CRC screening for their members.
Motivating factors for participating in mailed FIT program
Both health plans saw CRC screening as an increasingly important organizational priority. In addition, the growing numbers of Medicare members in both health plans meant that more of their members needed CRC screening (i.e., they were in the age-eligible population), and it made sense for the organizations to close this “care gap.” Both organizations also mentioned that CRC screening is a HEDIS measure and is one of the Medicare 5 STAR rating measures. Both organizations had tried smaller pilots of mailed FIT programs that they wanted to expand upon. Given these experiences, the mailed FIT approach proposed by the BeneFIT study was compatible with both organizations’ goals and perceived to be a “good cultural fit,” and allowed for a larger scale assessment than had been previously conducted.
Both organizations saw a mailed FIT program as a way to meet various patient needs (such as increasing education on the importance of screening) and to address the organizational barriers to increased CRC screening.
We are looking for just a different avenue to promote colorectal cancer screening within our populations. As we noted before, access can be an issue with our population… We hope to see if we could increase participation and outcomes in a population that was essentially underinsured or of lower socioeconomic levels…So the thought of at least opening it up to be able to mail out FIT kits and follow-up with those members, via a telephone call, seemed promising….—Washington
We understand the limitations of our population - the homelessness, or the single mom that works two or three jobs…So I think the more that they can be exposed to [CRC screening], the better off they’re going to be and the more likely they’re going to at least return the FIT test.—Oregon
Health plan leaders felt that implementing the program would be both an opportunity to further support practices in promoting CRC screening and potentially overcome the challenge of lack of time to discuss CRC screening during office visits. In addition, both organizations were motivated by the chance to systematically evaluate this type of population-based approach.
Some motivating factors were unique to each health plan. Health Plan Oregon had the unique motivation of CRC screening being one of the 17 state CCO quality metrics. They saw the program as a way to help them meet that state metric while reducing the clinic workload through a coordinated screening approach.
I think that it seems like it has a lot of potential to be a very effective intervention for something that we are obviously motivated as an organization to make sure that we are doing well on and that all of our members are getting the screenings that they need...there’s also the financial incentive behind it for us to also perform well [on State metrics]. And there is an ease of implementation - in theory, should be easy for the member also. It’s kind of like a win/win all around. Ease for the organization, ease for the provider and ease for the member.
Health Plan Washington saw a unique chance to work with the research team to learn best practices for CRC screening and evaluate progress and return on investment.
I think not only the ease to which we can implement this for our members, but also being able to collect the data as a result of a very targeted campaign, is really attractive for this particular intervention.
Each of the health plans designed their mailed FIT programs within the context of these motivations and organizational environments, leading to both similarities and differences in their designs.
Mailed FIT program designs
At the start of the BeneFIT study, the research team provided information about the design, implementation, and results of prior mailed FIT programs. The health plans established internal teams that designed their programs, specifying how the programs would work in their organizations and with their vendors. The health plans developed two distinct models for the mailed FIT program (see Table 1). One model used health clinic staff for key pieces of the intervention while centralizing the FIT mail-out and administration of the program (collaborative model). The other model operationalized virtually all of the intervention workflow at the health plan level (centralized model).
Table 1.
| Implementation model similarities and differences
| Key similarities for both health plans | |
|---|---|
| Eligibility data | Health plan data are used to generate lists of plan members due for screening |
| Mailing through a vendor | An independent vendor prepared and mailed the kits, thereby offloading this time-intensive activity from individual clinics and the health plans |
| Key differences | Centralized model/Washington | Collaborative model/Oregon |
|---|---|---|
| FIT kit return | Kits returned to central lab contracted through the health plan | Kits returned to clinics |
| Phone and mail reminders | Outreach through centralized vendor-supplied health coaches | Outreach through a variety of clinic/health plan staff or no phone outreach. Vendor mailed reminder postcards for all clinics. |
| Mailed materials | All members received the same FIT and explanatory information from the health plan via the vendor | Letters were co-branded by the health plan and the clinic, FIT type varied by clinic, and different materials (i.e., consent-to-treatment forms) were included depending on clinic |
| Lab results | Lab vendor sent FIT results to mail vendor. Mail vendor mailed a copy of the FIT results to the health plan and clinics. | Clinics received results directly and health plan does not receive FIT results |
| Follow-up care | Clinics needed to incorporate results into EHR for follow-up. Health plan provided phone follow-up to encourage members with positive FIT tests to see their primary care providers | Follow-up care followed usual clinic procedures |
FIT fecal immunochemical testing.
Collaborative model
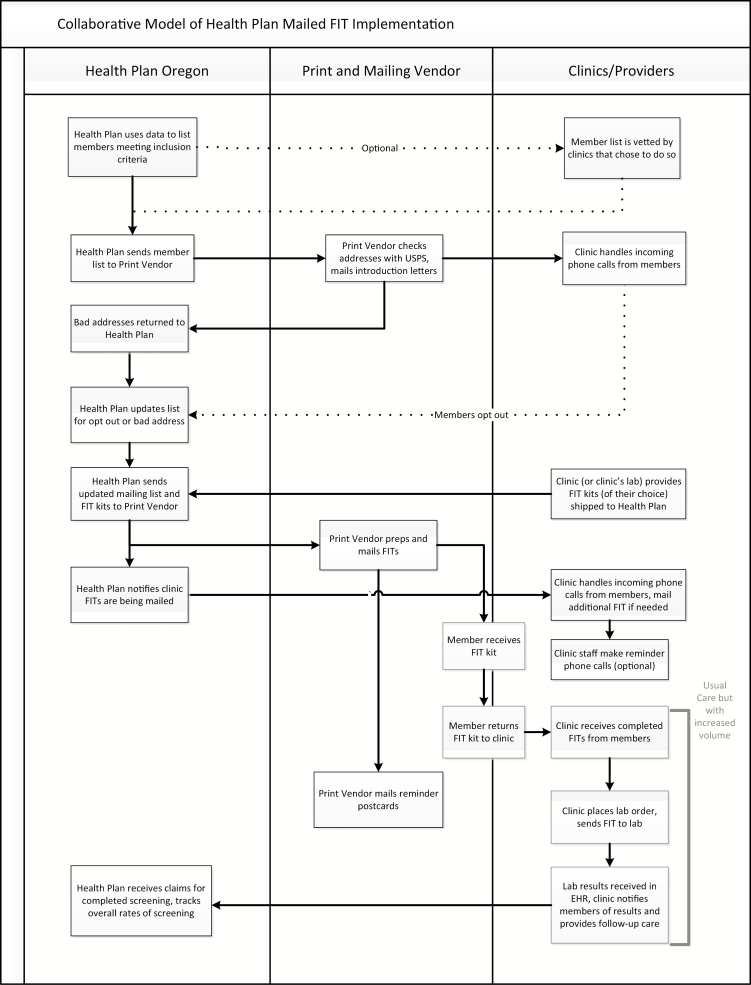
Health Plan Oregon developed a collaborative model (see Fig. 1) and approached their health centers or individual clinics, asking if they wanted to opt into the mailed FIT program. In Health Plan Oregon, six health centers, with 26 total clinics and 3,449 eligible patients, participated in the first year of the BeneFIT study. When a clinic or health center chose to be part of the program, a health plan staff member met with a clinic representative, who was typically a Quality Improvement or Operations Manager. For some small clinics, this person was a nursing manager or the director of the clinic. The health plan and clinic representatives customized how the program was going to work. They agreed on a number of logistical aspects, such as the type of FIT, how completed FIT kits were returned to the clinic from the patients, and how to customize materials for their clinic system (e.g., co-branding introductory letter, adding consent-to-treat forms or additional inserts).
Fig 1.
| Collaborative model of health plan mailed FIT intervention. FIT fecal immunochemical testing.
To support the FIT kit mailing, Health Plan Oregon created a list of health plan members who were assigned to receive care from each clinic and due for CRC screening. The clinics had the option to review this list and exclude patients whom they thought should not receive a mailed FIT (e.g., the member had already been screened for CRC or was not receiving care at the clinic). After the lists were reviewed by the clinics, Health Plan Oregon sent the lists of eligible members to a print and mail vendor, who checked the addresses against the U.S. Postal Service database and then printed and mailed a letter introducing the importance of FIT testing and telling members they were due for screening. Health Plan Oregon received undeliverable letters, removed those individuals from the list, and sent an updated list to the print vendor. The print vendor then assembled and mailed FIT kits to the remaining list. They mailed reminder postcards 2 weeks after the FITs. In addition, some clinics made phone call reminders to return the FIT, and in some cases, those phone calls were made by the health plan’s embedded panel managers.
Patients returned completed FITs to the clinic, either by mail or in person. The clinic’s staff placed lab orders and sent the FITs to their lab. Follow-up care and referrals to colonoscopy were handled through existing clinic processes. The health plan tracked overall screening rates using claims.
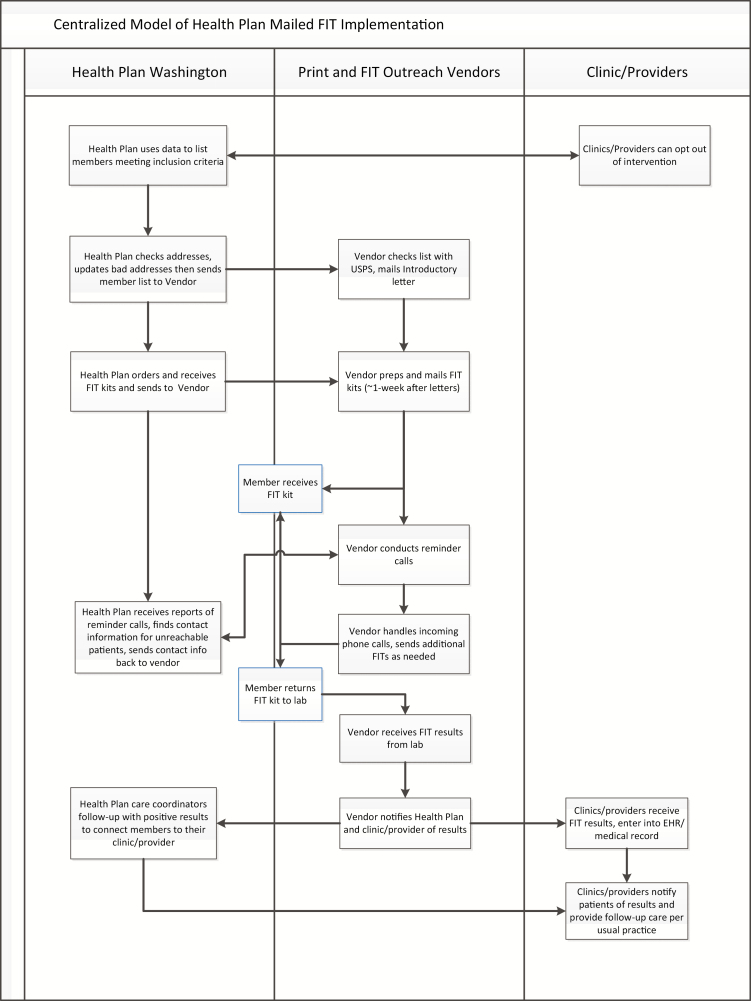
Centralized model
Health Plan Washington used a centralized model (see Fig. 2), outreaching to 8,551 members due for CRC screening in the first year of the program with virtually all the activities at the health plan or its contracted vendors. Health Plan Washington sent the lists of members due for CRC screening to a vendor to print and send introduction letters, place lab orders, and mail the FIT kits. The vendor also conducted reminder calls after the mailing to confirm that the patient received it, answer any questions, and mail a second kit if needed. A central lab received the completed FIT kits from members and sent results to the mail vendor. The mail vendor tracked their work (e.g., number of kits mailed, number of reminder calls completed) and reported back to the health plan. When a FIT kit was completed, the vendor received FIT results from the lab and sent the results to both the primary care provider and the Washington health plan. Health Plan Washington used its own care coordinators to follow-up on positive FITs by contacting the members and encouraging them to see their primary care provider. Health care providers followed up with patients based on usual clinical practice when they received FIT results.
Fig 2.
| Centralized model of health plan mailed FIT intervention. FIT fecal immunochemical testing.
Similarities and differences of the models
The models developed by the two health plans had similarities and differences that led each one to have its own advantages (see Table 1). Because the collaborative model closely involved the clinics in the program, the FIT kits sent were those that the clinics already used, thus enabling clinic staff to answer patient questions. The materials were co-branded with clinic and health plan logos. Clinics could review the health plan lists of patients not up-to-date for screening and remove patients who were current for screening according to their EHR. They also could validate that the health plan lists contained patients who correctly belonged to the clinic’s population, and update their medical records, as needed. The FIT test results came back to health clinics following each clinic’s usual process, ensuring documentation of the results in the patient record. This streamlined follow-up care. On the other hand, the clinics needed staff to successfully process the incoming FITs, handle lab billing, and answer patient questions. This collaborative model also required customized workflows for each clinic, which was a more intensive process for the health plan. For example, clinics used a variety of FIT types, each having different instructions, requiring the vendor to mail different tests and instructions to patients depending on their clinic designation. Finally, health plan data determined the clinic to which each member belonged, but these data were not always accurate. Some plan members may have been assigned to a particular clinic, but not yet established care at the clinic. Work processes were needed to address these discrepancies either before the mailed outreach or after the FITs were returned.
In the centralized model, on the other hand, virtually all the FIT program administration was handled by the health plan and its vendors, yielding economies of scale. Only one type of FIT kit and instructions was used, greatly simplifying the mail-out. Health Plan Washington was able to use their care coordinator team to follow-up on positive FIT results. The centralized model did not allow for identification of patients who were current for screening according to their EHR, but whose claims data suggested they were eligible to receive the FIT. Additionally, information about the BeneFIT mailing and its results was not available until the vendor provided these to the clinic and health plan. This could pose problems with clinicians receiving questions from their patients about the mailed FIT (which had been ordered by the contracted vendor, not the clinician) or challenges with follow-up of results. Because the plan’s contracted vendor ordered the FITs, usual integration of lab results into the clinic records did not apply, and clinics needed to enter the FIT results into their medical records once they were received from the vendor.
DISCUSSION
The BeneFIT study enabled two U.S. health plans to develop their own ways of implementing an evidence-based mailed FIT program. Health plans can potentially manage the logistics and costs of mailing more easily than individual clinics or smaller health centers. In this way, the BeneFIT models of mailed FIT can help scale up clinic-based efforts, which may be especially important in FQHC environments.
Centralized CRC screening interventions have been successful in many national health care programs [13, 15–17] and integrated health care organizations (i.e., a single-payer system), such as Kaiser Permanente or the Veteran’s Administration [40–44]. However, when shifting to a health care setting with multiple types of insurance, including U.S. Medicaid, outreach interventions are more complicated to implement and fund. Many non-single payer health insurance plans are working on initiatives to raise CRC screening rates [45], yet few have rigorously evaluated those programs.
We found one similar study that translated a research-based CRC screening intervention into a health plan-based outreach specifically in a U.S. Medicaid population. Staff in three New York Medicaid managed care organizations [26] used telephone screening reminders and increased CRC screening by 6.1% compared to usual care (n = 2,240 women aged 50–63). In this study, implementation success and screening rates were slightly lower than the research team’s original intervention results, which Dietrich et al. [26] attributed to a different patient population and staffing (i.e., health plan staff had competing priorities). Our study also translates a research-based outreach into a health plan environment, but with the health insurers as partners in the outreach to Medicare and Medicaid populations to improve CRC screening uptake.
The BeneFIT study was able to systematically look at motivations and how health plans came to develop and implement their own models of implementation. While many studies have compared centralized mailed FIT programs [46–48], few have examined different implementation strategies. Some population-based studies have compared different programs for delivering preventive care [49, 50]. However, they were somewhat different from our study, as they mainly compared different types of interventions. We did identify one UK study where implementation led to different service models (one centralized and one collaborative learning approach) for acute stroke services and that did influence implementation outcomes [51]. In our study, however, direct mailing of FITs to patients was the core program, but the strategies for delivering the mailed program were different, that is, centralized versus collaborative models.
The two health plans in this study had similar motivations for rolling out a mailed FIT program in their patient population. They both were generally adopting more population-based approaches that enabled them to streamline efforts. Both plans provided coverage for mainly Medicaid patients whose benefits included CRC screening and follow-up tests, and both plans were motivated to try a new CRC outreach approach in the face of a multitude of new state and federal requirements. The program design addressed many barriers to CRC screening programs and was a good fit for both health plans organizationally. Despite many similar barriers and motivations to conducting a mailed FIT program, the models the two health plans ultimately developed were very different. The two resulting models of implementation reflect both structural constraints and culturally unique characteristics of each of the two health plans. For example, the Oregon plan had previously relied on coaching of clinic-level work processes and panel managers embedded within each clinic, while the Washington plan had centralized care coordinators who could follow-up with high-risk individuals.
Adaptations to evidence-based programs that fit an organization’s structure and available resources are almost always needed to implement and potentially maintain interventions [28–32, 52], yet these adaptations are rarely systematically documented and studied. For the BeneFIT project, adaptation and tailoring of the mailed FIT program was central to its implementation in both health plans. The resulting variation in models we documented is important because it demonstrates how the implementation features arose from the internal organizational context rather than the research team’s solution, while also allowing fidelity to the core intervention element of mailing a FIT. The intervention was rolled out using existing organizational relationships within the health plans and care delivery systems, such as lab vendors, care coordinators and panel managers, and print vendors. Both models leveraged the health plans’ existing claims data and tools to select members eligible for mailed FITs. We observed that these two plans leveraged their own organizational strengths to implement an evidence-based program within a challenging setting.
Limitations and strengths
While we do not yet have data on the impact of each of these models on CRC screening rates or challenges faced while implementing these two models, we anticipate publishing these findings in the future. Our focus here is on the design and early implementation phases of the BeneFIT program. We ensured the credibility of our interview data [36, 38] by interviewing all leaders and staff at the health plan level involved in designing and implementing the programs. This group was a small number of people, leading to relatively few qualitative interviews. In addition, because we specifically set out to study a health plan-initiated intervention, our focus was to understand the health plan motivations and experiences, thus we did not solicit provider or patient feedback. However, we utilized an interview guide informed by CFIR, employed standard content analysis techniques [36–38], and reviewed notes from health plan development meetings. Despite these limitations, this research is intended to describe the motivations of two health plans for selecting different FIT program models within the context of an innovative naturalistic study of a much-needed intervention.
Our study was set in the United States and focused on a specific form of health insurance (U.S. Medicaid and Medicare) to address a population with low CRC screening rates. We do not know how these models might be applied outside of the United States and in the context of nationalized or single-payer health systems. Nonetheless, the models of implementation might be valuable for programs or organizations implementing a mailed FIT program in other healthcare settings.
According to Chambers et al.’s [53] Dynamic Sustainability Framework, interventions improve from ongoing optimization and need to be continually adapted to be institutionalized. Observing adaptation by the health insurance plans was built into the BeneFIT project, which enables us to study the process of what allows two different organizations to institutionalize the program, and our overall evaluation will include assessing the reach, effectiveness, costs, and lessons learned from implementation. Our future evaluations will also explore whether the programs were maintained after the research ended and what additional adaptations to implementing the mailed FIT program contributed to or hindered the health plans’ ability to sustain the program. By exploring the organizational context and details of these real-world approaches, we hope to help other health plan decision makers delivering direct-mail CRC screening outreach and to contribute to the implementation literature.
Compliance with Ethical Standards
Conflict of Interest: All authors declare they have no conflict of interest.
Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Verbal informed consent was obtained from all interviewed participants in the study.
Acknowledgements
The findings and conclusions in this presentation are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors would like to acknowledge the administrative assistance of Robin Daily and Emily Wood. This study was funded by a Health Promotion and Disease Prevention Research Center grant supported by Cooperative Agreement Number U48DP005013 from the Centers for Disease Control and Prevention.
References
- 1. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. J Am Med Assoc. 2016;315(23):2576–2594. [DOI] [PubMed] [Google Scholar]
- 2. Sabatino SA, White MC, Thompson TD, Klabunde CN; Centers for Disease Control and Prevention (CDC) Cancer screening test use—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(17):464–468. [PMC free article] [PubMed] [Google Scholar]
- 3. White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Health Resources and Services Administration (HRSA). 2016 National Health Center Data 2016. Available at https://bphc.hrsa.gov/uds/datacenter.aspx. Accessibility verified November 2, 2017.
- 5. Burnett-Hartman AN, Mehta SJ, Zheng Y, et al. ; PROSPR Consortium Racial/ethnic disparities in colorectal cancer screening across healthcare systems. Am J Prev Med. 2016;51(4):e107–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singal AG, Gupta S, Skinner CS, et al. Effect of colonoscopy outreach vs fecal immunochemical test outreach on colorectal cancer screening completion: a randomized clinical trial. J Am Med Assoc. 2017;318(9):806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berkowitz SA, Percac-Lima S, Ashburner JM, et al. Building equity improvement into quality improvement: reducing socioeconomic disparities in colorectal cancer screening as part of population health management. J Gen Intern Med. 2015;30(7):942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolf MS, Satterlee M, Calhoun EA, et al. Colorectal cancer screening among the medically underserved. J Health Care Poor Underserved. 2006;17(1):47–54. [DOI] [PubMed] [Google Scholar]
- 10. Honein-AbouHaidar GN, Kastner M, Vuong V, et al. Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2016;25(6):907–917. [DOI] [PubMed] [Google Scholar]
- 11. Coronado GD, Petrik AF, Spofford M, Talbot J, Do HH, Taylor VM. Clinical perspectives on colorectal cancer screening at Latino-serving Federally Qualified Health Centers. Health Educ Behav. 2015;42(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson CS, Oman M, Patel AM, Vega KJ. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol. 2016;7(Suppl. 1):S32–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benson VS, Atkin WS, Green J, et al. ; International Colorectal Cancer Screening Network Toward standardizing and reporting colorectal cancer screening indicators on an international level: the International Colorectal Cancer Screening Network. Int J Cancer. 2012;130(12):2961–2973. [DOI] [PubMed] [Google Scholar]
- 14. Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327–1337. [DOI] [PubMed] [Google Scholar]
- 15. Atkin WS, Benson VS, Green J, et al. Improving colorectal cancer screening outcomes: proceedings of the second meeting of the International Colorectal Cancer Screening Network, a global quality initiative. J Med Screen. 2010;17(3):152–157. [DOI] [PubMed] [Google Scholar]
- 16. Toes-Zoutendijk E, van Leerdam ME, Dekker E, et al. ; Dutch National Colorectal Cancer Screening Working Group Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology. 2017;152(4):767–775.e2. [DOI] [PubMed] [Google Scholar]
- 17. Kim S, Kwon S, Subramanian SV. Has the National Cancer Screening Program reduced income inequalities in screening attendance in South Korea?Cancer Causes Control. 2015;26(11):1617–1625. [DOI] [PubMed] [Google Scholar]
- 18. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coronado GD, Vollmer WM, Petrik A, et al. Strategies and opportunities to STOP colon cancer in priority populations: design of a cluster-randomized pragmatic trial. Contemp Clin Trials. 2014;38(2):344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coronado GD, Schneider JL, Petrik A, Rivelli J, Taplin S, Green BB. Implementation successes and challenges in participating in a pragmatic study to improve colon cancer screening: perspectives of health center leaders. Transl Behav Med. 2017;7(3):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coury J, Schneider JL, Rivelli JS, et al. Applying the Plan-Do-Study-Act (PDSA) approach to a large pragmatic study involving safety net clinics. BMC Health Serv Res. 2017;17(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coronado GD, Schneider JL, Sanchez JJ, Petrik AF, Green B. Reasons for non-response to a direct-mailed FIT kit program: lessons learned from a pragmatic colorectal-cancer screening study in a federally sponsored health center. Transl Behav Med. 2015;5(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiner BJ, Rohweder CL, Scott JE, et al. Using practice facilitation to increase rates of colorectal cancer screening in community health centers, North Carolina, 2012–2013: feasibility, facilitators, and barriers. Prev Chronic Dis. 2017;14:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klabunde CN, Riley GF, Mandelson MT, Frame PS, Brown ML. Health plan policies and programs for colorectal cancer screening: a national profile. Am J Manag Care. 2004;10(4):273–279. [PubMed] [Google Scholar]
- 25. Verma M, Sarfaty M, Brooks D, Wender RC. Population-based programs for increasing colorectal cancer screening in the United States. CA Cancer J Clin. 2015;65(6):497–510. [DOI] [PubMed] [Google Scholar]
- 26. Dietrich AJ, Tobin JN, Robinson CM, et al. Telephone outreach to increase colon cancer screening in Medicaid managed care organizations: a randomized controlled trial. Ann Fam Med. 2013;11(4):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CDC. Colorectal cancer tests save lives 2013. Available at https://www.cdc.gov/vitalsigns/colorectalcancerscreening/. Accessibility verified November 2, 2017.
- 28. Carvalho ML, Honeycutt S, Escoffery C, Glanz K, Sabbs D, Kegler MC. Balancing fidelity and adaptation: implementing evidence-based chronic disease prevention programs. J Public Health Manag Pract. 2013;19(4):348–356. [DOI] [PubMed] [Google Scholar]
- 29. Barrera M Jr, Berkel C, Castro FG. Directions for the advancement of culturally adapted preventive interventions: local adaptations, engagement, and sustainability. Prev Sci. 2017;18(6):640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bopp M, Saunders RP, Lattimore D. The tug-of-war: fidelity versus adaptation throughout the health promotion program life cycle. J Prim Prev. 2013;34(3):193–207. [DOI] [PubMed] [Google Scholar]
- 31. van Daele T, van Audenhove C, Hermans D, van den Bergh O, van den Broucke S. Empowerment implementation: enhancing fidelity and adaptation in a psycho-educational intervention. Health Promot Int. 2014;29(2):212–222. [DOI] [PubMed] [Google Scholar]
- 32. Janevic M, Bryant-Stephens T, Lara M, et al. Adaptation reconceptualized: “retrofitting” ongoing organizational activities with essential elements of evidence-based interventions. Implementation Sci 2015;10(Suppl 1): A35. doi:10.1186/1748-5908-10-S1-A35.
- 33. Oregon Health Authority. Coordinated Care Organizations (CCO) Available at http://www.oregon.gov/oha/HSD/OHP/Pages/Coordinated-Care-Organizations.aspx. Accessibility verified November 2, 2017.
- 34. Coronado GD, Vollmer WM, Petrik A, et al. Strategies and opportunities to STOP colon cancer in priority populations: pragmatic pilot study design and outcomes. BMC Cancer. 2014;14(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- 37. Strauss A, Corbin J.. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 3rd ed Thousand Oaks, CA: Sage Publications, Inc; 2008. [Google Scholar]
- 38. Bernard HR, Ryan GW.. Analyzing Qualitative Data: Systematic Approaches. Los Angeles, CA: SAGE; 2010. [Google Scholar]
- 39. ATLAS.ti. Version 6.0. Berlin, Germany: Scientific Software Development; 1999. [Google Scholar]
- 40. Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33(1):101–110. [DOI] [PubMed] [Google Scholar]
- 41. Charlton ME, Mengeling MA, Halfdanarson TR, et al. Evaluation of a home-based colorectal cancer screening intervention in a rural state. J Rural Health. 2014;30(3):322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mosen DM, Feldstein AC, Perrin N, et al. Automated telephone calls improved completion of fecal occult blood testing. Med Care. 2010;48(7):604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schlichting JA, Mengeling MA, Makki NM, et al. Veterans’ continued participation in an annual fecal immunochemical test mailing program for colorectal cancer screening. J Am Board Fam Med. 2015;28(4):494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Green BB, Anderson ML, Cook AJ, et al. A centralized mailed program with stepped increases of support increases time in compliance with colorectal cancer screening guidelines over 5 years: a randomized trial. Cancer. 2017;123(22):4472–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. National Colorectal Cancer Roundtable. Colorectal Cancer Screening Best Practices Handbook for Health Plans 2017. Available at http://nccrt.org/resource/handbook-health-plans/. Accessibility verified November 2, 2017.
- 46. Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bell RA, Arcury TA, Ip E, et al. Correlates of physician trust among rural older adults with diabetes. Am J Health Behav. 2013;37(5):660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coronado GD, Petrik AF, Vollmer WM, et al. Effectiveness of a mailed colorectal cancer screening outreach program in community health clinics: the STOP CRC cluster randomized clinical trial. JAMA Intern Med. 2018;178(9):1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kempe A, Saville AW, Dickinson LM, et al. Collaborative centralized reminder/recall notification to increase immunization rates among young children: a comparative effectiveness trial. JAMA Pediatr. 2015;169(4):365–373. [DOI] [PubMed] [Google Scholar]
- 50. Park ER, Quinn VP, Chang Y, et al. Recruiting pregnant smokers into a clinical trial: using a network-model managed care organization versus community-based practices. Prev Med. 2007;44(3):223–229. [DOI] [PubMed] [Google Scholar]
- 51. Fulop NJ, Ramsay AI, Perry C, et al. Explaining outcomes in major system change: a qualitative study of implementing centralised acute stroke services in two large metropolitan regions in England. Implement Sci. 2016;11(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tu SP, Chun A, Yasui Y, et al. Adaptation of an evidence-based intervention to promote colorectal cancer screening: a quasi-experimental study. Implement Sci. 2014;9(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]