Abstract
Screening programmes for BRCA1/2 Jewish Founder mutations (JFM) in the Jewish community have been advocated internationally. Implementation of these programmes could decrease morbidity and mortality of BRCA1/2 JFM carriers through the uptake of cancer screening strategies and risk-reducing surgery. An online programme offered to the Sydney Jewish community that delivers pre-test information and collects consent for BRCA1/2 JFM testing via a website is currently being evaluated (JeneScreen). Forty-three participants from JeneScreen were invited to participate in a sub-study, of semi-structured pre- and post-result telephone interviews. Eleven participants consented to the sub-study. The interviews explored their experiences regarding the online model of obtaining pre-test genetic information, giving consent and receiving results. Inductive thematic analysis was carried out on the interviews. Overarching themes identified include (1) embracing online testing, (2) the online pre-test experience, (3) the result notification experience, (4) concerns associated with online testing and (5) testing as a responsibility. Overall, participants were highly satisfied with online BRCA1/2 JFM testing, an indication that the a website for pre-test information provision is an acceptable alternative to in-person genetic counselling for BRCA1/2 JFM screening and represents a feasible model for future community screening efforts.
Electronic supplementary material
The online version of this article (10.1007/s12687-019-00450-7) contains supplementary material, which is available to authorized users.
Keywords: BRCA1, BRCA2, Founder mutations, Population testing, Online genetic testing, Jewish community, Qualitative research
Introduction
Mutations (pathogenic variants) in the BRCA1 and BRCA2 genes are associated with hereditary breast and ovarian cancers, accounting for about 5% of all breast cancers (Campeau et al. 2008) and 14% of all ovarian cancers in the general Australian population (Alsop et al. 2012). Within the Ashkenazi Jewish (AJ) population, specific Jewish founder mutations (JFM) (185delAG and 5382insC in BRCA1 and 6174delT in BRCA2) have been identified. These mutations in the AJ population are responsible for 12% of breast cancer (Warner et al. 1999) and approximately 35% of ovarian cancers (Moslehi et al. 2000). Furthermore, the AJ BRCA1/2 carrier frequency is known to be significantly elevated, with 1 in 40 individuals of AJ ancestry at risk to be BRCA1/2 carriers (Bahar et al. 2001), compared to the 1 in 200–400 rate for the general population (Manickam et al. 2018; Metcalfe et al. 2010b).
A BRCA1/2 JFM screening programme in the AJ population has been largely advocated internationally (Lieberman et al. 2016b; Manchanda et al. 2015b; Metcalfe et al. 2015). The screening programme fulfils the WHO criteria (Wilson and Jungner 1968) as BRCA1/2 JFM testing identifies carriers who can utilize highly effective screening (Riedl et al. 2015) and surgical strategies (Domchek et al. 2010) to decrease cancer morbidity and mortality, capable of significantly improving health outcomes (Ludwig et al. 2016). The prevalence of JFM, coupled with the low rate of de novo BRCA1/2 mutations and non-founder BRCA1/2 mutations (Rosenthal et al. 2015), further allows for a relatively straightforward and inexpensive screen. Pilot BRCA1/2 JFM screening programmes for the AJ community have been found to be highly cost-effective over the conventional family history-based testing approach (Manchanda et al. 2015a; Rubinstein et al. 2009).
Despite the proven benefits of BRCA1/2 JFM screening in the AJ population (Gabai-Kapara et al. 2014; Manchanda et al. 2015a), current guidelines remain conservative. BRCA1/2 JFM testing is only offered to individuals of AJ ancestry with a personal and/or family history of breast and/or ovarian cancer. For instance, the Human Genetic Society of Australasia (HGSA) currently recommends that a personal or family history of breast and/or ovarian cancer is required to be eligible for testing (HGSA 2014). Studies have found that it is not uncommon for AJ individuals to be unaware of their family history (Cousens et al. 2017; Hartge et al. 1999), a possible outcome of the Holocaust and/or family dispersal during migration (Metcalfe et al. 2015).
A web-based BRCA1/2 screening programme (JeneScreen) was launched in Sydney, Australia, in early 2018, to offer BRCA1/2 JFM screening to all individuals of AJ ancestry, regardless of their personal or family history of cancer. It uses a website to deliver pre-test education and collect informed consent, replacing in-person counselling and increasing cost and time efficiencies. Using an online platform to facilitate genetic testing uptake is hypothesised to be highly accessible for the Australian public, as 86% of households are reported to possess Internet access (Australian Bureau of Statistics 2016). Furthermore, there has been success in BRCA1/2 JFM screening trials conducted internationally with incorporation of similarly streamlined models of pre-test education utilized group counselling sessions (Wiesman et al. 2016), a pre-test genetics education pamphlet (Metcalfe et al. 2010b), written information (Lieberman et al. 2016b) or information packages consisting of both written and digital material (Manchanda et al. 2015b).
Most literature in the field has evaluated the streamlined experience and satisfaction of BRCA1/2 JFM screening quantitatively. A Canadian BRCA1/2 JFM screening study which evaluated satisfaction using Satisfaction with Decision (SWD) scale and author-designed questionnaires reported high satisfaction rates (> 90%) with the streamlined experience that utilized pre-test informational packages (Metcalfe et al. 2010a).
However, there is a lack of qualitative assessment of the streamlined and, specifically, the online experience – indicating a gap in current literature. This leads to the following research question in our study: is the streamlined online pre-test experience a satisfactory alternative to in-person pre-test genetic counselling and result notification? The primary objective of this qualitative study is to explore the experience and satisfaction of Jewish participants using online platforms for pre-test education, collection of consent and result notification.
Methods
JeneScreen programme
The ongoing JeneScreen programme offers free testing of three Jewish BRCA1/2 founder mutations to adult English-speaking individuals of the Sydney Jewish community who did not receive a diagnosis of cancer in the last 12 months or does not have a known BRCA1/2 mutation in their family. A previous study identified participants who indicated an interest to participate in BRCA1/2 JFM screening (Cousens et al. 2017).
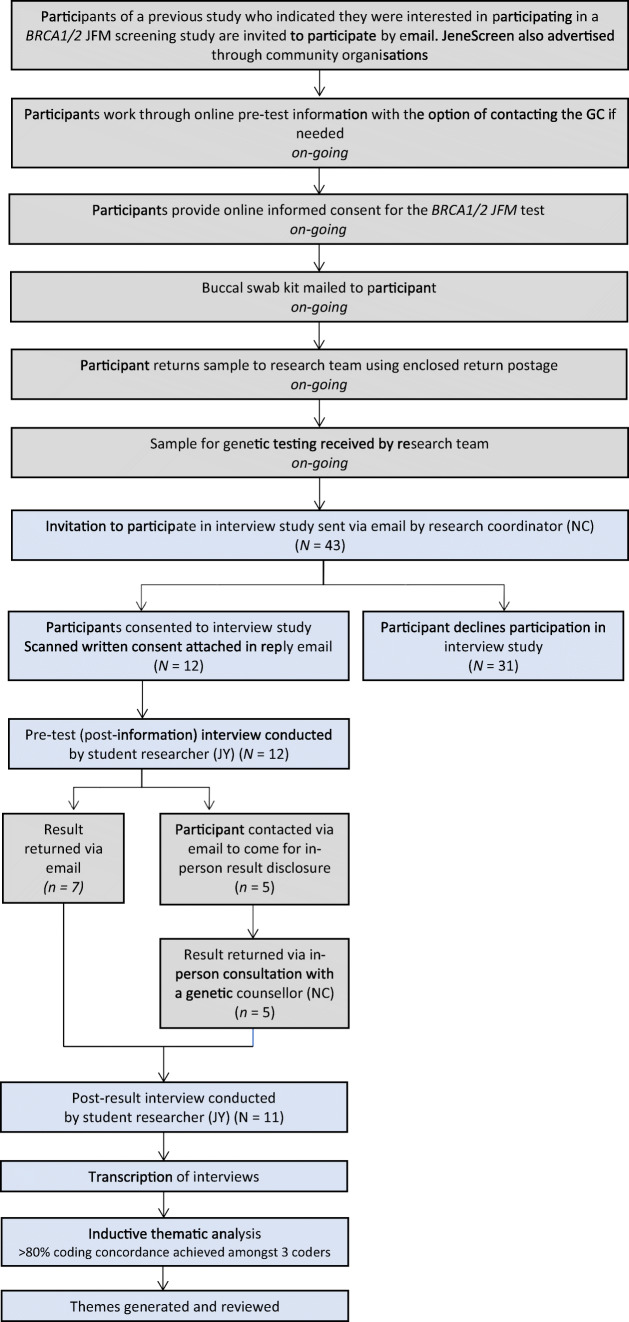
Participants of the JeneScreen programme receive pre-test information and provide consent via the JeneScreen website (https://www.genetics.wolper.com.au/brca). The design of the JeneScreen programme is outlined in Fig. 1. The sample for genetic testing is collected through a mailed cheek swab.
Fig. 1.
Flow diagram of JeneScreen Program (grey) and Interview Study (blue) Design
Most participants were informed of their negative results through an email. A random sample of participants who tested negative, participants who reported a significant family history of cancer and all participants who tested positive received an email to attend a genetic counselling appointment to receive their results.
Interview study design and participants
All participants of the JeneScreen programme who registered to participate between January and March 2018 were invited by email to participate in the interview study after they mailed back their cheek swab samples for genetic testing (Fig. 1). Email invitations with consent forms attached were sent out by the JeneScreen research coordinator (NC) who is a genetic counsellor. Participants who agreed to partake in the interview study returned completed consent forms by email. This research study was approved by Human Research Ethics Committee (HREC), Ethics Approval HREC ref. no: 17/235 (LNR/17/POWH/488).
Procedures
Consented participants underwent two semi-structured telephone interviews conducted at the pre-result (post-online information) and post-result time points. All telephone interviews were conducted by a master’s student researcher (JY), as part of the Masters of Genetic Counselling research requirement. There were 12 semi-structured pre-result (post-online information) telephone interviews conducted in January–March 2018 and 11 semi-structured post-result telephone interviews in March–July 2018, with the same participants. The pre-result interview was conducted 2–3 weeks after the participants obtained pre-test information through the interactive website and consented online to BRCA1/2 JFM testing. Pre-result interviews ranged between 20 and 50 min. Participants were contacted for their post-result interview 4–6 weeks after receiving their test results. Post-result interviews ranged between 5 and 20 min. All interviews were audio-recorded and transcribed verbatim.
Instrumentation: interview guide
The research team [JY, ROS (genetic counsellor), NC and LA (genetic medical clinician)] designed a semi-structured interview guide (Supplementary Materials 1) after a comprehensive analysis of current literature. The pre-result interview focused on the experience with using a website for pre-test education and collection of consent to undergo testing, while the post-result interview was intended to explore the experience and preferences with the way that results were returned (online/in person) and to gather feedback on the overall experience of online testing.
Data analysis
All interviews were analysed using NVIVO Pro v.11 software. An inductive thematic approach for analysis was undertaken, as it permits identification, description, analysis and reporting of themes and patterns within data (Braun and Clarke 2014). With few qualitative studies currently conducted in this area, this approach facilitates the identification of emerging themes (Braun and Clarke 2014), enabling a comprehensive exploration of the online BRCA1/2 screening experience.
Identification and characterization of themes was an ongoing process. After the coding of pre-result interviews, codes were organized into preliminary themes and subthemes depending on its content and motivation. Some codes were classified under multiple themes. Iterative coding was conducted on three transcripts to achieve > 80% concordance amongst three coders [JY, KBS (genetic counsellor; A/Professor of Masters of genetic counselling), NC]. JY coded the remaining transcripts once concordance was reached. The coding tree was also organized iteratively throughout the analysis phase as more transcripts were coded. The coding tree generated from pre-result responses was used to code the post-result transcripts. Recurring codes were arranged into themes, which were discussed and reviewed with the research team (KBS, NC and LA).
Results
Forty-three participants from the JeneScreen study were invited to participate in the interview sub-study, of which 12 participants consented to participate (27.9% response rate). There were 11 participants who were interviewed at both pre- and post-result time points. One participant was uncontactable for the post-result interview, whose responses were excluded from analysis.
Characterization of participants
Participant demographics, family history and BRCA1/2 testing results are shown in Table 1. Most participants (91%) were female and ranged in age from mid-40s to early 70s. The majority were highly educated with undergraduate degrees (27%) and postgraduate qualifications (45%). Most participants presented no (64%) or insignificant (27%) family histories (i.e. Manchester score < 15) of cancer (Evans et al. 2017). All participants received negative BRCA1/2 JFM testing results, of which six (55%) participants were notified of their results through an email and five (45%) participants attended a clinic appointment to receive their results in person.
Table 1.
Participant characteristics
| Participants interviewed at both time points | N = 11 (%) |
|---|---|
| Gender | |
| Female | 10 (91%) |
| Male | 1 (9%) |
| Age | |
| 40–50s | 2 (18%) |
| 50–60s | 3 (27%) |
| 60–70s | 3 (27%) |
| 70–80s | 3 (27%) |
| Education | |
| Year 12 | 1 (9%) |
| TAFE certificate/diploma/college | 2 (18%) |
| University undergraduate degree | 3 (27%) |
| Higher degree (postgraduate qualification) | 5 (45%) |
| Family history of cancer | |
| No family history of cancer | 7 (64%) |
| Insignificant a | 3 (27%) |
| Significant b | 1 (9%) |
| BRCA JFM test results | |
| Negative | 11 (100%) |
| Results notification method | |
| 6 (55%) | |
| In person (clinic appointment) | 5 (45%) |
TAFE = technical and further education.
JFM = Jewish founder mutation.
aManchester score < 15 (Evans et al. 2017): cancer family history indicates that the pre-test likelihood of finding a pathogenic BRCA1/2 mutation is less than 10%
bManchester score ≥ 15 (Evans et al. 2017): cancer family history indicates that the pre-test likelihood of finding a pathogenic BRCA1/2 mutation is more than 10%
Themes identified
Five major themes were identified, and subthemes reflect the participants’ experience and satisfaction with online BRCA1/2 JFM screening. The first three themes relate to the specific aspects of online testing: embracing online testing, the online pre-test experience and the result notification experience. The fourth theme is concerns with online genetic testing which revolves around the considerations identified with implementing online testing. The last theme is testing as a responsibility which encompasses the motivations for genetic testing and study participation.
Theme 1: embracing online testing
All participants expressed being satisfied with the overall process that they had undergone for online BRCA1/2 JFM screening. All were supportive of the online JeneScreen trial and expressed that they were likely to recommend or have already recommended their online experience with BRCA1/2 JFM screening to friends and family.
Furthermore, the majority (10/11) of participants indicated that it is their preference to receive pre-test information online rather than through an in-person pre-test counselling session:
Online, online… online, everybody prefers online (P9, 65-year-old female, with no family history)
Easier, more convenient and accessible
All participants agreed that the online process represented an easier and more straightforward alternative to in-person pre-test genetic counselling. Most participants (7/11) appreciated the ability to do it from the comfort of their homes. All participants felt that receiving information or results online was more convenient and accessible, removing the hassle and need for scheduling and attending clinic appointments. Those with knowledge of family history of cancer (4/4) were equally appreciative of the ease, convenience and accessibility of the online testing:
I was able to do it online, and they just sent the sample to my house. I just had to put the sample back into the post box. I didn’t have to … meet with anyone, or be somewhere in particular, so it was all at my convenience. (P5, 53-year-old female, with significant family history)
Time-efficient process
Most participants (9/11) complimented the online method of testing for being time-efficient. It was especially important to those who considered themselves to be time-poor (7/11), as they felt doing it online saved them time needed for the logistics of getting around to a genetic counselling appointment and/or time usually spent in a waiting room:
If it required me going somewhere to meet someone, then it probably would have taken me longer to get around to doing it because you need to find a suitable time to take time off work to go there to do all those kinds of things… the logistics might mean I wouldn’t do it … It was more likely that I did it the fact that it was online. (P8, 45-year-old female, with no family history)
Theme 2: the online pre-test experience
Satisfaction with online pre-test education and consent
All but one participant (10/11) was satisfied with the experience of receiving pre-test information and giving consent to BRCA1/2 JFM testing via a website:
I was very satisfied with that [the online process]. The alternative to do would be you go and meet someone face-to-face and sign a paper consent form, so yeah, it made it easy for me. (P8, 45-year-old female, with no family history)
The outlier participant cited old age as the reason for her preference of telephone counselling over online pre-test information:
Well, I am over 70, I prefer to do things where I am speaking with somebody. (P6, 71-year-old female, with no family history)
Self-paced learning
The freedom of self-paced learning using the website was favoured by most (9/11). Participants noted that the website enabled several features of learning that were not possible with a face-to-face pre-test appointment, processing the information at their own pace, rereading/revisiting the information on the website if desired and looking up additional information while progressing through the pre-test information on the website:
I also remember when I was doing it [reading the pre-test website information] I went to google something, there was some statistic that I read about, the increased incidence of you know… the BRCA test result? And I kind of went, oh I wonder what the overall incidence is? so I could google relevant information … had I been face-to-face, I would find I couldn’t have done. So, it's actually really good that way. (P10, 48-year-old female, with no family history)
Relevant, staged pre-test education
Nonetheless, there was feedback (7/11) that there was too much pre-test information to process on the website. Some (4/11) suggested providing the information in a staged manner. For instance, details relating to cancer prevention strategies were regarded as irrelevant at the pre-test stage and would only be applicable if they were found to be carriers:
I felt that some of it [the information on the website] would become more important if you got a particular result. But you may not get that result, so you wouldn't need to go into as much detail about that topic. So only if you need the information after receiving your results, then you'll need to know. And at that point you should learn of it, instead of before going through the test? (P8, 45-year-old female, with no family history)
Well-informed online decision-to-test
Most (8/11) reported that their online decision to undergo BRCA1/2 JFM testing was well-informed and supported. All of whom noted having received sufficient information to make their decision, though, for some (3/11), it triggered certain considerations they had not previously considered (i.e. insurance issues). Ultimately, they felt that the information and the considerations triggered did better equip them to make their decision to undergo testing. Additionally, a subset of participants (4/11) highlighted that the option to contact the study coordinator/genetic counsellor (NC) also played a significant role in ensuring that their decision felt well-supported:
I came away feeling supported in my decision to go ahead with this. It didn’t seem like a hardship at all… there were enough phone numbers there that I could have rung up and said, hang on a second what does this mean and what do you mean by that? So, I could have done that anyway. (P4, 66-year-old female, with no family history, who sought email clarification with NC regarding the pre-test process on the website)
Theme 3: the result notification experience
Satisfaction with the result notification process
The majority of participants (9/11) (5/6 notified of test results via email and 4/5 notified in person) were satisfied with the method results that were returned:
I am happy with it, and the way it came, I don't have a problem. (P2, 70-year-old female, with insignificant family history, who received results via email)
Notwithstanding, we observed two participants (2/5) who attended clinic appointments to receive results, highlighted feeling anxious or worried, as they felt that the clinic appointment could be an indication of positive results. These participants, upon receiving negative results, emphasized that such anxiety that they experienced was unnecessary and recommended that all participants should be informed that the default method for result notification would be an appointment. Other arrangements (i.e. an email for negative results) can be made as per participant’s preference depending on the result.
Email is satisfactory if negative only
There is consensus (5/6) that an email notification would suffice for negative results. One participant, however, who stated in her pre-result interview that telephone counselling was her preferred method to undergo pre-test procedures, felt that the email notification came across as too impersonal or cold. Conversely, one participant who received results face to face emphasized that he would have liked to receive his results through an email:
Since I was negative, I would have been happy to have it online. It would save me a trip. (P3, 71-year-old male, who received test result in person)
However, all participants, who were notified via email, stated that they might not have felt satisfied if they were to receive positive results through an email. The need for an in-person consultation to cope with potential anxiety or answer questions was raised by all participants:
No, I think I would have liked it personally if I was positive, I can imagine being anxious (P1, 61-year-old female, who was notified of negative results by email)
There is value in receiving negative results in person
Of those who received their results in person, some (3/5) on hindsight saw value in receiving negative results in person. They appreciated the opportunity to ask personalized questions and to receive information specific to themselves and their families. One participant also highlighted that the time taken to attend the appointment was an opportunity to reflect on her possible reactions to the result:
Having to go to the appointment, …it meant that on my way there and coming back, … I could give myself space to think about it… Even it was a negative result … it still was worthwhile going face to face because … the person I’m speaking to can assess my reaction and provide some additional clarification about things. It’s an opportunity to ask questions and you sort of understand the implications I think (P8, 45-year-old female, with no family history who received negative results in person)
Theme 4: concerns with online genetic testing
Despite the support for and satisfaction with online BRCA1/2 JFM screening amongst the participants, there were concerns raised regarding the prospects of online population screening. A few (3/11) were concerned about the privacy of their information and results that were shared online. Others (3/11) underlined the dangers of making online decisions to test without sufficient deliberation:
It is easy to skim the information and not read it properly, I suppose from that perspective, it's a bit of a disadvantage for it to be online… It was a lot less of a decision… you know what I mean… you could go a lot further online before making a decision. (P10, 48-year-old female, with no family history)
Online testing may be unsuitable for everyone
All emphasized that online screening may not be suitable for everyone. Some stated that doing it online might only be suitable for the computer literate (3/11) or those comfortable with reading medical information (5/11). One participant cautioned that people with a family history of related cancers may experience heightened anxiety and would require more support to undertake the decision to undergo testing:
It depends on the person. For me, it was no problem, but for someone who has a family history and they're worried they might be positive … there are a whole lot of other issues that go on from that, which they may want to consult someone on. (P3, 71-year-old male, with no family history)
Theme 5: testing as a responsibility
The majority (9/11) of participants believed that undergoing BRCA1/2 JFM screening is a responsibility to their families (7/11) and their Jewish community (3/11). Similarly, most (9/11) wanted to participate in the programme to contribute to medical research:
Well, you know, the reason a lot of the Jewish people, a lot of their ancestors died, because they didn’t know what illnesses they carried, because they were killed at a younger age. So, I suppose this is one way of finding out – that is the reason why I’m doing this – for the data to be available to other people (P2, 70-year-old female, with insignificant family history)
Discussion
This is the first study to qualitatively assess the use of an online model for pre-test education, consent and return of results for clinical BRCA1/2 JFM screening. It was designed to evaluate if such an online model for pre-test information delivery and result notification is a satisfactory alternative to in-person genetic counselling for clinical BRCA1/2 JFM screening.
Most participants indicated high overall satisfaction with the entire process for online testing. All participants were satisfied with the use of a website to deliver pre-test education and obtain consent for clinical BRCA1/2 JFM testing. Similarly, high satisfaction rates (> 90%) were identified in studies that offered pre-test materials in the form of informational packages, brochures or digital information to replace traditional in-person pre-test genetic counselling for BRCA1/2 testing (Lieberman et al. 2016a; Metcalfe et al. 2010a). Longitudinal studies of women with breast cancer who received pre-test educational material in place of in-person genetic counselling reported high satisfaction and low distress rates (Sie et al. 2016). The acceptance and satisfaction with online pre-test education provision are not surprising as the process of delivering online pre-test education has long been offered by direct-to-consumer (DTC) companies marketing genetic testing, for which its demand continues to grow – which could be perceived as a form of acceptance for online pre-test education modules for genetic testing (Ramos and Weissman 2018).
The use of digital information to provide pre-test education for clinical genetic testing is gaining popularity, with supportive attitudes for online pre-test education observed in cancer genetic services of Israel (Lieberman et al. 2016b) and Australia (Ratnayake et al. 2011). This aligns with our findings; most participants prefer the use of the website, over telephone and in-person pre-test genetic counselling, to receive pre-test information to empower their clinical genetic testing decisions (Xu et al. 2018).
The use of a website for pre-test genetic education provision was well-received as it was perceived as a convenient and time-efficient way to access genetic testing, eliminating the hassle and time taken to obtain a referral to schedule and attend a clinic appointment. A similar study evaluating a previsit website for breast cancer genetic counselling reported consistent findings. Participants appreciated the features of convenience and efficiency that accompanied the use of the website, regarding its equivalent to in-person counselling (Albada et al. 2012).
The participants highlighted several advantages of an online BRCA1/2 JFM screening model, which could remove the main barriers to uptake, the need for physician referral (Hafertepen et al. 2017) and the long wait for a genetics consultation (Delikurt et al. 2015). Furthermore, the website enabled self-paced learning, a feature that was largely commended by participants, where the freedom of doing it at their own time and pace was appreciated and seemed to provide a more conducive environment for learning (de Jonge et al. 2015).
Another study that designed its own pre-test, self-directed, online education module to facilitate decision-making in parents considering the uptake of clinical genomic testing to diagnose autism spectrum disorders (ASD) reported that the online module was effective in improving the parent’s knowledge regarding the uses and implications of genomic testing for ASD (Xu et al. 2018). Likewise, while most participants reported that the pre-test website was informative, empowering their decisions regarding undergoing testing, there were considerable suggestions to streamline the pre-test information on a need-to-know basis. There was feedback that information provided was overly detailed and irrelevant to the stage of pre-testing. This finding is concordant to another qualitative study that offered written pre-test information (Lieberman et al. 2016a), where stepwise knowledge was preferred. In this construct, only the information necessary to make an informed decision for testing should be provided, which differs from the information that should be provided after being identified as a carrier (i.e. risk-reducing medications, cancer screening, prophylactic surgery). Streamlining the current website with stepwise knowledge could further reduce the time needed for pre-test education, possibly promoting greater time efficiencies and subsequent uptake to BRCA1/2 JFM screening, appealing to those who are time-poor or those who may be previously overwhelmed by the amount of information on the website.
The study also explored how an online model (i.e. email) of negative result disclosure compares with in-person (meeting with a genetic counsellor) methods. Most of the participants were satisfied with the method in which their negative results were returned to them. There was consensus that an email would suffice for the return of negative results from those who were notified by email. In other studies exploring the return of negative results, concordant findings were observed; there were no indications of psychological distress that accompanied the return of negative results (Butterfield et al. 2018; Hamilton et al. 2009). Unfortunately, these perspectives and outcomes are only representative of noncarriers. Notwithstanding, in a randomized non-inferiority trial that compared the disclosure of positive exome testing results via email or in person from a genetic counsellor, it established the non-inferiority of email result disclosure amongst post-reproductive, healthy adults (Biesecker et al. 2018). However, this is confounded by a quantitative study that explored participants’ preferences for the return of negative BRCA1/2 results, where a minority (32%) was open to receiving result via Web-based platforms, citing disadvantages which included the impersonal nature of an email, the risk of misinterpreting the result and the inability to ask questions (Gaieski et al. 2019). These disparate findings highlight the need to replicate such studies in larger sample sizes, representative of carriers and noncarriers.
In the DTC realm, the result disclosure process is commonly done via email or a company-specific online platform, where there has been much controversy on how appropriate such methods are for the communication of genetic test results; there have been cases of significant psychological distress, and therein lies the possibility of misinterpretation from online modes of result disclosure. Dohany et al. (2012) highlighted a case of significant psychological distress in a woman who received a 1.5-page email outlining her DTC genetic test result which identified her carrier status of a BRCA2 AJ founder mutation (Dohany et al. 2012). She recounted her experience with shock, anxiety and confusion which led to distancing oneself from family to avoid sharing her positive result. While this highlights the detriments and dangers of returning a positive result via email, this model of BRCA1/2 JFM screening is designed to minimize such an experience as all identified carriers are invited (via email) to schedule an in-person result disclosure appointment with a genetic counsellor and/or clinical geneticist. All carriers (identified through JeneScreen) received a post-result consultation to aid in the understanding and adaptation to the new knowledge of what it means to be a carrier. Furthermore, we can expect that a targeted screening programme, specific to BRCA1/2 JFM, would present a lower likelihood for the return of unexpected results, as participants who consented to testing would have received compulsory pre-test website education, which outlined the possibility of a positive BRCA1/2 result, minimizing the chance for shock on receipt.
In a bid to meet the resource challenges for the increasing demand for genetic testing services, Web-based tools have been developed to manage the return of genetic test results. My46 is an online tool designed to manage the return of genetic test results; it allows clients to log their result return preferences (Tabor et al. 2017). It supports the result disclosure process as indicated by the client with an in-built option to connect to a genetic counsellor, provides accompanying education regarding results and enables client assessment on their experience to gather feedback. Minimally, this model of BRCA1/2 JFM screening could benefit from allowing preferences for result disclosure to be indicated and an in-built option to connect to a local genetic counsellor would be an improvement to the current model. Such Web-based tools could be an effective platform for the return of results that minimizes the likelihood for psychosocial distress but nonetheless would require further research on carriers and noncarriers to understand the acceptability and satisfaction associated with its utilization for clinical practice.
This model of clinical BRCA1/2 JFM screening is aligned with the shift away from the traditional in-person counselling model and towards telehealth genetic services (Zierhut et al. 2018) to increase accessibility of genetic counselling and testing (Rayes et al. 2019). In a recent study evaluating telephone genetic counselling services in women affected with high-grade serous ovarian cancer, both BRCA1/2 carriers and noncarriers reported accepting, satisfactory attitudes and minimal regret towards testing (Tutty et al. 2019), which are largely consistent with the positive responses of our cohort. It was also noted that carriers were not observed to carry additional psychosocial burden as compared to noncarriers. A main feature of this model, which provides a more convenient yet satisfactory alternative to in-person genetic services, has also been highlighted by other studies offering telephone genetic counselling (Kinney et al. 2014; Schwartz et al. 2014).
Lastly, our findings reveal that a personal responsibility to health was a main motivator to undergo genetic screening. It is consistent with the motivations to uptake traditional clinical genetic testing (Brunstrom et al. 2016). Interestingly, in this study, the participants expressed an overarching altruistic motivation to contribute their data to research or to help others in the Jewish community. This differs from studies that found people tended to altruistic participation in genetic testing mainly for the benefit of family members, instead of unrelated members of a community (Baskovich et al. 2016; Garg et al. 2016; Raz and Schicktanz 2009). Such altruistic motivation within the Jewish community in the Sydney is encouraging and could indicate the success of a screening programme in this community, as they are highly motivated and receptive to genetic testing conducted for the benefit of their health and community research.
Practice implications: genetic counselling
Traditionally, individuals would attend an hour-long pre-test genetic counselling session prior to genetic testing – an approach that would be time- and resource-intensive if BRCA1/2 JFM population screening is to become a reality (Metcalfe et al. 2015). With concurrent efforts advocating for BRCA1/2 JFM screening in the Jewish community, our findings supplement other clinical and DTC findings that an online model for the pre-test education and collection of consent could be an acceptable approach for clinical BRCA1/2 JFM screening within the Jewish community. This could significantly help to alleviate time and resource challenges faced by current genetic services (Johnstone et al. 2016) by reducing the need for in-person pre-test genetic counselling for BRCA1/2 JFM population screening.
While this model incorporates the use of email to return negative results and an email invitation to identified carriers for an in-person result disclosure session with a genetic counsellor, the satisfactory stance on the result notification process is limited to that of noncarriers. Our result suggests that the return of negative results via email could be feasible model for the design of a BRCA1/2 JFM screening programme.
Study limitations
There are some limitations to this study. Selection bias arising from our recruitment is a limitation, as interviewees were individuals who had previously indicated their interest to undergo BRCA1/2 screening (Cousens et al. 2017). These participants may be generally more interested in issues related to the accessibility of BRCA1/2 testing, more so than other members in the Jewish community. Therefore, our findings cannot be generalized to the entire Jewish population in Sydney, Australia, due to the following reasons: sample size was small, majority was females and there were no carriers identified. The prospective recruitment of participants into this interview study failed to ensure the inclusion of carriers. Our findings may inadvertently be an overestimation of the positivity and acceptance towards an online platform for pre-test education and the return of results; carriers may be inclined to experience higher levels of cancer-related distress (Bredart et al. 2013; Farrelly et al. 2013) and may then prefer in-person methods for pre-test counselling. Notwithstanding, BRCA1/2 JFM carriers are expected to make up only 2–3% of participants (Bahar et al. 2001; Manchanda et al. 2017); this study has been designed to evaluate the experience of the JeneScreen programme on this majority of participants. A separate evaluation of carriers is underway. Lastly, the cohort recruited was highly educated, which may be atypical, yet may be representative of future participants in clinical screening (Lieberman et al. 2016b). In this respect, this study provides insights to the profile of participants who would be satisfied with undergoing online BRCA1/2 JFM screening in place of in-person genetic counselling.
Research implications
While this study offers evidence that online pre-test provision of information for BRCA1/2 JFM testing could be an acceptable and satisfactory alternative to in-person pre-test genetic counselling, additional studies are required to corroborate this in carriers. There is a need to qualitatively assess the experience of carriers identified through online BRCA1/2 JFM screening – an experience expected to differ from noncarriers due to the need for post-test genetic counselling regarding risk-reducing strategies and familial implications.
As this is one of the few studies utilizing a Web-based platform for result disclosure, it would be meaningful to explore which models of result disclosure are suitable for carriers and noncarriers in a model of screening that uses alternative telehealth methods for pre-test information provision.
Although attitudes towards receiving pre-test information via a website are positive and promising, more work is required to understand those who might otherwise be unsuitable for online testing, if factors, such as age, perception of cancer, family history of cancer, computer or health literacy, may affect one’s suitability in using Web-based platforms to facilitate pre-test education and informed consent to genetic testing. For instance, women aged in their forties are more likely to be impacted by a positive result, as compared to postmenopausal women, as their options for risk-reducing screening and/or surgery would be immediately apparent – indicating the need to stratify further research based on the consequences that a positive BRCA1/2 result might have on one’s risk management options.
Lastly, considering the small sample size of this study, larger sample sizes of quantitative research are still needed to measure satisfaction, knowledge, distress, decisional conflict and decisional regret to further assess the psychosocial outcomes arising from online models offering alternative to in-person pre-test counselling and the subsequent return of results to carriers and noncarriers. This is currently being carried out in an ongoing study.
Conclusions
Our model of BRCA1/2 JFM screening that uses a website for pre-test education and consent, replacing in-person pre-test counselling, resulted in high participant satisfaction and a strong preference for the website over in-person counselling. This suggests that a website for pre-test education and collection of informed consent could be an acceptable approach for the design of BRCA1/2 JFM screening within the Jewish community. A model of BRCA1/2 JFM screening that uses a website for pre-test education in place of in-person counselling could provide greater time and resource efficiencies for participants and genetic counsellors.
While the subsequent result disclosure process to noncarriers via email or in-person methods was similarly well-received, further research is needed to explore the experience of carriers identified from models of screening that utilizes telehealth methods to replace in-person pre-test counselling and post-test result disclosure.
Electronic supplementary material
(PDF 519 kb).
Acknowledgements
We thank all individuals who participated in this study for their invaluable contribution. Thank you to Ovarian Cancer Australia and Murdoch Children’s Research Institute for the collaboration in this study. Thank you to Wolper Jewish Hospital, Jewish Communal Appeal and all philanthropic donors, for this study could not be possible without them.
Compliance with ethical guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of informed consent
Informed consent was obtained from all patients for being included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Albada A, van Dulmen S, Ausems MG, Bensing JM. A pre-visit website with question prompt sheet for counselees facilitates communication in the first consultation for breast cancer genetic counseling: findings from a randomized controlled trial. Genet Med. 2012;14:535–542. doi: 10.1038/gim.2011.42. [DOI] [PubMed] [Google Scholar]
- Alsop K, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian ovarian cancer study group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/jco.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Bureau of Statistics, ABS (2016) 8146.0 - Household Use of Information Technology, Australia, 2014–15. http://www.abs.gov.au/ausstats/abs@.nsf/mf/8146.0. Accessed 2017 18 May
- Bahar AY, et al. The frequency of founder mutations in the BRCA1, BRCA2, and APC genes in Australian Ashkenazi Jews. Cancer. 2001;92:440–445. doi: 10.1002/1097-0142(20010715)92:2<440::AID-CNCR1340>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Baskovich B, et al. Expanded genetic screening panel for the Ashkenazi Jewish population. Genet Med. 2016;18:522–528. doi: 10.1038/gim.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker BB, Lewis KL, Umstead KL, Johnston JJ, Turbitt E, Fishler KP, Patton JH, Miller IM, Heidlebaugh AR, Biesecker LG. Web platform vs in-person genetic counselor for return of carrier results from exome sequencing: a randomized clinical trial. JAMA Intern Med. 2018;178:338–346. doi: 10.1001/jamainternmed.2017.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Clarke V (2014) What can “thematic analysis” offer health and wellbeing researchers? Int J Qual St Health Well-being 9. 10.3402/qhw.v9.26152 [DOI] [PMC free article] [PubMed]
- Bredart A, et al. Short-term psychological impact of the BRCA1/2 test result in women with breast cancer according to their perceived probability of genetic predisposition to cancer. Br J Cancer. 2013;108:1012–1020. doi: 10.1038/bjc.2012.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstrom K, Murray A, McAllister M. Experiences of women who underwent predictive BRCA 1/2 mutation testing before the age of 30. J Genet Couns. 2016;25:90–100. doi: 10.1007/s10897-015-9845-5. [DOI] [PubMed] [Google Scholar]
- Butterfield RM et al (2018) Returning negative results to individuals in a genomic screening program: lessons learned. Genet Med. 10.1038/s41436-018-0061-1 [DOI] [PMC free article] [PubMed]
- Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008;124:31–42. doi: 10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- Cousens N, Kaur R, Meiser B, Andrews L. Community attitudes towards a Jewish community BRCA1/2 testing program. Fam Cancer. 2017;16:17–28. doi: 10.1007/s10689-016-9918-0. [DOI] [PubMed] [Google Scholar]
- de Jonge M, Tabbers HK, Pecher D, Jang Y, Zeelenberg R. The efficacy of self-paced study in multitrial learning. J Exp Psychol Learn Mem Cogn. 2015;41:851–858. doi: 10.1037/xlm0000046. [DOI] [PubMed] [Google Scholar]
- Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2015;23:739–745. doi: 10.1038/ejhg.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohany L, Gustafson S, Ducaine W, Zakalik D. Psychological distress with direct-to-consumer genetic testing: a case report of an unexpected BRCA positive test result. J Genet Couns. 2012;21:399–401. doi: 10.1007/s10897-011-9475-5. [DOI] [PubMed] [Google Scholar]
- Domchek SM, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DG, et al. Pathology update to the Manchester scoring system based on testing in over 4000 families. J Med Genet. 2017;54:674–681. doi: 10.1136/jmedgenet-2017-104584. [DOI] [PubMed] [Google Scholar]
- Farrelly A, et al. Unmet support needs and distress among women with a BRCA1/2 mutation. Fam Cancer. 2013;12:509–518. doi: 10.1007/s10689-012-9596-5. [DOI] [PubMed] [Google Scholar]
- Gabai-Kapara E, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaieski JB, Patrick-Miller L, Egleston BL, Maxwell KN, Walser S, DiGiovanni L, Brower J, Fetzer D, Ganzak A, McKenna D, Long JM, Powers J, Stopfer JE, Nathanson KL, Domchek SM, Bradbury AR. Research participants' experiences with return of genetic research results and preferences for web-based alternatives. Mol Genet Genom Med. 2019;7:e898. doi: 10.1002/mgg3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Vogelgesang J, Kelly K. Impact of genetic counseling and testing on altruistic motivations to test for BRCA1/2: a longitudinal study. J Genet Couns. 2016;25:572–582. doi: 10.1007/s10897-015-9911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafertepen L, Pastorino A, Morman N, Snow J, Halaharvi D, Byrne L, Cripe M. Barriers to genetic testing in newly diagnosed breast cancer patients: do surgeons limit testing? Am J Surg. 2017;214:105–110. doi: 10.1016/j.amjsurg.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol. 2009;28:510–518. doi: 10.1037/a0014778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA 1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet. 1999;64:963–970. doi: 10.1086/302320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HGSA, Australasia HGSo (2014) Pre-symptomatic and predictive testing for genetic disorders. Alexandria
- Johnstone B, Kaiser A, Injeyan MC, Sappleton K, Chitayat D, Stephens D, Shuman C. The relationship between burnout and occupational stress in genetic counselors. J Genet Couns. 2016;25:731–741. doi: 10.1007/s10897-016-9968-3. [DOI] [PubMed] [Google Scholar]
- Kinney AY et al (2014) Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst 106. 10.1093/jnci/dju328 [DOI] [PMC free article] [PubMed]
- Lieberman S, Lahad A, Tomer A, Cohen C, Levy-Lahad E, Raz A (2016a) Population screening for BRCA1/BRCA2 mutations: lessons from qualitative analysis of the screening experience. Genet Med. 10.1038/gim.2016.175 [DOI] [PubMed]
- Lieberman S et al (2016b) Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: proactive recruitment compared with self-referral. Genet Med. 10.1038/gim.2016.182 [DOI] [PubMed]
- Ludwig KK, Neuner J, Butler A, Geurts JL, Kong AL. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg. 2016;212:660–669. doi: 10.1016/j.amjsurg.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Manchanda R, Legood R, Burnell M, McGuire A, Raikou M, Loggenberg K, Wardle J, Sanderson S, Gessler S, Side L, Balogun N, Desai R, Kumar A, Dorkins H, Wallis Y, Chapman C, Taylor R, Jacobs C, Tomlinson I, Beller U, Menon U, Jacobs I. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi Jewish women compared with family history-based testing. J Natl Cancer Inst. 2015;107:380. doi: 10.1093/jnci/dju380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchanda R, Loggenberg K, Sanderson S, Burnell M, Wardle J, Gessler S, Side L, Balogun N, Desai R, Kumar A, Dorkins H, Wallis Y, Chapman C, Taylor R, Jacobs C, Tomlinson I, McGuire A, Beller U, Menon U, Jacobs I. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: a randomized controlled trial. J Natl Cancer Inst. 2015;107:379. doi: 10.1093/jnci/dju379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchanda R et al (2017) Cost-effectiveness of population based BRCA testing with varying Ashkenazi Jewish ancestry. Am J Obstet Gynecol. 10.1016/j.ajog.2017.06.038 [DOI] [PubMed]
- Manickam K, Buchanan AH, Schwartz MB, et al. Exome sequencing–based screening for brca1/2 expected pathogenic variants among adult biobank participants. JAMA Network Open. 2018;1:e182140. doi: 10.1001/jamanetworkopen.2018.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe KA, et al. Patient satisfaction and cancer-related distress among unselected Jewish women undergoing genetic testing for BRCA1 and BRCA2. Clin Genet. 2010;78:411–417. doi: 10.1111/j.1399-0004.2010.01499.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Poll A, Royer R, Llacuachaqui M, Tulman A, Sun P, Narod SA. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28:387–391. doi: 10.1200/JCO.2009.25.0712. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Eisen A, Lerner-Ellis J, Narod SA. Is it time to offer BRCA1 and BRCA2 testing to all Jewish women? Curr Oncol. 2015;22:e233–e236. doi: 10.3747/co.22.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslehi R, et al. BRCA1 and BRCA2 mutation analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am J Hum Genet. 2000;66:1259–1272. doi: 10.1086/302853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos E, Weissman SM. The dawn of consumer-directed testing. Am J Med Genet C Semin Med Genet. 2018;178:89–97. doi: 10.1002/ajmg.c.31603. [DOI] [PubMed] [Google Scholar]
- Ratnayake P, Wakefield CE, Meiser B, Suthers G, Price MA, Duffy J, Tucker K. An exploration of the communication preferences regarding genetic testing in individuals from families with identified breast/ovarian cancer mutations. Fam Cancer. 2011;10:97–105. doi: 10.1007/s10689-010-9383-0. [DOI] [PubMed] [Google Scholar]
- Rayes N, et al. MAGENTA (making genetic testing accessible): a prospective randomized controlled trial comparing online genetic education and telephone genetic counseling for hereditary cancer genetic testing. BMC Cancer. 2019;19:648. doi: 10.1186/s12885-019-5868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz AE, Schicktanz S. Diversity and uniformity in genetic responsibility: moral attitudes of patients, relatives and lay people in Germany and Israel. Med Health Care Philos. 2009;12:433–442. doi: 10.1007/s11019-009-9215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea MK, Rudas M, Singer CF, Helbich TH. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33:1128–1135. doi: 10.1200/jco.2014.56.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E, Moyes K, Arnell C, Evans B, Wenstrup RJ. Incidence of BRCA1 and BRCA2 non-founder mutations in patients of Ashkenazi Jewish ancestry. Breast Cancer Res Treat. 2015;149:223–227. doi: 10.1007/s10549-014-3218-x. [DOI] [PubMed] [Google Scholar]
- Rubinstein WS, Jiang H, Dellefave L, Rademaker AW. Cost-effectiveness of population-based BRCA1/2 testing and ovarian cancer prevention for Ashkenazi Jews: a call for dialogue. Genet Med. 2009;11:629–639. doi: 10.1097/GIM.0b013e3181afd322. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, Nusbaum R, Huang AT, Chang Y, Graves K, Isaacs C, Wood M, McKinnon W, Garber J, McCormick S, Kinney AY, Luta G, Kelleher S, Leventhal KG, Vegella P, Tong A, King L. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32:618–626. doi: 10.1200/jco.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie AS, et al. High satisfaction and low distress in breast cancer patients one year after BRCA-mutation testing without prior face-to-face genetic counseling. J Genet Couns. 2016;25:504–514. doi: 10.1007/s10897-015-9899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor HK, et al. My46: a Web-based tool for self-guided management of genomic test results in research and clinical settings. Genet Med. 2017;19:467–475. doi: 10.1038/gim.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutty E, et al. Evaluation of telephone genetic counselling to facilitate germline BRCA1/2 testing in women with high-grade serous ovarian cancer. Eur J Hum Genet. 2019;27:1186–1196. doi: 10.1038/s41431-019-0390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, Ozcelik H, Goss P, Allingham-Hawkins D, Hamel N, di Prospero L, Contiga V, Serruya C, Klein M, Moslehi R, Honeyford J, Liede A, Glendon G, Brunet JS, Narod S. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst. 1999;91:1241–1247. doi: 10.1093/jnci/91.14.1241. [DOI] [PubMed] [Google Scholar]
- Wiesman C, Rose E, Grant A, Zimilover A, Klugman S, Schreiber-Agus N (2016) Experiences from a pilot program bringing BRCA1/2 genetic screening to the US Ashkenazi Jewish population. Genet Med. 10.1038/gim.2016.154 [DOI] [PubMed]
- Wilson JM, Jungner YG. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam. 1968;65:281–393. [PubMed] [Google Scholar]
- Xu L, Richman AR, Mitchell LC, Luo H, Jiang YH, Roy S, Floyd AE. Evaluating Web-based educational modules on genetic testing for autism among parents of children with autism. Am J Health Behav. 2018;42:3–12. doi: 10.5993/ajhb.42.4.1. [DOI] [PubMed] [Google Scholar]
- Zierhut HA, MacFarlane IM, Ahmed Z, Davies J. Genetic counselors’ experiences and interest in telegenetics and remote counseling. J Genet Couns. 2018;27:329–338. doi: 10.1007/s10897-017-0200-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 519 kb).