Abstract
The electrochemical CO2 reduction reaction (CO2RR) represents a very promising future strategy for synthesizing carbon-containing chemicals in a more sustainable way. In spite of great progress in electrocatalyst design over the last decade, the critical role of wettability-controlled interfacial structures for CO2RR remains largely unexplored. Here, we systematically modify the structure of gas-liquid-solid interfaces over a typical Au/C gas diffusion electrode through wettability modification to reveal its contribution to interfacial CO2 transportation and electroreduction. Based on confocal laser scanning microscopy measurements, the Cassie-Wenzel coexistence state is demonstrated to be the ideal three phase structure for continuous CO2 supply from gas phase to Au active sites at high current densities. The pivotal role of interfacial structure for the stabilization of the interfacial CO2 concentration during CO2RR is quantitatively analysed through a newly-developed in-situ fluorescence electrochemical spectroscopic method, pinpointing the necessary CO2 mass transfer conditions for CO2RR operation at high current densities.
Subject terms: Electrocatalysis, Energy, Electrocatalysis, Characterization and analytical techniques
Great advances have been made in CO2 electroreduction, however, the role of wettability-controlled interfacial structures remains poorly understood. Here, the authors apply confocal laser scanning microscopy to gain deeper understanding of these phenomena in gas diffusion electrodes.
Introduction
Anthropogenic CO2 emissions arising from the chemical industry (e.g. cement and ammonia synthesis) and the combustion of fossil fuels for electricity generation and transportation are causative effects of global warming. One of the most promising approaches to reduce CO2 emissions is to capture CO2 and transform it into valuable commodity chemicals and hydrocarbon fuels1,2. A wide variety of technologies are currently being explored for achieving this, amongst which the electrochemical CO2 reduction reaction (CO2RR) is arguably the most promising, especially when the electricity used to drive the reduction is generated sustainably (i.e. from photovoltaics, hydro or wind turbines, geothermal power stations, etc.)3–5.
Over the past decade, enormous effort has been directed towards the design of electrocatalysts with improved CO2RR efficiencies6–9. Most studies have used an electrochemical cell based on a liquid–solid double phase contact (DPC) system, utilizing CO2 dissolved in the electrolyte. However, hydrogen evolution as competition is dominant for most DPC systems at high current densities as a result of fast water reduction kinetics and CO2 mass-transfer limitations (poor CO2 solubility and a low CO2 diffusion coefficient in liquid electrolytes)10–15. It is well known that the diffusion coefficient of CO2 in the gas phase (~0.1 cm2 s−1) is approximately four orders of magnitude higher than that of CO2 in the liquid phase16,17, thereby making CO2 in gas phase a more promising source for electrochemical CO2RR. Since gas-phase CO2 itself cannot partake in the electrochemical reaction in the absence of aqueous media, researchers have focussed attention on the development of three-phase contact (TPC) systems for CO2RR, wherein gaseous CO2, the electrolyte and the electrocatalyst are all in intimate contact18–22. TPC systems offer many advantages for electrochemical CO2RR, for example allowing the use of high pH electrolytes (that cannot readily be applied in DPC systems) to promote CO2RR electron transfer kinetics23–25. By this approach, efficient CO2 electrolysis to valuable products (e.g. CO, formic acid) can be achieved at high current densities, the use of the latter being an essential requirement for potential industrial scale applications26–28. However, due to the severe non-Faradaic consumption of CO2 in alkali electrolytes, recent efforts have been focused on the structure and micro-environment (i.e. layer thickness, electric field, pressure, etc.) of electrodes to increase the effective CO2 concentration at interfaces8,29,30. However, studying gas–liquid–solid three-phase interfaces is challenging, with knowledge being extremely limited about interfacial structures and CO2 transport behaviour under nonequilibrium conditions (i.e. conditions where the rate of CO2 consumption by electrochemical reaction and CO2 supply from bulk to the surface of the electrodes should be simultaneously considered).
The wettability of gas–liquid–solid interfaces has been extensively explored in the past few years31–33, providing new understanding of the mechanisms of wettability-controlled electrochemical reactions. It has been shown that the catalytic rate of various electrochemical reactions that involve a gas-phase reactant, such as the oxygen reduction and hydrogen evolution reactions, have an intimate relationship to the surface wettability of electrodes34–37. Through internal contact angle and microscopic analysis, the wetting property of gas diffusion layers (GDL) have recently been investigated38–40. In theory, variations of wettability over three-phase interfaces can dramatically alter the interfacial transportation behaviour of gaseous reactants/products and the contact between catalytic sites and ions in electrolyte, thus influencing gas diffusion and electron transfer processes as the rate determining steps in electrochemical reaction kinetics41. Accordingly, through wettability control it should be possible to simplify the complicated variables in TPC systems to independently investigate the relationship between interfacial structures, CO2 transportation and CO2 electroreduction, obtaining necessary information for the rational design of more efficient CO2RR systems.
Here we use a typical Au/C electrode as a model to demonstrate the role of wettability to electrochemical CO2RR, with the results expected to be transferrable to other gas diffusion electrode systems. By applying confocal laser scanning microscopy (CLSM) to image three-phase interfaces on Au/C electrode with interfacial wettabilities ranging from superhydrophobic to hydrophilic, we reveal the importance of the Cassie-Wenzel coexistence wetting state for promoting interfacial CO2 transportation and maintaining close contact between catalytic active sites and the electrolyte. Time-dependent in situ fluorescence electrochemical spectroscopy (FES) is developed to quantitatively analyse the interfacial CO2 transportation process during CO2RR. Our findings show that in TPC systems, the electrochemical CO2RR efficiency at large current densities is greatly influenced by the CO2 concentration at interfaces, which is fundamentally determined by the efficiency of CO2 mass transfer over wettability-controlled interface structures.
Results
Characterization of the Au/C electrodes
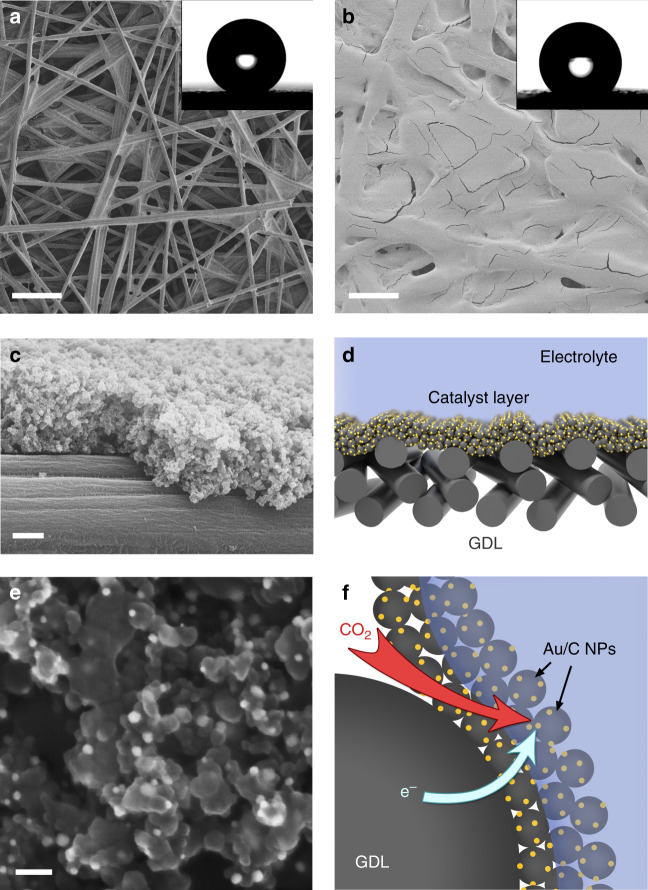
Gold-based nanostructures are reported to offer efficient and stable CO2RR activities, along with high selectivity for the production of CO42–47. In this work, we prepared monodisperse gold nanoparticles (Au NPs) with diameters of 8.95 ± 0.52 nm, then mixed these with carbon black to produce a cathodic catalyst (Au/C NPs). Detailed characterization data for both the Au NPs and Au/C NPs are provided in Supplementary Fig. 1 (see also the Methods section for the synthesis of these materials). The Au/C electrodes were then prepared by depositing the Au/C NPs as a thin film onto a polytetrafluoroethylene (PTFE)-modified carbon fibre paper. Figure 1a shows a typical scanning electron microscopy (SEM) image of the PTFE-modified carbon fibre paper, which possessed an external water contact angle (CA) of 151 ± 2° and was used here as a superhydrophobic porous GDL. As shown in Fig. 1b, after the deposition of Au/C NPs onto the PTFE-modified GDL, the water CA decreased to 135 ± 3°. The Au/C NPs catalyst layer with an average thickness of 1.2 ± 0.1 μm is supported by the carbon fibres without blocking the internal pores of the GDL (Fig. 1c, d and Supplementary Fig. 2). This architecture is crucial as rapid transport of gaseous CO2 from the bulk gas phase to the surface of the Au/C NPs through the porous electrode was required to achieve a stable interfacial CO2 concentration when operating at high current densities (Fig. 1e, f).
Fig. 1. Structural characterization of the Au/C electrode.
a SEM image of a PTFE-modified carbon fibre GDL, scale bar: 100 μm. b SEM image of the electrode after coating with an Au/C NPs film, scale bar: 100 μm. Insets in (a–b) show photographs of water droplets on each electrode. c Cross-sectional SEM image of the Au/C electrode, scale bar: 500 nm. d Schematic illustration of the TPC electrochemical cathode. e SEM image of the Au/C electrode at high magnification, scale bar: 50 nm. f Schematic illustration of the gas–liquid–solid three-phase interfaces of a TPC system for electrochemical CO2RR. Source data are provided as a Source data file.
Wettability-controlled electrochemical CO2RR
We then prepared Au/C electrodes with a range of wettabilities through surface modifications of the catalyst layer. As is shown in Fig. 2a, one modification involved coupling a fluorine-terminated silane to the carbon black in Au/C catalyst layer (the resulting electrode is denoted as Au/C-F)48, thus producing a superhydrophobic electrode (water CA of 158 ± 3°). Conversely, other electrodes were prepared containing more oxygen groups on the surface of carbon black via air plasma treatments (the resulting electrodes were labelled as Au/C-P-x, where x represents the plasma treatment time in min)49. Fourier-transform infrared spectroscopy (FTIR) revealed that the amount of surface oxygen-containing functional groups on the electrodes increased with plasma treatment time (Supplementary Fig. 3). This gave electrodes that were more hydrophilic, with water CAs decreasing from 107 ± 3° (Au/C-P-0.5) to 21 ± 5° (Au/C-P-2.5). Through X-ray photoelectron spectroscopy (XPS) and Au L3-edge X-ray absorption fine structure (XAFS) characterization studies, we demonstrated that the surface modifications had little influence on the chemical composition or structure of the Au nanoparticles (Supplementary Figs. 4 and 5).
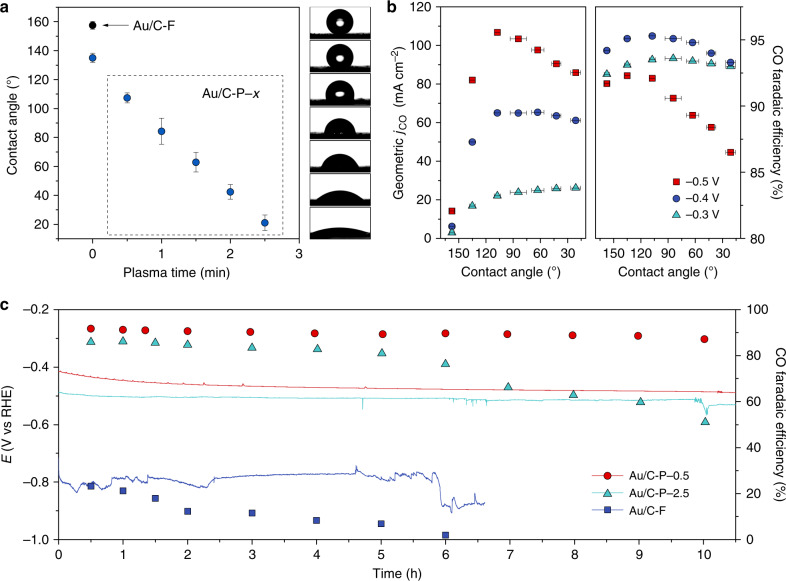
Fig. 2. Effect of interfacial wettability on electrochemical CO2RR performance.
a Plot of average water droplet contact angles on different Au/C electrodes and photographs of water droplets on each electrode. b Geometric jCO and CO Faradaic efficiency of Au/C electrodes with various water CAs at −0.3, −0.4 and −0.5 V vs. RHE. Error bars represent the standard deviation of three independent experiments. c Cathode chronopotentiometry tests for Au/C-F, Au/C-P-0.5 and Au/C-P-2.5 in 1 M KOH at a constant current density of 100 mA cm−2 (without iR correction). Source data are provided as a Source data file.
The electrochemical CO2RR performance of the different Au/C electrodes in TPC systems were investigated using a gas-phase-connected H-type electrochemical cell, using 1 M KOH as electrolyte (see Methods section). The potentials were referenced to the reversible hydrogen electrode (RHE). The linear sweep voltammetry (LSV) curves and chronoamperometry tests for the Au/C electrodes show an intimate relationship with the surface modifications, amongst which Au/C-P-0.5 exhibited the best performance (Supplementary Fig. 6). As seen in Fig. 2b, the wettability of Au/C catalyst layer greatly impacted the CO partial current density (jCO) and CO Faradaic efficiency, especially under large applied bias potentials. For example, at −0.3 V, jCO gradually increased as the catalyst layer becomes more hydrophilic, which can be attributed to the increased electrochemical surface area (ECSA) of the Au/C electrodes (Supplementary Figs. 7–9)50. For CO2RR at −0.4 V, slightly decreased jCO and CO Faradaic efficiencies were observed for electrodes with CA < 60°. At −0.5 V, the jCO reached 106.7 mA cm−2 for Au/C-P-0.5 with a CA = 107 ± 3°, followed by a sharp reduction to 85.9 mA cm−2 for Au/C-P-2.5 with a CA = 21 ± 5°. Ongoing from Au/C-P-0.5 to Au/C-P-2.5, the CO Faradaic efficiency decreased from 92.1 to 86.5%. Based on the small overpotential and high CO Faradaic efficiency, Au/C-P-0.5 afforded excellent cathodic energy efficiency for CO production amongst reported gold-based electrochemical CO2RR systems (Supplementary Fig. 10 and Table 1). To probe the long-term stability, the CO2RR performance of three typical electrodes (i.e. Au/C-F, Au/C-P-0.5 and Au/C-P-2.5) were tested at a constant current density of 100 mA cm−2. As shown in Fig. 2c, the three electrodes with different wettabilities exhibited very different cathodic potentials and CO2RR stabilities. For Au/C-F, the superhydrophobic properties of the electrode gave a negative potential with very low CO Faradaic efficiency (decreasing from 23.2 to 1.9% after 6-h operation), implying poor contact between catalyst and electrolyte. The water CAs of both the front side and reverse side of the Au/C-F electrode decreased by more than 20° after the test, indicating the superhydrophobic property was not stable at such negative potentials (Supplementary Fig. 11). On the contrary, the cathodic potential of Au/C-P-0.5 was maintained at −0.47 ± 0.02 V during the stability test. The CO Faradaic efficiency decreased slightly from 91.8 to 87.3% over 10-h operation, which is explained by the increased hydrophilicity of the catalyst layer (the water CA decreased to 74°). It is worth noting that the reverse side of the Au/C-0.5 retained its superhydrophobic property. For Au/C-P-2.5, the water CA of the reverse side decreased slightly to 140° after the stability test. This indicates that the electrolyte penetrated through the hydrophilic catalyst layer and partially blocked the pores of GDL, thus decreasing the CO Faradaic efficiency from 86.0 to 51.0% after 10-h operation. Regulating the initial wettability of the catalyst layer is therefore significant for reducing the CO2RR overpotential and improving the CO2RR stability when operating at high current densities.
Wettability-controlled TPC interfaces
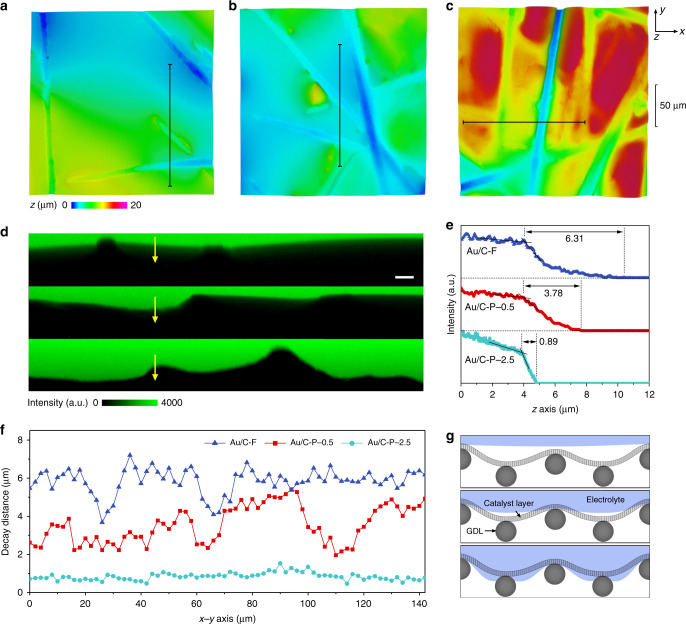
To understand the fundamental role of interfacial wettability, the relationship between electrochemical performance and the wettability-controlled structure of TPC interfaces was further investigated. We applied confocal laser scanning microscopy (CLSM) for the first time to directly observe the structure of TPC interfaces (see Methods and Supplementary Fig. 12 for experiment details)51. The 3D reconstruction images of Au/C-F, Au/C-P-0.5 and Au/C-P-2.5 are shown in Fig. 3a–c. The profile roughness of the images reflects the penetration degree of electrolyte into the catalyst layer along the z axis. Intuitively, the results indicate that the electrolyte penetration depth increased with surface hydrophilicity of the electrode. For example, Au/C-F provided an almost flat profile, with only a small amount of electrode textual observed, indicating that the liquid phase was supported by the top of catalyst layer. Conversely, for Au/C-P-2.5, the hydrophilic nature of the catalyst layer resulted in a richly textured profile, indicating that the liquid phase penetrated deep into the electrode. In order to ascertain the actual wetting states of the three electrodes, cross-sectional images along z axis were selected for comparison. As shown in Fig. 3d, the cross-sectional images show the interfacial structures of Au/C-F, Au/C-P-0.5 and Au/C-P-2.5 (from top to bottom). The bright green region indicates occupancy by the luminous electrolyte phase, while the dark non-fluorescent regions indicate occupancy by either the gas phase or the solid electrode phase. To distinguish the liquid–gas interface and the liquid–solid interface, which is important to reveal the nature of the three-phase structures, fluorescence decay behavior across the interface was examined. Due to the resolution limitation of CLSM, the fluorescence intensity does not decay to zero at the boundary of the liquid phase. Instead, a trailing effect occurs, where the decay distance is highly dependent on the properties of the second phase. In our experiments, the typical decay distance over the liquid–solid interface (<1 μm) is significantly shorter than that of the liquid–gas interface (>6 μm) due to the strong light absorption and blocking effects of the solid phase (Supplementary Fig. 13). Fluorescence intensity line scans along the z axis from the cross-sectional images (yellow arrows) are plotted in Fig. 3e. The decay distances of Au/C-F (6.31 μm) refers to a typical liquid–gas interface. While for Au/C-P-0.5 and Au/C-P-2.5, the decay distance was reduced to 3.78 and 0.89 μm, respectively, indicating that the liquid–solid interface gradually replaced the liquid–gas interface. Figure 3f provides the statistical data of the decay distances over the entire cross-sectional area. Most part of Au/C-F showed the specific decay distance of a liquid–gas interface, demonstrating that the catalyst layer is mostly exposed to the gas phase without contact with the electrolyte. The catalyst layer is therefore a gas-solid interface dominated by the Cassie state52. On the contrary, liquid–solid interfaces occupied the whole region for Au/C-P-2.5, revealing a Wenzel state that the catalyst layer is immersed by the electrolyte. Meanwhile, a Cassie-Wenzel coexistence state was observed for Au/C-P-0.5, since the fluorescence decay distance fluctuated between the typical decay distance of the liquid–gas and liquid–solid interfaces. In brief, as schematically depicted in Fig. 3g, the Cassie state, Cassie-Wenzel coexistence state and Wenzel state were the dominant wetting states for Au/C-F, Au/C-P-0.5 and Au/C-P-2.5, respectively.
Fig. 3. Characterization of gas–liquid–solid interfaces.
a–c Confocal 3D reconstruction images of Au/C-F, Au/C-P-0.5 and Au/C-P-2.5, respectively. d Cross-sectional fluorescence images scanned from labelled regions (black lines) in (a–c), scale bar: 10 μm. From top to bottom are Au/C-F, Au/C-P-0.5 and Au/C-P-2.5, respectively. e Corresponding z axis fluorescence intensity line scans of labelled regions (yellow arrows) in (d). f Statistics of fluorescence decay distance from entire area of the cross-sectional fluorescence images in (d). g Schematic illustration of interfacial structures of Au/C-F (top), Au/C-P-0.5 (middle) and Au/C-P-2.5 (bottom), respectively. Source data are provided as a Source data file.
Beyond the variation of ECSA caused by different contact modes between catalyst and electrolyte, the interfacial structure and wettability was expected to affect the efficiency of CO2 transportation, leading to a fundamentally different CO2RR performance when operating at high current densities. Quantitative evidence of interfacial CO2 transportation under electrochemical nonequilibrium conditions is thus required to provide a firm foundation for rationalizing the influence of interfacial structures of electrochemical CO2RR performance, as is explored below.
Discussion
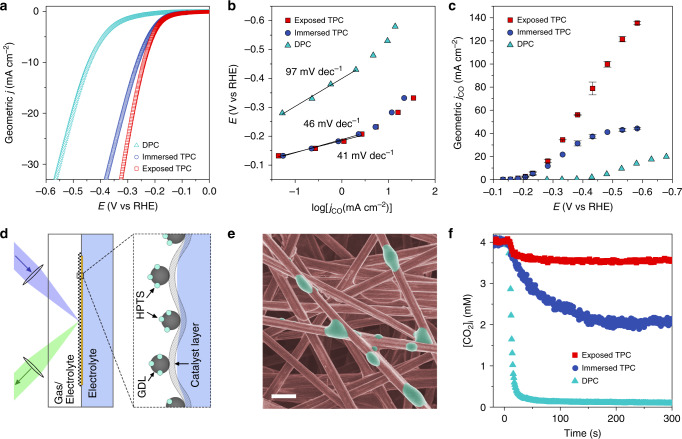
To demonstrate the effect of CO2 transportation on electrochemical CO2RR performance, we developed two typical TPC systems, denoted here as the immersed TPC system and the exposed TPC system (the former is also known as an underwater aerophilic system)53, and a traditional DPC system as depicted schematically in Supplementary Fig. 14. From the LSV curves shown in Fig. 4a, the Au/C-P-0.5 electrode in both of the TPC systems exhibited similar performance below 10 mA cm−2. The difference in the total current density (j) between the two TPC systems became larger as the cathodic potential increased, implying a gradual change in the rate determining step at higher current densities. CO production Tafel plots further confirmed similar CO2RR electrochemical kinetics for the two TPC systems at low current densities (Fig. 4b), achieving Tafel slopes of 41 and 46 mV dec−1 for exposed and immersed TPC systems, respectively. In contrast, the DPC system showed a −0.2 V shift in the onset potential for CO2RR relative to the TPC systems with a Tafel slope of 97 mV dec−1, which can be ascribed to the limited electron transfer rate in the neutral-pH carbonate electrolyte23.
Fig. 4. Electrochemical CO2RR over different interfacial structures.
a LSV of Au/C-P-0.5 electrodes in 1 M KOH for the TPC systems and in CO2-saturated 1 M KHCO3 for the DPC system. b CO production Tafel plot of Au/C-P-0.5 electrodes over exposed TPC, immersed TPC and DPC systems. c Geometric jCO of Au/C-P-0.5 over the three systems versus applied cathodic potentials. Error bars represent the standard deviation of three independent experiments. d Schematic illustration of in situ fluorescence electrochemical spectroscopy measurements. e Colour-modified SEM image of HPTS-labelled gas diffusion layer. Bright green is the CO2-sensitive HPTS gel immobilized on the surface of carbon fibres, scale bar: 20 μm. f Time-dependent [CO2]i during CO2RR at 50 mA cm−2. Time = 0 s represents the start of electrolysis. Source data are provided as a Source data file.
The electrochemical CO2 reduction products were subsequently examined at fixed potentials. As shown in Fig. 4c, the jCO of Au/C-P-0.5 in the exposed TPC system increased quickly to 99.9 ± 2.5 mA cm−2 at −0.48 V, achieving a steady CO Faradaic efficiency of 94.3 ± 2.5% from −0.2 to −0.5 V (Supplementary Fig. 15). The immersed TPC system showed similar CO2RR activity to the exposed TPC system below −0.2 V. But the jCO gradually stopped growing at more negative potentials (maximized at ~45 mA cm−2), accompanied by a dramatically drop in CO Faradaic efficiency to 81.7% at −0.48 V. For the DPC system, the maximum jCO was observed around 23 mA cm−2. It is worth noting that the current density of H2 evolution is similar between the exposed and immersed TPC systems, implying the different CO2RR activity is mainly due to CO2 transport effects rather than a surface area effect. We therefore hypothesize that at a critical current density threshold, CO2 transportation became the rate determining step in CO2RR, with the threshold being highly dependent on the interfacial structure of the electrode used.
Interfacial mass transfer is one of the key factors to consider when evaluating CO2RR at large current densities (i.e. conditions relevant for industrial electroreduction of CO2)27. Although distinct electrochemical performance has been observed, the fundamental CO2 transportation behaviour at different interfaces remains largely unexplored. Due to the electrochemical conversion of CO2 on the surface of catalysts, the interfacial CO2 concentration ([CO2]i) should be lower than that in the bulk gas phase ([CO2]0). For TPC systems, the interfacial CO2 mass-transfer flux N is a function of mass-transfer coefficient k and the difference in concentration between [CO2]0 and [CO2]i, as described by the following equation16:
| 1 |
[CO2]0 is an external variable that can be adjusted though pressure control54, whereas k is closely linked to the interfacial structure of an electrode, n is the electron transfer number of electrode reaction for each CO2 molecule (=2 for CO production), and F is the Faraday constant. Both the interfacial effective velocity and diffusivity of CO2 under electrochemical CO2RR are key factors that can influence the value of k (see Supplementary Methods). Because the interfacial region is thin in TPC systems, the CO2 mass-transfer flux N in the gas phase will be equal to that in the liquid16. It means that N is proportional to the current density of CO2RR (i.e., jCO) when non-Faradaic consumption of CO2 is neglected. Thus, [CO2]i is the only parameter needed to quantify k and examine the interfacial CO2 transportation behaviour. However, traditional characterization methods are not capable of tracking the local CO2 concentration at the microscale, especially under electrochemical conditions. Fluorescence probes show very high sensitivity to trace amounts of CO2, high spatial resolution, fast time responses and specific CO2 molecular target recognition in complex environments, though have never previously been used to study electrochemical systems for CO2RR55–58. To this end, we developed here the technique of in situ fluorescence electrochemical spectroscopy (FES) to monitor the [CO2]i during CO2RR.
8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) is a typical fluorescence probe used to detect local CO2 concentrations, the basic detection principle of which is described in the Supplementary Methods. A HPTS-based probe was deposited onto the reverse side of the GDL (Fig. 4d, e). We then fixed the HPTS-labelled Au/C working electrode into an electrochemical cell equipped with light pass channels for excitation and emission of light (Supplementary Fig. 16). The detected CO2 concentration can be approximated to the [CO2]i according to the Fick’s first law (see Methods and Supplementary Methods for details). Considering the detection limit of HPTS and the relatively low CO2 solubility in electrolyte due to the salting out effect59, the initial [CO2]0 was fixed at 4.1 mM for all systems using CO2/Ar mixtures as the gas source (see Supplementary Methods). As shown in Fig. 4f, under 50 mA cm−2 chronopotentiometry the measured [CO2]i for all the systems decreased at the beginning of electrolysis, achieving a new steady state after a certain period of electrolysis. The exposed TPC system showed the smallest decrease in [CO2]i (going from 4.1 to 3.5 mM), becoming stable within 50 s of electrolysis, while for the immersed TPC system the [CO2]i decreased continuously and eventually stabilized at 2.0 mM after 200 s electrolysis. The DPC system showed the largest decrease in [CO2]i during electrolysis, with an equilibrium [CO2]i of only 0.1 mM. Mass-transfer coefficients k were calculated to be 0.27, 0.07 and 0.02 cm s−1 for exposed TPC, immersed TPC and DPC systems, respectively. For the DPC system, k was very low owing to the poor CO2 diffusivity in the liquid phase. For the two TPC systems, which shared the same diffusivity, the difference in k mainly originated from the different effective CO2 flow velocities over the porous GDL. As such, a comprehensive analysis of the relationship between the cathodic reactions and [CO2]i is clearly required to correlate the very fast CO2 transportation in the exposed TPC system to its superior CO2RR performance.
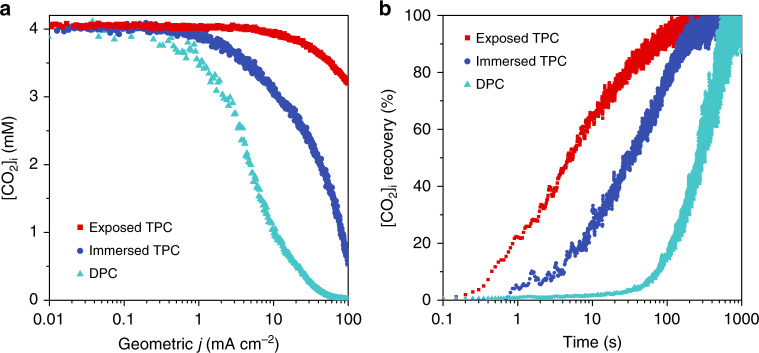
The electrochemical reaction rate can be easily controlled through potential regulation. We therefore performed LSV at slow scan rates (2 mV s−1) to monitor the [CO2]i variation for all systems (Supplementary Fig. 17). We assumed that a slow scan rate would create a quasi-equilibrium state for small potential windows, allowing the relationship between [CO2]i, cathodic potential and the corresponding current density to be revealed. As seen in Fig. 5a, all the systems showed negligible change in the [CO2]i at low current densities. However, a 50% [CO2]i reduction was found for the DPC and immersed TPC systems at 5 and 40 mA cm−2, respectively. In contrast, the [CO2]i of the exposed TPC system decreased very slowly along with the increased cathodic potential, retaining 80% of its initial concentration even at 100 mA cm−2. These trends closely matched those seen in the electrochemical CO2RR performance experiments (Fig. 4a–c), confirming that CO2 transportation dominates the reaction kinetics at high current densities.
Fig. 5. Effect of CO2 transportation on electrochemical CO2RR.
a [CO2]i versus geometric current density showing the best CO2 concentration stability is achieved in the exposed TPC system, especially at high current densities. b Time-dependent [CO2]i recovery after stopping electrolysis. Source data are provided as a Source data file.
In order to probe the interfacial CO2 transportation behaviour independently from Faradaic processes, we further examined the time-dependent [CO2]i recovery after stopping electrochemical reaction. After the reaction is halted, the [CO2]i will recover to the initial equilibrium state value. As seen in Fig. 5b, the exposed TPC system showed a fast recovery rate, with over 50% of the [CO2]i lost during electrolysis being re-supplied from bulk gas phase within 5 s. The same recovery required 32 and 280 s for the immersed TPC and DPC systems, respectively. Thus, we can conclude that effective CO2 transportation from the bulk phase to the optimized three-phase interfaces is a key factor for the stabilization of nonequilibrium [CO2]i, thus achieving non-diffusion controlled CO2RR performance at high current densities.
In summary, a typical metal/C electrode comprising an Au/C NPs film immobilized on the gas diffusion layer were successfully prepared and evaluated for CO2RR. Confocal laser scanning microscopy provided valuable information for the interface structures of wettability-controlled three-phase interfaces. The quantification of interfacial CO2 transportation behaviour though in situ fluorescence electrochemical spectroscopy further revealed the fundamental role of interfacial CO2 transportation in determining the stability of CO2 equilibrium concentration during electrochemical reaction. The TPC system with Cassie-Wenzel coexistence wetting state was identified as the ideal interface structure for CO2RR, evidenced by an interfacial CO2 mass-transfer coefficient of 0.27 cm s−1 which was sufficient to maintain 80% of the initial CO2 concentration at the interface when operating at current densities above 100 mA cm−2. Our results thus provide valuable new insights for the rational design of TPC systems for non-diffusion controlled CO2RR, as well as TPC systems for other electrochemical processes that use gaseous reactants.
Methods
Characterization
Transmission electron microscopy (TEM) images were obtained on a JEOL-2100 microscope or a JEOL2100F microscope, with both instruments operated at an accelerating voltage of 200 kV. Field emission scanning electron microscopy (FESEM) images were obtained on a Hitachi S-4800 instrument. Fourier-transform infrared (FTIR) spectra were collected on Excalibur 3100 (Varian, USA) spectrophotometer using an attenuated total reflection mode over the range 4000–600 cm−1 at a resolution of 4 cm−1. X-ray photoelectron spectroscopic (XPS) data were obtained on a Quantum 2000 Scanning ESCA Microprobe (Physical Electronics) using monochromatic Al-Kα radiation (hν = 1486.6 eV) as the excitation source. X-ray absorption fine structure (XAFS) data were collected at the Beijing Synchrotron Radiation Facility (BSRF), with the raw fluorescence mode data processed via background-subtraction, normalization and Fourier transformations using the standard procedures within the ATHENA program. Fluorescence spectra were recorded on F-4600 (Hitachi, Japan) luminescence spectrometer.
Au/C electrode preparation
Au NPs with diameters of 8.95 ± 0.52 nm were prepared using a seed growth method60. Next, 1 mL of a dispersion of the Au NPs in cyclohexane (8 mg mL−1) was added to 40 mL of a dispersion of carbon black (VULCAN XC-72, Cabot, USA) in cyclohexane (1 mg mL−1), followed by ultrasonication for 30 min. The resulting dispersion was then heated to dryness under an infrared lamp. The resulting as-prepared Au/C NPs were then calcined at 200 °C for 12 h to remove surface organic ligands on the Au NPs. The Au weight ratio in Au/C NPs product was determined to be 16.1 wt.% by thermogravimetric analysis.
Working electrode preparation
The preparation and surface modification steps are schematically shown in Supplementary Fig. 18. Commercial carbon fibre papers (TGP-H-060, Toray, Japan) were modified with 1% poly(tetrafluoroethylene) (PTFE) and used as a gas diffusion layer (GDL). A catalyst ink (1 mg mL−1) comprising Au/C NPs dispersed in a water-ethylene glycol-n-propyl alcohol mixed solution (volume ratio 1:1:1) was then drop cast onto the GDL to achieve a loading of 120 μgAu cm−2. For the surface hydrophobicity modification, the as-prepared Au/C electrode was treated with fluorine-terminated silane coupling agent (1,1,2,2-perfluorodecyltrimethoxysilane). Briefly, the Au/C electrode was placed into a stainless steel vessel containing 10 μL of coupling agent. Then, the vessel was evacuated and maintained at 90 °C for 10 min, producing the sample known as Au/C-F. Surface hydrophilicity modifications involved subjecting Au/C electrodes to air plasma (PDC-002, Harrick Plasma, USA) treatment for different times were then performed (the resulting electrodes are labelled herein as Au/C-P-x, where x represents the plasma treatment time in min). The chamber is evacuated for 2 min, after which plasma treatment (low level) was applied.
Electrochemical measurements
The electrochemical CO2RR experiments were performed in a gas-phase-connected H-type electrochemical flow cell, with data collected using a CHI660E electrochemical workstation (Shanghai Chenhua, China). The diameter and width of the channels in the flow cell are 20 and 12 mm, respectively. The electrode potentials after iR compensation were rescaled to the reversible hydrogen electrode (RHE) using the following equation:
| 2 |
For cathodic CO2RR tests, platinum and Ag/AgCl (saturated KCl) were used as counter electrode and reference electrode, respectively. 1 M KOH was were used as electrolytes (pH = 12.42, estimated from surface pH simulation by Dinh29) for three-phase electrochemical systems, While, for double phase electrochemical system, CO2 saturated 1 M KHCO3 was used as electrolyte (pH = 7.96). The gas flow rate was controlled at 20 sccm. The catholyte and anolyte flow rates were controlled at 5 mL min−1 via a peristaltic pump. The cathode and anode were separated via a proton exchange membrane (N-117, Dupont, USA). The length of all chronoamperometry experiments were fixed at 30 min before the gas products were collected for analysis. Gas-phase products were quantified using a gas chromatograph (GC-2014C, Shimadzu, Japan) equipped with thermal conductivity detector (Supplementary Fig. 19). The CO geometric partial current density jCO was calculated using the following formula:
| 3 |
In which n is the number of electrons transferred, F is Faraday’s constant, m is the mole fraction of CO quantified by gas chromatography, V is the total molar flow rate in the gas phase (mol s−1), S is the geometric area of the electrode. The Faradaic efficiency, FE, to CO was calculated using the following formula:
| 4 |
where jtot is the total current density. The cell energy efficiency, EE, for CO2RR was calculated using the following formula:
| 5 |
The cathodic energy efficiency was calculated for the cathodic half-cell, where the overpotential of the oxygen evolution reaction is assumed as zero.
Contact angle measurements
The contact angles were measured using a contact angle system (OCA 20, Dataphysics, Germany) at ambient temperature, with the probe liquid being 2 μL of water. All contact angle images were taken 5 s after the application of the liquid droplet on the surface of samples. The average contact angle was obtained by measuring three different positions of the same sample.
Fluorescent probe molecule preparation
8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) shows a characteristic emission peak at 520 nm in the presence of CO2, the intensity of which shows a strong CO2 concentration dependence56. HPTS was used as a pH-sensitive fluorescence dye here for the determination of interfacial CO2 concentrations. Briefly, a methanolic solution of tetraoctylammonium hydroxide (TH, 0.5 M) was prepared from a methanolic solution of tetraoctylammonium bromide (0.5 M), with silver oxide being used to facilitate the anion exchange. Next, 3.8 mg of HPTS was added to 1 mL of methanolic 0.5 M TH solution, followed by the addition of a further 2.5 mL of methanol to form the fluorescent probe solution. Then, 30 μL of the probe solution was deposited on the reverse side of gas diffusion layer (i.e. the opposite side to the Au/C NPs film) and dried under an infrared lamp for about 5 min. A hydrophobic silane-based sol–gel was then prepared and dabbed onto the surface of fluorescent probe to prevent leaching of HPTS when in contact with electrolyte56.
Fluorescence electrochemical spectroscopy (FES)
The in situ FES measurements utilized an electrochemical workstation (CHI660E, Shanghai Chenhua, China) integrated with a fluorescence spectrophotometer (F-4600, Hitachi, Japan). A homemade electrochemical cell equipped with light input/output channels and a gas flow system was used. Excitation and emission wavelengths for HPTS were 485 and 520 nm, respectively. For standard curve measurements, a time scan PL intensity curve was collected without electrolysis (Supplementary Fig. 20). During the measurement, the CO2 concentration in gas phase was switched from 0 to 10% in 1% increments using Ar as the carrier gas (while maintaining a total gas flow rate of 50 mL min−1). A fluorescence intensity versus CO2 concentration standard curve was fitted using an exponential decay equation. The standard curve was calibrated against the PL intensity of pure Ar gas before each measurement. For three-phase contact (TPC) systems, 1 M KOH was used as electrolyte and a 10.0% CO2 in Ar gas mixture used (equal to 4.1 mM CO2 concentration). For the double phase system (DPC system), the electrolyte was 1 M KHCO3 and a 17.8% CO2/Ar gas mixture used in order to achieve the same initial local CO2 concentration as in the TPC system (see Supplementary Methods). In order to minimize the interference of convective bulk flow, thereby allowing the intrinsic Faradaic current-induced CO2 transportation at interfaces to be studied, both the gas side and liquid side of the cell were was sealed without external flow before the measurements.
Confocal laser scanning microscopy (CLSM)
Owing to the high resolution and fast response of CLSM, we can directly image the structures of the gas–liquid–solid three-phase interfaces in the TPC systems through the analysis of a series of CLSM images at different depths within the catalyst layer. All measurements were carried out on an N-C2-SIM (Nikon, Japan) CLSM. In detail, 100 μL of fluorescein-labelled 1 M KOH was deposited into a confocal dish, followed by placing a 8 × 8 mm Au/C electrode (Au/C NPs side down) onto the liquid droplet, as is shown in Supplementary Fig. 12. A 405-nm laser was then used as the excitation source, with the confocal microscope being equipped with a ×60 (water) objective lens (numerical aperture = 1.27, pinhole = 0.5, airy units).
Supplementary information
Acknowledgements
The authors are grateful for financial support from the National Key Projects for Fundamental Research and Development of China (2017YFA0206904, 2017YFA0206900, 2016YFB0600901, 2018YFB1502002), the National Natural Science Foundation of China (51825205, U1662118, 51772305, 51572270, 21871279, 21802154, 21902168), the Beijing Natural Science Foundation (2191002, 2194089, 2182078), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB17000000), the Beijing Municipal Science and Technology Project (Z181100005118007), the Royal Society-Newton Advanced Fellowship (NA170422), the International Partnership Program of Chinese Academy of Sciences (GJHZ1819, GJHZ201974), the K. C. Wong Education Foundation and the Youth Innovation Promotion Association of the CAS. G.I.N.W. acknowledges funding support from the University of Auckland Faculty Research Development Fund, the Energy Education Trust of New Zealand, and the MacDiarmid Institute for Advanced Materials and Nanotechnology. The XAFS experiments were conducted in 1W1B beamline of Beijing Synchrotron Radiation Facility (BSRF).
Source data
Author contributions
R.S., L.J., L.S. and T.Z. conceived the idea for the project. R.S. performed the design of electrochemical cell. J.G. and Y.Z. performed the structural characterization. R.S., X.Z. and C.Z. conducted the performance measurements and analysed the data. Z.H. and Y.Z. performed the electrochemical analysis. R.S., G.I.N.W., Z.H. and T.Z. wrote the paper. L.J. and T.Z. supervised the project. All authors discussed the results and commented on the paper at all stages.
Data availability
The source data underlying Figs. 1a-c, 2a-c, 3a-f, 4a–c,e,f and 5a,b and Supplementary Figs. 1-13, 15-17, 19 and 20 are provided as a Source data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Thomas Burdyny, Antoni Forner Cuenca and Thomas Doneux for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-16847-9.
References
- 1.Gao P, et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017;9:1019–1024. doi: 10.1038/nchem.2794. [DOI] [PubMed] [Google Scholar]
- 2.Wei J, et al. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017;8:15174. doi: 10.1038/ncomms15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshio H, Katsuhei K, Shin S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985;14:1695–1698. [Google Scholar]
- 4.Bushuyev OS, et al. What should we make with CO2 and how can we make it? Joule. 2018;2:825–832. [Google Scholar]
- 5.Álvarez A, et al. Challenges in the greener production of formates/formic acid, methanol, and dme by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 2017;117:9804–9838. doi: 10.1021/acs.chemrev.6b00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang HB, et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy. 2018;3:140–147. [Google Scholar]
- 7.Zhou Y, et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 2018;10:974–980. doi: 10.1038/s41557-018-0092-x. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature. 2016;537:382–386. doi: 10.1038/nature19060. [DOI] [PubMed] [Google Scholar]
- 9.Ma M, Djanashvili K, Smith WA. Controllable hydrocarbon formation from the electrochemical reduction of CO2 over Cu nanowire arrays. Angew. Chem. Int. Ed. 2016;55:6680–6684. doi: 10.1002/anie.201601282. [DOI] [PubMed] [Google Scholar]
- 10.Ooka H, Figueiredo MC, Koper MTM. Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Langmuir. 2017;33:9307–9313. doi: 10.1021/acs.langmuir.7b00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day TJF, Schmitt UW, Voth GA. The mechanism of hydrated proton transport in water. J. Am. Chem. Soc. 2000;122:12027–12028. [Google Scholar]
- 12.Tackett BM, Gomez E, Chen JG. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019;2:381–386. [Google Scholar]
- 13.Mistry H, et al. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. J. Am. Chem. Soc. 2014;136:16473–16476. doi: 10.1021/ja508879j. [DOI] [PubMed] [Google Scholar]
- 14.Kim C, et al. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc. 2015;137:13844–13850. doi: 10.1021/jacs.5b06568. [DOI] [PubMed] [Google Scholar]
- 15.Rosen BA, et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science. 2011;334:643–644. doi: 10.1126/science.1209786. [DOI] [PubMed] [Google Scholar]
- 16.Cussler, E. L. Diffusion: Mass Transfer in Fluid Systems 238–263 (Cambridge University Press, 2009).
- 17.Carroll JJ, Slupsky JD, Mather AE. The solubility of carbon dioxide in water at low pressure. J. Phys. Chem. Ref. Data. 1991;20:1201–1209. [Google Scholar]
- 18.Jouny M, Luc W, Jiao F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 2018;1:748. [Google Scholar]
- 19.Li J, et al. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat. Catal. 2018;1:592–600. [Google Scholar]
- 20.Weekes DM, Salvatore DA, Reyes A, Huang A, Berlinguette CP. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 2018;51:910–918. doi: 10.1021/acs.accounts.8b00010. [DOI] [PubMed] [Google Scholar]
- 21.Zheng T, et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule. 2019;3:265–278. [Google Scholar]
- 22.Albo J, Irabien A. Cu2O-loaded gas diffusion electrodes for the continuous electrochemical reduction of CO2 to methanol. J. Catal. 2016;343:232–239. [Google Scholar]
- 23.Verma S, et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 2017;3:193–198. [Google Scholar]
- 24.Kim B, Ma S, Molly Jhong H-R, Kenis PJA. Influence of dilute feed and pH on electrochemical reduction of CO2 to CO on Ag in a continuous flow electrolyzer. Electrochim. Acta. 2015;166:271–276. [Google Scholar]
- 25.Verma S, Lu X, Ma S, Masel RI, Kenis PJ. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2016;18:7075–7084. doi: 10.1039/c5cp05665a. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, et al. Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities. Nat. Commun. 2020;11:593. doi: 10.1038/s41467-020-14402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Arquer FPG, et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science. 2020;367:661–666. doi: 10.1126/science.aay4217. [DOI] [PubMed] [Google Scholar]
- 28.Verma S, Kim B, Jhong HR, Ma S, Kenis PJA. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem. 2016;9:1972–1979. doi: 10.1002/cssc.201600394. [DOI] [PubMed] [Google Scholar]
- 29.Dinh C-T, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science. 2018;360:783–787. doi: 10.1126/science.aas9100. [DOI] [PubMed] [Google Scholar]
- 30.Gabardo CM, et al. Combined high alkalinity and pressurization enable efficient CO2 electroreduction to CO. Energy Environ. Sci. 2018;11:2531–2539. [Google Scholar]
- 31.Liu M, Wang S, Jiang L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017;2:17036. [Google Scholar]
- 32.Wang S, Liu K, Yao X, Jiang L. Bioinspired surfaces with superwettability: new insight on theory, design, and applications. Chem. Rev. 2015;115:8230–8293. doi: 10.1021/cr400083y. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Lu Z, Sun X, Jiang L, Duan X. Superwetting electrodes for gas-involving electrocatalysis. Acc. Chem. Res. 2018;51:1590–1598. doi: 10.1021/acs.accounts.8b00070. [DOI] [PubMed] [Google Scholar]
- 34.Lu Z, et al. Superaerophilic carbon-nanotube-array electrode for high-performance oxygen reduction reaction. Adv. Mater. 2016;28:7155–7161. doi: 10.1002/adma.201504652. [DOI] [PubMed] [Google Scholar]
- 35.Forner-Cuenca A, et al. Engineered water highways in fuel cells: radiation grafting of gas diffusion layers. Adv. Mater. 2015;27:6317–6322. doi: 10.1002/adma.201503557. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, et al. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution. Adv. Funct. Mater. 2015;25:1737–1744. [Google Scholar]
- 37.Lu Z, et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014;26:2683–2687. doi: 10.1002/adma.201304759. [DOI] [PubMed] [Google Scholar]
- 38.Leonard ME, Clarke LE, Forner‐Cuenca A, Brown SM, Brushett FR. Investigating electrode flooding in a flowing electrolyte, gas‐fed carbon dioxide electrolyzer. ChemSusChem. 2020;13:400–411. doi: 10.1002/cssc.201902547. [DOI] [PubMed] [Google Scholar]
- 39.Fishman JZ, Leung H, Bazylak A. Droplet pinning by PEM fuel cell GDL surfaces. Int. J. Hydrog. Energy. 2010;35:9144–9150. [Google Scholar]
- 40.Parry V, Berthomé G, Joud J-C. Wetting properties of gas diffusion layers: application of the Cassie-Baxter and Wenzel equations. Appl. Surf. Sci. 2012;258:5619–5627. [Google Scholar]
- 41.Cai Z, et al. Selectivity regulation of CO2 electroreduction through contact interface engineering on superwetting Cu nanoarray electrodes. Nano Res. 2018;12:345–349. [Google Scholar]
- 42.Zhu W, et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013;135:16833–16836. doi: 10.1021/ja409445p. [DOI] [PubMed] [Google Scholar]
- 43.Kuhl KP, et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014;136:14107–14113. doi: 10.1021/ja505791r. [DOI] [PubMed] [Google Scholar]
- 44.Hansen HA, Varley JB, Peterson AA, Norskov JK. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO. J. Phys. Chem. Lett. 2013;4:388–392. doi: 10.1021/jz3021155. [DOI] [PubMed] [Google Scholar]
- 45.Todoroki N, et al. Surface atomic arrangement dependence of electrochemical CO2 reduction on gold: Online electrochemical mass spectrometric study on low-index Au(hkl) surfaces. ACS Catal. 2019;9:1383–1388. [Google Scholar]
- 46.Chen Y, Li CW, Kanan MW. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 2012;134:19969–19972. doi: 10.1021/ja309317u. [DOI] [PubMed] [Google Scholar]
- 47.Dunwell M, et al. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold. J. Am. Chem. Soc. 2017;139:3774–3783. doi: 10.1021/jacs.6b13287. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, et al. Fabricating surfaces with tunable wettability and adhesion by ionic liquids in a wide range. Small. 2015;11:1782–1786. doi: 10.1002/smll.201403021. [DOI] [PubMed] [Google Scholar]
- 49.Sheng X, et al. Enhanced photocatalytic reaction at air-liquid-solid joint interfaces. J. Am. Chem. Soc. 2017;139:12402–12405. doi: 10.1021/jacs.7b07187. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, et al. Sub-3 nm ultrafine monolayer layered double hydroxide nanosheets for electrochemical water oxidation. Adv. Energy Mater. 2018;8:1703585. [Google Scholar]
- 51.Wu L, et al. Printing patterned fine 3D structures by manipulating the three phase contact line. Adv. Funct. Mater. 2015;25:2237–2242. [Google Scholar]
- 52.Wang P, et al. Highly boosted oxygen reduction reaction activity by tuning the underwater wetting state of the superhydrophobic electrode. Small. 2017;13:1601250. doi: 10.1002/smll.201601250. [DOI] [PubMed] [Google Scholar]
- 53.Burdyny T, et al. Nanomorphology-enhanced gas-evolution intensifies CO2 reduction electrochemistry. ACS Sustain. Chem. Eng. 2017;5:4031–4040. [Google Scholar]
- 54.Kohjiro H, Tadayoshi S. Large current density CO2 reduction under high pressure using gas diffusion electrodes. Bull. Chem. Soc. Jpn. 1997;70:571–576. [Google Scholar]
- 55.Mills A, Chang Q. Fluorescence plastic thin-film sensor for carbon dioxide. Analyst. 1993;118:839–843. [Google Scholar]
- 56.Nivens DA, Schiza MV, Angel SM. Multilayer sol–gel membranes for optical sensing applications: single layer pH and dual layer CO2 and NH3 sensors. Talanta. 2002;58:543–550. doi: 10.1016/s0039-9140(02)00323-5. [DOI] [PubMed] [Google Scholar]
- 57.Wolfbeis OS, Weis LJ, Leiner MJP, Ziegler WE. Fiber-optic fluorosensor for oxygen and carbon dioxide. Anal. Chem. 2002;60:2028–2030. [Google Scholar]
- 58.Amao Y, Nakamura N. Optical CO2 sensor with the combination of colorimetric change of α-naphtholphthalein and internal reference fluorescent porphyrin dye. Sens. Actuat. B-Chem. 2004;100:347–351. [Google Scholar]
- 59.Gupta N, Gattrell M, MacDougall B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 2005;36:161–172. [Google Scholar]
- 60.Peng S, et al. A facile synthesis of monodisperse Au nanoparticles and their catalysis of CO oxidation. Nano Res. 2008;1:229–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data underlying Figs. 1a-c, 2a-c, 3a-f, 4a–c,e,f and 5a,b and Supplementary Figs. 1-13, 15-17, 19 and 20 are provided as a Source data file. Source data are provided with this paper.