Abstract
Germline mutations in the cylindromatosis gene (CYLD) are associated with a rare autosomal dominant disease known as CYLD cutaneous syndrome (CCS). Patients present multiple neoplasms originating from skin appendages. Here, we investigated the main clinical and molecular features of a large family with CCS having lived in a small Brazilian town for 6 generations, making its prevalence significantly high. We observed a predominance of the disease among males and a wide phenotypic variation. A high frequency of basal cell carcinomas among affected people was found. The mutation c.2806C>T, p.Arg936* in the CYLD gene was detected in all patients. In this work, a geographical cluster of CCS was found, which raised some community genetics issues related not only to the high prevalence of a rare disease in a limited area but also to the strong social stigma associated with the disease.
Keywords: CYLD, Trichoepithelioma, Cylindroma, Brooke-Spiegler, Basal cell carcinoma, Medical population genetics
Introduction
Germline mutations in the cylindromatosis (CYLD) gene (NCBI RefSeq: NM_015247.2) are associated with three rare skin appendage autosomal dominant genodermatoses with tumor predisposition: familial cylindromatosis (FC; OMIM 132700), multiple familial trichoepithelioma 1 (MFT1; OMIM: 601606), and Brooke-Spiegler syndrome (BSS; OMIM 605041) (Parren et al. 2018).
Typically, FC is characterized by the presence of multiple cylindromas (benign tumors with differentiation towards apocrine sweat glands) located on the scalp and face, while MFT1 presents various trichoepitheliomas (numerous firm skin-colored papules originating from hair follicles) mainly at the center of the face. Patients with BSS, on the other hand, usually present cylindromas, trichoepitheliomas, and/or other skin appendage tumors, such as spiradenomas (Nagy et al. 2015). Although they are usually benign diseases, an association with malignancy has been reported (Kallam et al. 2016), and lesions might cause social and psychological issues due to cosmetic disfigurement, which can affect the quality of life of patients (Karimzadeh et al. 2018).
Currently, FC, MFT1, and BSS are considered a phenotypic spectrum of the same disorder, since members of the same family with the CYLD mutation may present any of the three phenotypes (Bowen et al. 2005; Parren et al. 2018). The clinical differentiation among FC, MFT1, and BSS has no prognostic or diagnostic value, and the term “CYLD cutaneous syndrome” (CCS) for individuals carrying a CYLD mutation has been proposed (Rajan et al. 2009).
The CYLD gene is located at 16q12.1 and contains 20 exons. Generally, a single heterozygous mutation within exons 9–20 is responsible for the phenotype (Verhoeft et al. 2016). CYLD is a tumor suppressor gene that encodes an enzyme with deubiquitinase activity that regulates many cellular and signaling pathways, especially the nuclear factor-κB (NF-κB) signaling pathway (Farkas et al. 2016; Verhoeft et al. 2016). However, many biological aspects of the cutaneous syndrome related to mutations in CYLD remain poorly understood, such as the molecular mechanisms of phenotypic variation, the significance of frameshift and splice-site mutations, and the complex interacting network of the CYLD protein (Nagy et al. 2015).
Clinical, molecular, and epidemiological research of CCS has been limited in Brazil and Latin America (Nagy et al. 2015). Here, we report the prevalence of the disease in a Brazilian town where a large family affected by this disease lives, with a discussion of the molecular and clinical descriptions, phenotypic variation, and sex ratio distortion.
Materials and methods
Subjects and pedigree building
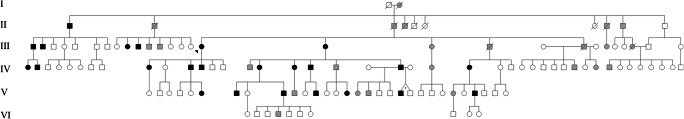
The family investigated here lives in the Brazilian northeast coastal town of Aracati (state of Ceará; 4° 33′ 43″ S, 37° 46′ 12″ W). After consecutive meetings with its members, a six-generation pedigree was built (Fig. 1) using Progeny v.7.0 software. To estimate the prevalence of the disease in Aracati in 2017, data from the Brazilian Institute of Geography and Statistics were collected. This study was approved by the Federal University of Rio Grande do Sul Ethics Committee (CAEE 30802513.0.0000.5347), and informed consent was obtained from all participants or their guardians.
Fig. 1.
Pedigree of the family with CYLD cutaneous syndrome (CCS). Black symbols represent individuals who were clinically diagnosed with CCS, and gray symbols represent individuals who were reported to have signs compatible with the syndrome
Clinical evaluation
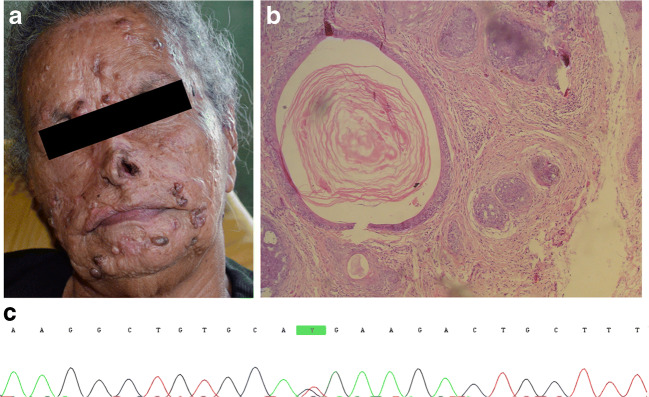
Family members were evaluated by a medical geneticist and a dermatologist who performed physical evaluation and dermatoscopy of the lesions. Skin biopsy for histological analyses was performed in the proband (III-16), a 69-year-old woman with signs compatible with CCS since the age of 7 years (Fig. 2a).
Fig. 2.
Clinical, histopathological, and molecular features. a Proband’s face showing numerous papules as well as scars of esthetic treatments performed previously. b Histopathological features of a lesion removed from proband showing islands of basaloid cells with keratinized cysts surrounded by follicular stroma. c Mutation c.2806C>T (p.R936X) in exon 20 of CYLD gene
Molecular screening
Saliva samples from 31 individuals (24 affected and 7 unaffected) were collected with the Oragene® DNA kit (Genotek, Ottawa, Ontario, Canada), and DNA extraction was performed according to the manufacturer’s recommendations. PCR was performed using primers specific for all 20 exons and exon-intron boundaries of the CYLD gene. The amplified products were purified following Exonuclease I and Alkaline Phosphatase (Amersham Biosciences–GE Healthcare, Piscataway) protocols. Both DNA strands were sequenced using an ABI 3730/3730x Sequence Analyzer (Life Technologies, Carlsbad, CA). Putative mutations were resequenced for confirmation. DNA sequence analysis was performed with CodonCode Aligner software. All statistical analyses were carried out using SPSS v18.0 software.
Results
Prevalence estimate and pedigree analysis
The six-generation pedigree confirmed autosomal dominant inheritance, with at least 48 people reportedly affected (Fig. 1). However, there was a predominance of males among those affected (30/48 or 62.5%). We personally evaluated 24 patients (14 men/10 women). Therefore, the minimum prevalence of the disease for Aracati (CE) was 3.5/10,000 (24/69,159 inhabitants). If we consider all individuals living in the town and those who were reported as affected, the prevalence may reach 5.1/10,000 (35/69,159).
Clinical and histological evaluation
The main clinical characteristics of the 24 individuals are summarized in Table 1. The reported average age of onset of the lesions was 10.8 years (range 3–18, SD 4.4) for men and 10.5 years (range 1–21, SD 5.7) for women. Histopathological findings of the proband’s skin biopsy showed basaloid cell aggregates with keratinized cysts surrounded by specific follicular stroma, typical of trichoepithelioma (Fig. 2b) (Karimzadeh et al. 2018). Dermatological examination revealed numerous, firm, and dome-shaped skin papules mainly on the forehead, nose, nasolabial folds, and perioral region, which ranged in size from 2 to 10 mm (mean 4.0 mm), with the formation of coalescent lesions in 18 cases. The most common site where lesions appeared was the face, but lesions off the face were found in 13 patients, mainly on the shoulders, arms, back, and chest. In addition, lesions on the scalp were found in only one patient.
Table 1.
Main clinical characteristics of all affected subjects
| Case | Gender | Age (years) | Age of onset (years) | Mean lesion size (mm) | Out of the face location | Confluence of adjacent lesions | Presence of BCC | Associated inflammatory process |
|---|---|---|---|---|---|---|---|---|
| IV-1 | F | 33 | 1 | 4 | yes | yes | no | yes |
| IV-2 | M | 18 | 3 | 3 | no | yes | no | no |
| III-1 | M | 56 | 10 | 7 | yes | yes | yes | yes |
| III-2 | M | 45 | 13 | 5 | yes | yes | no | no |
| II-1 | M | 92 | 18 | 8 | yes | no | no | no |
| III-16 | F | 69 | 7 | 5 | yes | yes | yes | yes |
| IV-9 | F | 35 | 13 | 3 | no | yes | no | no |
| IV-11 | M | 50 | ? | 5 | no | yes | no | no |
| IV-12 | M | 31 | ? | 5 | no | yes | no | no |
| V-6 | F | 29 | 8 | 4 | yes | yes | yes | no |
| III-17 | F | 72 | 12 | 10 | yes | yes | yes | yes |
| IV-21 | M | 46 | 9 | 3 | yes | yes | yes | yes |
| IV-16 | F | 49 | 10 | 3 | yes | yes | yes | yes |
| V-7 | M | 25 | 14 | 2 | no | no | no | no |
| IV-17 | F | 40 | ? | 3 | no | yes | no | no |
| IV-18 | M | 51 | 10 | 2 | yes | yes | yes | yes |
| V-9 | M | 29 | ? | 3 | no | no | no | no |
| V-12 | M | 27 | 6 | 2 | no | no | no | no |
| V-20 | M | 18 | 15 | 2 | yes | no | no | yes |
| V-15 | F | 15 | 12 | 3 | no | no | no | no |
| IV-24 | F | 44 | 21 | 2 | no | yes | no | no |
| V-27 | M | 27 | ? | 3 | yes | yes | no | no |
| III-9 | F | 43 | ? | 4 | no | yes | no | no |
| III-10 | M | 41 | 10 | 3 | yes | yes | no | no |
| Total men | 14 | 39.7 (19.6)† | 10.8 (4.4)† | 3.8 (1.9)† | 8 (57.1%)‡ | 9 (64.3%)‡ | 3 (21.4%)‡ | 4 (28.6%)‡ |
| Total women | 10 | 42.9 (17.3)† | 10.5 (5.7)† | 4.1 (2.2)† | 5 (50%)‡ | 9 (90%)‡ | 4 (40%)‡ | 4 (40%)‡ |
?, unavailable; BCC, basal cell carcinoma; †, mean (standard deviation); ‡, number of “yes” (percentage inside gender)
The number and severity of the lesions was variable regardless of age and sex. For instance, the 56-year-old male (III-1) had a greater number of lesions than the 92-year-old male (II-1), while the 29-year-old (V-6) female had more lesions than the 33-year-old female (IV-24). Four women (III-16, III-17, IV-16, and V-6) and three men (III-1, IV-18, and IV-21) were diagnosed with basal cell carcinoma close to the lesions.
Molecular evaluation
The nonsense mutation c.2806C>T, p.Arg936* in exon 20 of the CYLD gene (Fig. 2c) was detected in all affected family members and none of the unaffected relatives. The C to T substitution results in arginine being replaced by a stop codon that is predicted to lead to premature chain termination (Young et al. 2006).
Discussion
CCS is a rare genetic disease, and its exact prevalence around the world is unknown (Karimzadeh et al. 2018). The prevalence of CYLD germline mutations in the UK population was estimated to be ~ 1:100,000 (Dubois et al. 2015), much lower than its minimum prevalence estimated in Aracati (3.5/10,000). A geographical cluster, such as this one, prompts the implementation of tailored health policies directed towards this community (Giugliani et al. 2019). In addition to genetic counseling and clinical support offered to family members, it is important to implement educational strategies aimed at the whole community to avoid stigmatization of the family members and the community itself. Clusters of genetic diseases are not uncommon in Brazil, particularly in the northeast region, where cultural habits associated with poverty frequently keep people in the same geographical location (Cardoso et al. 2018). Moreover, the stigma related to the disease (deforming, fear of being contagious) might prevent affected individuals from moving from their local residence.
Clinical and histopathological characteristics indicated a higher presence of trichoepitheliomas; however, the disease was widely variable among individuals. The main variable characteristics observed among affected people were the location of the lesions, the presence of the inflammatory process and basal cell carcinoma. This phenotypic variation of CCS has been extensively reported elsewhere (Young et al. 2006; Rajan et al. 2009; Parren et al. 2018). Modifier genotypes, epigenetic mechanisms, and environmental factors may contribute to the phenotypic variation of the disease. The family reported here lives in a coastal city in northeast Brazil, only 4 ° south of the Equator, with strong exposure to sunlight nearly all year (Corrêa 2015). Cumulative ultraviolet exposure plays an important role in photodamage, and it has been extensively related to the etiopathogenesis of multiple cutaneous disorders, including cancer, abnormal pigmentation, and aging (Massoumi 2010; Schuch et al. 2017; Atzori et al. 2018). Thus, intense exposure to sunlight may have had an effect on the phenotypic expression in this family and may explain the high prevalence of basal cell carcinoma.
Interestingly, we observed sex differences in the frequency of the lesions. Other reports also observed a sex distortion, with an excess of female affected individuals (Rajan et al. 2009; Karimzadeh et al. 2018). Here, the higher prevalence was in males. We believe that this could be partially explained by the fact that among the 113 individuals within the family, 61 (54%) are men. Another explanation might be that in the members of this family, the majority of men work as fishermen or on farmlands, while women keep more domestic activities and consequently have less exposure to sunlight.
Several reports have described patients with benign skin appendageal tumors carrying germline mutations in CYLD, as reviewed by Nagy et al. (2015). The nonsense mutation c.2806C>T (p.R936X) in exon 20 of the CYLD gene was already described in other studies, and it has been associated with MFT1 (Zhang and Liang 2015) or FC alone (Bignell et al. 2000; Saggar et al. 2008) and in the spectrum of BSS (Bowen et al. 2005; Young et al. 2006; Saggar et al. 2008; Grossmann et al. 2013; Nagy et al. 2013). The absence of the mutation in all six unaffected individuals points to a complete penetrance phenotype in this family. Nonsense mutations account for approximately 27% of the CYLD mutations identified thus far (Nagy et al. 2015), leading to a truncated protein with potential loss of function of the CYLD protein, losing its catalytic function of deubiquitinating other target proteins, especially the NF-κB signaling pathway, such as TRAF2, TRAF6, NEMO, and BCL3 (Farkas et al. 2016; Parren et al. 2018).
In our study, seven affected individuals were also diagnosed with basal cell carcinoma. This finding strengthens the association of mutations in CYLD with malignancy, which has been associated in some studies not only with basal cell carcinoma but also with head and neck tumorigenesis and salivary gland malignancy (Young et al. 2006; Kallam et al. 2016; Verhoeft et al. 2016). In fact, CYLD is involved in the regulation of biological processes such as cell proliferation and inflammation, and loss of the deubiquitinating activity of CYLD protein has been correlated with tumorigenesis (Liang et al. 2005; Nagy et al. 2015). Therefore, the awareness of the potential development of carcinoma in patients carrying CYLD mutations is important (du Toit et al. 2016; Kallam et al. 2016). Consequently, guidance on photoprotection was provided to all study participants.
There is still no cure for CCS. The patients reported here have had uncomfortable lesions or suspected malignancy removed through repeated surgeries. We were able to offer suitable genetic counseling to anyone who wished it. In addition, we are developing informative booklets on basic aspects of the disease to be shared with the family and the town’s health care settings. Future research in this and other families with CCS around the world may help to reveal key aspects of the disease, as well as provide important insights into human tumorigenesis and treatment.
This study also raises some interesting aspects of monogenic disorders at the community level (Giugliani et al. 2019). Autosomal recessive diseases are known to be associated with isolation and endogamy, and clusters of these disorders can be found in the Brazilian northeast at a relatively high frequency (dos Santos et al. 2013; Cardoso et al. 2018). Here, we studied an autosomal dominant disease in a large family with many members having lived in the same small town for 6 generations, making its prevalence significantly high. A few members have now emigrated to Fortaleza, the capital of Ceará, but the majority still live in Aracati, as they did about 100 years ago. Although we provided individual genetic counseling for the family members who wanted it, we should also provide knowledge for the community. A strong prejudice is pervasive in the community since CCS produces deforming lesions (whose effects can be even more severe with the presence of BCC), and there is an unfounded fear of the lesions being contagious. Moreover, an autosomal dominant trait evidently runs in the family and can reinforce their exclusion from the community. Consequently, our efforts were also directed to spread information to health professionals—both for appropriate medical care and for understanding the biological basis of the disease.
Conclusion
CYLD cutaneous syndrome is considered rare around the world, but a cluster due to a large family in a coastal town in the Brazilian northeast was found. The investigation of six generations of affected individuals with CCS permitted the study of important aspects of the disease, such as a sex ratio distortion and a high frequency of basal cell carcinomas among affected people. Moreover, we explored the impact of the disease when many individuals in one large family in a small community are affected. This work reinforces the need for health professionals to be prepared to host, care, and provide genetic counseling for those patients.
Acknowledgments
The authors thank the whole family who always received us with great care and joy.
Funding information
This work was supported by the National Institute of Population Medical Genetics (INAGEMP; CNPq 465549/2014-4).
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Federal University of Rio Grande do Sul Ethics Committee, CAEE 30802513.0.0000.5347) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anderson Pontes Arruda and Augusto César Cardoso-dos-Santos contributed equally to this work.
References
- Atzori L, Corbeddu M, Fumo G et al (2018) Trichoepithelioma arising in a congenital melanocytic nevus of an adult: a diagnostic pitfall. Res Clin Dermatology 01. 10.35841/clinical-dermatology.1.1.2-4
- Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- Bowen S, Gill M, Lee DA, et al. Mutations in the CYLD gene in Brooke–Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype–phenotype correlation. J Invest Dermatol. 2005;124:919–920. doi: 10.1111/j.0022-202X.2005.23688.x. [DOI] [PubMed] [Google Scholar]
- Cardoso GC, de Oliveira MZ, Paixão-Côrtes VR et al (2018) Clusters of genetic diseases in Brazil. J Community Genet:1–8 [DOI] [PMC free article] [PubMed]
- Corrêa MDP. Solar ultraviolet radiation: properties, characteristics and amounts observed in Brazil and south America. An Bras Dermatol. 2015;90:297–313. doi: 10.1590/abd1806-4841.20154089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos SC, Melo US, Lopes SS d S, et al. A endogamia explicaria a elevada prevalência de deficiências em populações do Nordeste brasileiro? Cien Saude Colet. 2013;18:1141–1150. doi: 10.1590/S1413-81232013000400027. [DOI] [PubMed] [Google Scholar]
- du Toit JP, Schneider JW, Visser WI, Jordaan HF. The clinicopathological spectrum of trichoepitheliomas: a retrospective descriptive study. Int J Dermatol. 2016;55:270–277. doi: 10.1111/ijd.12855. [DOI] [PubMed] [Google Scholar]
- Dubois A, Wilson V, Bourn D, Rajan N (2015) CYLD Genetic testing for Brooke-Spiegler syndrome, familial cylindromatosis and multiple familial trichoepitheliomas. PLoS Curr 7 [DOI] [PMC free article] [PubMed]
- Farkas K, Deák BK, Sánchez LC, et al. The CYLD p.R758X worldwide recurrent nonsense mutation detected in patients with multiple familial trichoepithelioma type 1, Brooke-Spiegler syndrome and familial cylindromatosis represents a mutational hotspot in the gene. BMC Genet. 2016;17:36. doi: 10.1186/s12863-016-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliani R, Bender F, Couto R et al (2019) Population medical genetics: translating science to the community. Genet Mol Biol AHEAD. 10.1590/1678-4685-GMB-2018-0096 [DOI] [PMC free article] [PubMed]
- Grossmann P, Vanecek T, Steiner P, Kacerovska D, Spagnolo DV, Cribier B, Rose C, Vazmitel M, Carlson JA, Emberger M, Martinek P, Pearce RL, Pearn J, Michal M, Kazakov DV. Novel and recurrent germline and somatic mutations in a cohort of 67 patients from 48 families with Brooke–Spiegler syndrome including the phenotypic variant of multiple familial trichoepitheliomas and correlation with the histopathologic findings in 379. Am J Dermatopathol. 2013;35:34–44. doi: 10.1097/DAD.0b013e31824e7658. [DOI] [PubMed] [Google Scholar]
- Kallam AR, Satyanarayana MA, Aryasomayajula S, Krishna BAR. Basal cell carcinoma developing from trichoepithelioma: review of three cases. J Clin Diagn Res. 2016;10:PD17–PD19. doi: 10.7860/JCDR/2016/15432.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimzadeh I, Namazi MR, Karimzadeh A. Trichoepithelioma: a comprehensive review. Acta Dermatovenerol Croat. 2018;26:162–165. [PubMed] [Google Scholar]
- Liang YH, Gao M, Sun LD, Liu LJ, Cui Y, Yang S, Fan X, Wang J, Xiao FL, Zhang XJ. Two novel CYLD gene mutations in Chinese families with trichoepithelioma and a literature review of 16 families with trichoepithelioma reported in China. Br J Dermatol. 2005;153:1213–1215. doi: 10.1111/j.1365-2133.2005.06960.x. [DOI] [PubMed] [Google Scholar]
- Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem Sci. 2010;35:392–399. doi: 10.1016/j.tibs.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Nagy N, Rajan N, Farkas K, Kinyó A, Kemény L, Széll M. A mutational hotspot in CYLD causing cylindromas: a comparison of phenotypes arising in different genetic backgrounds. Acta Derm Venereol. 2013;93:743–745. doi: 10.2340/00015555-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Farkas K, Kemény L, Széll M. Phenotype–genotype correlations for clinical variants caused by CYLD mutations. Eur J Med Genet. 2015;58:271–278. doi: 10.1016/j.ejmg.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Parren LJMT, Giehl K, van Geel M, Frank J. Phenotype variability in tumor disorders of the skin appendages associated with mutations in the CYLD gene. Arch Dermatol Res. 2018;310:599–606. doi: 10.1007/s00403-018-1848-2. [DOI] [PubMed] [Google Scholar]
- Rajan N, Langtry JAA, Ashworth A, et al. Tumor mapping in two large multigeneration families with CYLD mutations: implications for patient management and tumor induction Europe PMC Funders Group. Arch Dermatol. 2009;145:1277–1284. doi: 10.1001/archdermatol.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggar S, Chernoff KA, Lodha S, et al. CYLD mutations in familial skin appendage tumours. J Med Genet. 2008;45:298–302. doi: 10.1136/jmg.2007.056127. [DOI] [PubMed] [Google Scholar]
- Schuch AP, Moreno NC, Schuch NJ, et al. Sunlight damage to cellular DNA: focus on oxidatively generated lesions. Free Radic Biol Med. 2017;107:110–124. doi: 10.1016/j.freeradbiomed.2017.01.029. [DOI] [PubMed] [Google Scholar]
- Verhoeft KR, Ngan HL, Lui VWY. The cylindromatosis (CYLD) gene and head and neck tumorigenesis. Cancers Head Neck. 2016;1:10. doi: 10.1186/s41199-016-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Kellermayer R, Szigeti R, et al. CYLD mutations underlie Brooke-Spiegler, familial cylindromatosis, and multiple familial trichoepithelioma syndromes. Clin Genet. 2006;70:246–249. doi: 10.1111/j.1399-0004.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q-G, Liang Y-H. A recurrent R936X mutation of CYLD gene in a Chinese family with multiple familial trichoepithelioma. Indian J Dermatol Venereol Leprol. 2015;81:192–194. doi: 10.4103/0378-6323.152298. [DOI] [PubMed] [Google Scholar]