Abstract
Anthocyanins, a subclass of flavonoids, are synthesized at the cytoplasmic surface of the endoplasmic reticulum (ER), which then accumulate in vacuoles. Plant glutathione S-transferase (GST) genes are involved in anthocyanin transportation. Here, a total of 52, 42, 50, and 29 GST genes were identified from apple, pear, peach, and strawberry, respectively, through a comprehensive genome-wide survey. Based on phylogenetic analyses, the GST proteins of the four crops could be divided into the classes Phi, Tau, DHAR, TCHQD, and Lambda. The structure and chromosomal distribution of apple GST genes were further analyzed. The GST gene family expansion in apple likely occurred through tandem duplications, and purifying selection played a pivotal role in the evolution of GST genes. Synteny analysis showed strong microsynteny between apple and Arabidopsis/strawberry, but no microsynteny was detected between apple/strawberry/Arabidopsis and rice. Aminolevulinic acid (ALA), a key precursor of tetrapyrrole compounds, can significantly improve anthocyanin accumulation in fruits, Using RNA-seq and qRT-PCR analysis, we found that ALA treatment led to the differential expression of GST genes in apples. MdGSTF12 was strongly induced by ALA, suggesting that MdGSTF12 may play a role in ALA-induced anthocyanin accumulation. These results provide a detailed overview of GST genes in four Rosaceae species and indicate that GSTs are involved in ALA-induced anthocyanin accumulation.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02299-x) contains supplementary material, which is available to authorized users.
Keywords: Aminolevulinic acid, Anthocyanin, Apple, Bioinformatics, GST
Introduction
Glutathione S-transferases (GSTs) are a large, ancient and diverse group of multi-functional proteins that are found in almost all aerobic organisms, from bacteria to human.
Plant GSTs were first identified in maize and have been extensively investigated to date (Frear and Swanson 1970). In most plants, GSTs are cytosolic and exist as monomers, homo- or heterodimers of 23–30 kDa subunits, depending on the type of GST. Each subunit contains an N-terminal domain with a glutathione binding (G-site), and a C-terminal domain that determines second-substrate specificity (C-site) (Edwards et al. 2000). GSTs also have a noncatalytic ligand-binding site (l-site), where hydrophobic substrates and natural products can bind (Dixon et al. 2010). Plant GSTs are divided into 11 classes based on their active site residues, protein sequence similarity and gene organization as follows: Tau, Phi, Theta, Zeta, Lambda, dehydroascorbate reductase (DHAR), tetrachlorohydroquinone dehalogenase (TCHQD), elongation factor 1 (EF1G), microsomal prostaglandin e synthase type 2 (mPGES2), glutathione transferases with two thioredoxin (GST2N), and glutathionyl hydroquinone reductase (GHR) (Sheehan et al. 2001). Among these classes of GSTs, Tau, Phi, Lambda, and TCHQD are plant specific.
GSTs mainly catalyze the conjugation of reduced tripeptide (γ-Glu-Cys-Gly) glutathione (GSH) to various reactive electrophiles. In plants, GST proteins are essential for proper development and physiological function. They play an important role in stress response (Roxas et al. 2000; Csiszár et al. 2014), and are involved in insecticide and herbicide degradation (Hayes et al. 2005). GSTs are responsive to plant hormones, such as auxins, ethylene, salicylic acid, abscisic acid and jasmonic acid (Moons 2005; Marrs 1996). Moreover, GSTs can also regulate the transport and metabolism of secondary compounds such as anthocyanin, flavonoids, and porphyrins (Dixon et al. 2010; Dixon and Edwards 2010). In recent years, genome-wide analyses have revealed that there are 53 GST genes in Arabidopsis (Sappl et al. 2009), 79 in rice (Jain et al. 2010; Soranzo et al. 2004), 25 in soybean, 42 in maize (McGonigle et al. 2000), 90 in tomato (Islam et al. 2017), 85 in pepper (Islam et al. 2019), 32 in pumpkin (Kayum et al. 2018), 23 in sweet orange (Licciardello et al. 2014), 90 in potato (Islam et al. 2018), and 20 in Dracaena cambodiana (Zhu et al. 2016).To date, despite the high number of whole genome sequences available, the structure of the GST gene family in Rosaceae remains unclear.
Anthocyanins belong to a class of flavonoids that is widely present in higher plants. They are crucial in attracting seed dispersers (Vogt et al. 1994), protecting against UV radiation (Sarma and Sharma 1999), and play a role in response to biotic and abiotic stresses (Chalker-Scott 1999). For many species, anthocyanins are a marker of fruit ripening, and fruit with higher anthocyanin content have higher market value and greater health benefits (Boyer and Liu 2004). Anthocyanins are synthesized in the cytosol and transported to the vacuole for storage. Compared with the biosynthetic process, the understanding of the transportation mechanism of anthocyanins is limited. Recently, three distinct models have been proposed for anthocyanin transport to the vacuole: vesicle trafficking, membrane transporters, and a GST mediated pathway, and these processes act in an integrative manner (Zhao 2015). GSTs transport anthocyanin from the endoplasmic reticulum (ER) to the vacuole (Dixon et al. 2010; Zhu et al. 2016; Sun et al. 2012; Alfenito et al. 1998; Mueller et al. 2000), and this function was first demonstrated in the maize bronze-2 (bz2, GST-like protein) mutant, which has a brown to bronze pigmentation phenotype because of the disruption of anthocyanin uptake into vacuoles (Marrs et al. 1995). Subsequently, a number of genes with similar functions have been confirmed in different plant species, such as Arabidopsis (TT19) (Sun et al. 2012), petunia (AN9) (Alfenito et al. 1998; Mueller et al. 2000), grape (VviGST1 and VviGST4) (Conn et al. 2008), carnation (Fl3) (Larsen et al. 2003), Litchi (LcGST4) (Hu et al. 2016) and cyclamen (CkmGST3) (Kitamura et al. 2012). GSTs likely act as carrier proteins rather than catalyzing the anthocyanin-GSH conjugation (Dixon and Edwards 2010; Sun et al. 2012; Zhao and Dixon 2010).
Aminolevulinic acid (ALA) is an essential biosynthetic precursor of tetrapyrrole compounds, and is widely applied to plants in agricultural production to improve photosynthesis (Youssef and Awad 2008; Memon et al. 2009), plant growth (Nguyen et al. 2016), and resistance to stress (Korkmaz et al. 2010; Akram and Ashraf 2013; Nguyen et al. 2016). Treatment with ALA also leads to increased anthocyanin accumulation in several fruit crops, such as apple (Feng et al. 2016; Zheng et al. 2017), pear (Changcheng et al. 2012), peach (Guo et al. 2013) and litchi (Feng et al. 2015). However, the biological mechanisms underlying the increase in anthocyanin accumulation after ALA treatment is poorly understood. Because of the known role of GSTs in regulating anthocyanin accumulation (Marrs et al. 1995; Mueller et al. 2000; Pérez-Díaz et al. 2016; Sun et al. 2012), a genome-wide analysis of GST genes was performed in four Rosaceae species, with a focus on apple. Additional information on physiochemical properties, subcellular localization, chromosomal location, phylogenetic relationship, and gene duplication events for each member has also been further analyzed. Moreover, the expression of apple GSTs after ALA treatment was analyzed. Our results provide foundational work for studying ALA-induced anthocyanin accumulation in apple.
Materials and methods
Plant materials and chemical treatments
The calli of ‘Fuji’ apple (Malus × domestica Borkh.) was cultured on Murashige and Skoog (MS) medium containing 1 mg/L 6-BA and 1 mg/L 2,4-D, and sub-cultured for 20 day intervals at 23 °C in the dark (Akram and Ashraf 2013). To study the expression profile of apple GSTs under ALA treatment, the control calli were treated with deionized water, while the calli was soaked in 50 mg/L ALA solution for 3 h in a shaking incubator. The calli were then retransferred to new MS medium culture dishes, and the dishes were cultured under continuous light at 22 °C for 72 h.
Identification of GSTs in apple and other species
The protein sequence, nucleotide sequence, and gene annotation files were obtained from the following online databases. Chinese white pear (Pyrus bretschneideri) datasets were downloaded from Pear Genome Project database (V1, https://peargenome.njau.edu.cn). Multiple datasets of Arabidopsis thaliana (TAIR10) and Prunus persica (Prupe1.0) were downloaded from the EnsemblPlants database (https://plants.ensembl.org/). Multiple datasets of Fragaria vesca (V1.1) and Oryza sativa (v7.0) were downloaded from JGI database (https://genome.jgi.doe.gov/portal/). Apple (Malus domestica) datasets were downloaded from the GDR database (https://www.rosaceae.org/, V3.0).
To identify GSTs in the apple, pear, strawberry, and peach genomes, BlastP and HMM methods were used. Arabidopsis and rice GST protein sequences were used as query against the protein databases of apple, pear, strawberry, and peach. The GST domain files (GST_N, PF00043; GST_C, PF02798) were downloaded from Pfam (https://pfam.xfam.org), and used to search against the four protein databases. The two search results were combined, redundant genes were removed, and the resulting putative GSTs were searched in the SMART database (https://smart.embl-heidelberg.de) to confirm the presence of common GST protein domains.
Bioinformatics analysis
Length of the protein sequence, genomic position, isoelectric point (pI), subcellular localization, protein molecular weight (MW), and subcellular location were analyzed using ProtParam and CELLO (https://web.expasy.org/protparam/ and https://cello.life.nctu.edu.tw/). Domain analysis of the protein sequences, protein sequence alignment, phylogenetic analysis, gene distribution, and gene structure display were done through MEGA, MapGene2Chro (https://mg2c.iask.in/mg2c_v2.0/), and GSDS (https://gsds.cbi.pku.edu.cn/index.php), respectively. MCScanX was used to analyze duplicate pairs and types, and the results were visualized using Circos (Wang et al. 2012). The number of non-synonymous substitutions per non-synonymous site (Ka) and the number of synonymous substitutions per synonymous site (Ks) for duplicated MdGST homologues was calculate using ParaAT and KaKs_Calculator (Zhang et al. 2012).
Measurement of anthocyanin
The pigments of apple calli were extracted from two grams (fresh weight) of samples using 1% (v/v) HCl-methanol at room temperature in the dark for 24 h. Pigments were measured using the absorbance at 530 nm of the extract solution with a spectrophotometer. The nmol of cyanidin-3-galactoside in 1 g of fresh sample was used to present the total anthocyanin content with a molar extinction coefficient of 3.43 × 104 (Ubi et al. 2006). Three replicates were performed to calculate the mean values.
RNA isolation and RT-qPCR
The calli were harvested at 24, 48, and 72 h after ALA treatment to analyze gene expression patterns. Total RNA was extracted using the RNA simply Total RNA Kit (Tiangen, and Beijing, China) following the manufacturer’s protocol. The Revert Aid™ First-Strand cDNA Synthesis Kit (Transgen Biotech, China) was used to synthesize first-strand cDNA. cDNA products were diluted 30-fold in double-distilled H2O for RT-qPCR. RT-qPCR reactions were conducted using diluted cDNA, forward and reverse primers, 2 × SYBER GREEN Master Mix and sterile water, for a final volume of 20 μl. The housekeeping gene MdUBQ (CTCCGTGGTGGTTTTTAAGT, GGAGGCAGAAACAGTACCAT) was used as an internal control. Primers for quantitative RT-PCR are listed in Supplemental Table 1. PCR was conducted using the following cycling conditions: 95 °C for 10 min, then 35 cycles at 95 °C for 15 s, followed by 62 °C for 1 min, and stored at 4 °C. The 2−ΔΔCt method was used to calculate the expression levels for each sample (Livak and Schmittgen 2001).
Results
Identification and characterization of the GST gene family
To identify GST genes in apple, Chinese white pear, peach and strawberry, BlastP and HMM searches were conducted using protein sequence and domains from Arabidopsis and rice GSTs. The rice GST genes were selected based on past studies; however, the obsolete entries in the TIGR database, OsGSTU3 (LOC_Os10g38501) and OsGSTU4 (LOC_Os10g38495), were removed (Jain et al. 2010). Moreover, the candidate GST protein sequences from apple, pear, peach, and strawberry were verified using SMART. The combined Blastp and HMMER search resulted in all candidate proteins with complete GST-N- and GST-C-terminal domains, suggesting that our search was effective in identifying GSTs in these species. Finally, 52, 42, 50, 29, 77 and 53 genes were identified as possible members of the apple, pear, peach, strawberry, rice and Arabidopsis GST superfamily, respectively, and were used for further analysis (Table 1, Supplemental Tables 2–6). Remarkably, there were significantly more GST genes in rice compared to the other species included in this study, followed by Arabidopsis. Peach and apple had a similar number of GST genes, whereas strawberry had the fewest GSTs in the four studied species. We did not find an association between the number of GSTs and genome size. For example, the peach genome contains more GSTs compared to strawberry, even though the strawberry genome (240 Mb) is larger than the peach genome (224.6 Mb).
Table 1.
Detail information of apple GSTs
| Gene subfamilies | Gene name | Gene identifier | Size (AA) | Genomics position | Theoretical pI | Mw (kDa) | Predicted Subcellular localization |
|---|---|---|---|---|---|---|---|
| PHI | MdGSTF1 | MD17G1133800 | 214 | Chr17:11942168–11946015 | 5.55 | 24,814.63 | Cytoplasmic |
| MdGSTF2 | MD03G1282900 | 213 | Chr03:36322193–36324159 | 7.06 | 23,854.87 | Cytoplasmic | |
| MdGSTF3 | MD03G1282700 | 232 | Chr03:36305324–36,312,277 | 5.99 | 26,088.18 | Cytoplasmic | |
| MdGSTF4 | MD09G1147400 | 122 | Chr09:11549037–11551449 | 5.09 | 14,042.22 | Cytoplasmic | |
| MdGSTF5 | MD17G1134200 | 205 | Chr17:11987456–11988923 | 5.97 | 23,718.27 | Cytoplasmic | |
| MdGSTF6 | MD11G1303600 | 217 | Chr11:41895006–41896381 | 5.03 | 24,517.14 | Cytoplasmic | |
| MdGSTF7 | MD03G1283000 | 217 | Chr03:36329385–36330432 | 5.28 | 24,738.29 | Cytoplasmic/ER | |
| MdGSTF8 | MD09G1147100 | 204 | Chr09:11539783–11,541,140 | 5.98 | 23,565.13 | Cytoplasmic | |
| MdGSTF9 | MD09G1147300 | 225 | Chr09:11,544,507–11546275 | 5.48 | 25,990.84 | Cytoplasmic | |
| MdGSTF10 | MD17G1133600 | 214 | Chr17:11930350–11932418 | 5.73 | 24,716.38 | Cytoplasmic | |
| MdGSTF11 | MD08G1006600 | 218 | Chr08:537193–538944 | 6.84 | 24,561.02 | Cytoplasmic | |
| MdGSTF12 | MD17G1272100 | 215 | Chr17:33407349–33409236 | 5.67 | 24,490.29 | Cytoplasmic | |
| MdGSTF13 | MD06G1012200 | 217 | Chr06:1531953–1534667 | 6.13 | 24,284.89 | Cytoplasmic | |
| MdGSTF14 | MD17G1134300 | 278 | Chr17:11997619–11999641 | 6.37 | 32,125.13 | Cytoplasmic | |
| TAU | MdGSTU1 | MD16G1011600 | 225 | Chr16:894058–894918 | 6.11 | 25,831.67 | Cytoplasmic |
| MdGSTU2 | MD10G1172200 | 226 | Chr10:26459270–26460291 | 5.93 | 25,946.76 | Cytoplasmic | |
| MdGSTU3 | MD05G1184400 | 223 | Chr05:31287827–31289215 | 5.92 | 25,308.05 | Cytoplasmic | |
| MdGSTU4 | MD10G1172300 | 223 | Chr10:26469318–26470297 | 6.26 | 25,813.95 | Cytoplasmic | |
| MdGSTU5 | MD05G1184300 | 221 | Chr05:31259973–31261200 | 5.81 | 25,603.52 | Cytoplasmic | |
| MdGSTU6 | MD10G1172100 | 219 | Chr10:26448956–26449772 | 5.71 | 25,337.27 | Cytoplasmic | |
| MdGSTU7 | MD05G1184200 | 221 | Chr05:31257878–31258927 | 5.47 | 25,196.00 | Cytoplasmic | |
| MdGSTU8 | MD04G1139500 | 222 | Chr04:22765396–22774986 | 5.43 | 25,524.45 | Cytoplasmic | |
| MdGSTU9 | MD00G1136300 | 223 | Chr00:29541189–29539434 | 5.49 | 25,283.17 | Cytoplasmic | |
| MdGSTU10 | MD14G1232200 | 216 | Chr14:31238440–31239843 | 5.78 | 24,965.81 | Cytoplasmic | |
| MdGSTU11 | MD16G1081100 | 231 | Chr16:5706295–5707923 | 7.68 | 25,368.41 | Cytoplasmic | |
| MdGSTU12 | MD13G1227600 | 225 | Chr13:22267032–22268321 | 7.02 | 25,882.17 | Cytoplasmic | |
| MdGSTU13 | MD04G1139400 | 221 | Chr04:22756386–22758195 | 5.31 | 25,410.2 | Cytoplasmic | |
| MdGSTU14 | MD04G1139600 | 221 | Chr04:22779085–22780589 | 5.21 | 25,496.3 | Cytoplasmic | |
| MdGSTU15 | MD02G1236000 | 219 | Chr02:28232819–28234618 | 5.32 | 25,084.04 | Cytoplasmic | |
| MdGSTU16 | MD02G1236100 | 219 | Chr02:28241902–28242900 | 5.22 | 25,094.06 | Cytoplasmic | |
| MdGSTU17 | MD13G1081900 | 234 | Chr13:5750247–5751759 | 5.67 | 25,621.66 | Cytoplasmic | |
| MdGSTU18 | MD13G1082000 | 231 | Chr13:5752474–5755270 | 5.86 | 25,423.48 | Cytoplasmic | |
| MdGSTU19 | MD05G1209700 | 306 | Chr05:34122009–34124732 | 5.78 | 35,439.18 | Cytoplasmic | |
| MdGSTU20 | MD10G1197100 | 219 | Chr10:29507624–29509326 | 6.03 | 25,392.42 | Cytoplasmic | |
| MdGSTU21 | MD17G1271700 | 220 | Chr17:33239918–33240984 | 5.41 | 24,980.12 | Cytoplasmic | |
| MdGSTU22 | MD10G1196900 | 221 | Chr10:29492441–29499355 | 6.15 | 25,880.07 | Cytoplasmic | |
| MdGSTU23 | MD17G1286500 | 176 | Chr17:34609141–34610079 | 5.03 | 19,876.07 | Cytoplasmic | |
| MdGSTU24 | MD10G1196300 | 219 | Chr10:29448059–29449491 | 5.41 | 25,510.73 | Cytoplasmic | |
| MdGSTU25 | MD05G1210700 | 219 | Chr05:34204834–34206719 | 5.89 | 25,484.56 | Cytoplasmic | |
| MdGSTU26 | MD05G1211000 | 219 | Chr05:34224366–34225801 | 5.18 | 25,524.41 | Cytoplasmic | |
| MdGSTU27 | MD09G1279700 | 220 | Chr09:35654801–35655805 | 5.4 | 25,031.99 | Cytoplasmic | |
| MdGSTU28 | MD04G1033800 | 225 | Chr04:3807094–3807959 | 5.82 | 26,262.47 | Cytoplasmic | |
| MdGSTU29 | MD05G1210600 | 218 | Chr05:34198838–34200200 | 5.89 | 25,397.54 | Cytoplasmic | |
| MdGSTU30 | MD05G1210100 | 219 | Chr05:34154934–34156291 | 5.53 | 25,490.49 | Cytoplasmic | |
| MdGSTU31 | MD05G1210200 | 219 | Chr05:34165150–34166225 | 5.59 | 25,307.26 | Cytoplasmic | |
| MdGSTU32 | MD05G1210400 | 220 | Chr05:34178564–34182130 | 6.02 | 25,614.82 | Cytoplasmic | |
| MdGSTU33 | MD05G1210000 | 219 | Chr05:34146212–34147596 | 5.38 | 25,357.42 | Cytoplasmic | |
| DHAR | MdDHAR1 | MD15G1438400 | 420 | Chr15:53802878–53806470 | 6.58 | 47,450.67 | Cytoplasmic |
| MdDHAR2 | MD08G1244100 | 419 | Chr08:30929758–30932924 | 6.58 | 47,568.86 | Cytoplasmic | |
| MdDHAR3 | MD15G1403200 | 422 | Chr15:50419135–50422795 | 6.11 | 48,043.31 | Cytoplasmic | |
| TCHQD | MdTCHQD1 | MD15G1133600 | 267 | Chr15:9698962–9701045 | 9.63 | 31,372.40 | Mitochondrial |
| Lambda | MdGSTL2 | MD12G1129400 | 236 | Chr12:20460252–20463131 | 5.85 | 27,398.18 | Cytoplasmic |
The length of protein sequence, isoelectric point (pI), subcellular localization genomics position, and the protein molecular weight (MW) were analyzed. In apple, the smallest GST protein was MdGSTF4 (MD09G1147400) with 122 amino acids (aa), and MdDHAR3 (MD15G1403200) was the largest with 422 aa. The range of molecular weights and pI of the predicted apple GST proteins ranged from 14.04 to 48.04 kDa, and from 5.03 to 9.63, respectively. Most GSTs were predicted to localize in the cytoplasm, except MdCHQD1 (MD15G1133600), which was localized in the mitochondria. The properties of predicted GSTs from other species are listed in Supplemental Tables 2–4.
Classification and phylogenetic analysis of GST family genes.
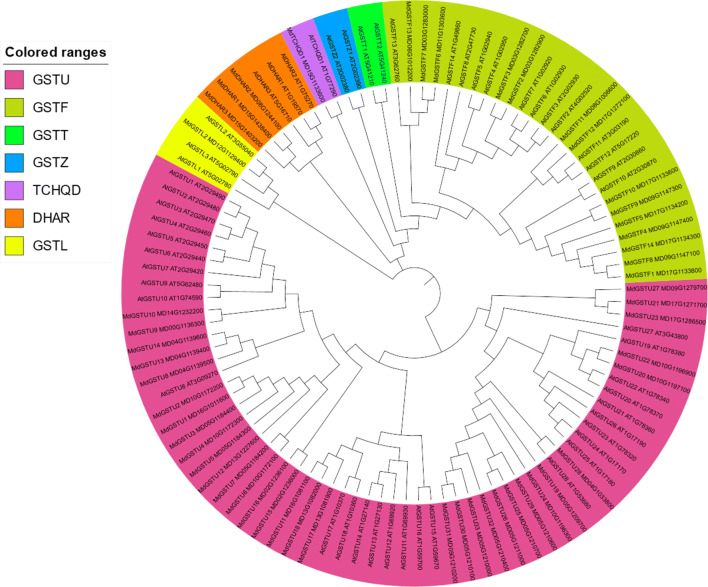
To examine phylogenetic relationships of GST genes, the putative GST protein sequences from apple, pear, peach, and strawberry were aligned with Arabidopsis (Fig. 1 and Supplemental Figs. 1–3). All studied Rosaceae GST members were named according their protein homology with Arabidopsis. Phylogenetic analysis revealed that apple and pear contained the same five GST classes: Phi, Tau, DHAR, TCHQD, and Lambda. Peach contained the classes Phi, Tau, TCHQD, Lambda, and Theta, whereas strawberry only had four classes: Phi, Tau, TCHQD and Lambda. The Rosaceae species included in this analysis contained four GST classes specific to plants: Phi, Tau, TCHQD, and Lambda. Interestingly, the Tau subfamily was the largest group and accounted for more than half of the total number of GSTs in the studied species, while subfamily of TCHQD, Lambda or Theta were relatively small. For example, among the 52 MdGSTs in apple, 33 proteins (MdGSTU1–MdGSTU33) were divided into Tau, whereas only one protein (MdTCHQD1) was classified as belonging to TCHQD. These results suggest that the GST Tau subfamily members expanded more rapidly in plants, and likely play varied roles. In contrast, compared with Arabidopsis and rice, some Rosaceae species lost GST subfamilies like Zeta and EF1G, suggesting that Zeta and EF1G subfamilies were lost during evolution or after diverged in the last common ancestor. In general, most GSTs belonging to the four species included in the study were distinct from Arabidopsis homologues in the phylogenetic analysis, implying that most gene duplication events happened after their divergence from Arabidopsis.
Fig. 1.
Unrooted phylogenetic tree representing relationships among GST protein of apple and Arabidopsis. The neighbor-joining method in MEGA 7.0 was used to construct the unrooted phylogenetic tree, with 1000 bootstrap replicates. Different subfamilies were marked as different colors
Structure analysis of GST transcripts
Intron gain or loss can alter gene structure, playing a vital role during the evolution of gene families (Xu et al. 2012). To characterize the structural diversity of the GST family, the four Rosaceae exon–intron organization of GSTs was analyzed. Exon–intron numbers and positions varied in the four Rosaceae fruits genome (Fig. 2 and Supplemental Figs. 4–6). Overall, a similar number of exons and introns was found in most genes that were clustered in the same group, suggesting that gene structure was conserved within the same group. The number of introns of GSTs varied from one to nine. Almost all Tau subfamily GSTs in the four Rosaceae species contained one intron, some had two introns like MdGSTU19 and PpGSTU10, suggesting that the Tau subfamily was conserved during evolution. Phi subfamily GSTs generally had two introns in apple, pear, and peach but not strawberry. It may indicated the functional diversity of the strawberry Tau subfamily during the evolutionary process. Notably, the DHAR and Lambda subfamily GSTs had more introns.
Fig. 2.
Exon–intron structures of apple GST genes. The gene structures were drawn using the GSDS program. Yellow indicates protein-coding sequences (CDSs); blue indicates upstream/downstream sequences; black line indicates introns
Chromosomal location analysis
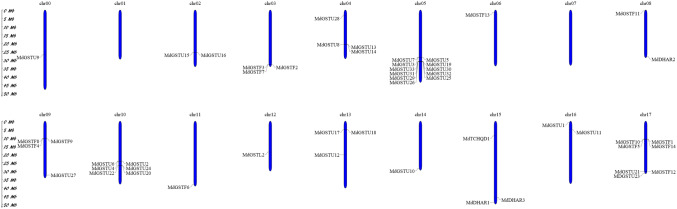
The 52 non-redundant MdGSTs were randomly distributed across the 17 chromosomes of apple, while according to the available genome, MdGSTU9 was not located in any chromosome according to the available genome. This may be because of the high level of heterozygosity or the quality of the apple genome sequence (Fig. 3). Chromosome 05 contained 11 GSTs, followed by chromosome 17 and chromosome 10, which had seven and six GSTs, respectively. Both chromosome 04 and 09 contained four GSTs. Chromosomes 03, 13 and 15 had three GSTs. Chromosomes 02, 08, and 16 contained two GSTs each. In contrast, there was only one GST located on chromosome 06, 11, 12, and 14, whereas GSTs were not identified on chromosome 01 and 07.
Fig. 3.
Chromosomal locations of GST genes in the genomes of apple. Blue strips represent chromosomes and chromosome number is labeled on the top of each chromosome, Chr00 is the gene scaffold. The scale represents megabases (Mb)
Gene duplication events, microsynteny analysis, and selection pressure on duplicated GSTs
Genome duplication events have occurred frequently during the course of plant genome evolution (Rensing 2014). Tandem and segmental duplication events often underlie the expansion of gene families (Cannon et al. 2004). Gene duplication events in the apple genome were investigated to elucidate the mechanism behind the expansion of the apple GST family. A total of 17 duplicated pair of genes were identified across the genome, including six segmental duplication pairs and 11 tandem duplication events (Table 2). Among the six segmental GST gene pairs, four belonged to the Tau class, and DHAR and Phi class had one each. In the 11 tandem duplicated gene pairs, 10 belonged to the Tau class, which may have contributed to the high number of Tau class GSTs in the apple genome, and only one pair belonged to the Phi subfamily. These results suggest that tandem duplication events played a significant role in the expansion of the Tau class GSTs in apple, and this pattern is similar to the expansion of GSTs in other plant species (Jain et al. 2010; Dong et al. 2016). In general, both kinds of duplication events were important for the GST gene family expansion in apple. Furthermore, the approximate date of the duplication event was calculated, and we found that duplications of GST genes in apple occurred from 0.20 Mya to 49.15 Mya (Table 2).
Table 2.
Ka/Ks analysis and estimation of the absolute dates of the duplication events for the duplicated GST gene pairs from apple
| Duplicate pair (Gene ID) | Duplicate pair (Gene name) | Duplicate type | Ka | Ks | Ka/Ks | Purifying selection | Duplicate dates (million years) |
|---|---|---|---|---|---|---|---|
| MD02G1236000–MD02G1236100 | MdGSTU15–MdGSTU16 | Tendem | 0.021 | 0.006 | 3.390 | No | 0.203 |
| MD04G1139400–MD04G1139500 | MdGSTU13–MdGSTU8 | Tendem | 0.054 | 0.153 | 0.353 | Yes | 5.114 |
| MD04G1139500–MD04G1139600 | MdGSTU8–MdGSTU14 | Tendem | 0.080 | 0.150 | 0.532 | Yes | 4.998 |
| MD05G1184200–MD05G1184300 | MdGSTU7–MdGSTU5 | Tendem | 0.310 | 1.475 | 0.210 | Yes | 49.154 |
| MD05G1184200–MD10G1172100 | MdGSTU7–MdGSTU6 | Segmental | 0.087 | 0.125 | 0.696 | Yes | 4.170 |
| MD05G1184300–MD05G1184400 | MdGSTU5–MdGSTU3 | Tendem | 0.250 | 1.408 | 0.177 | Yes | 46.918 |
| MD05G1210000–MD05G1210100 | MdGSTU33–MdGSTU30 | Tendem | 0.040 | 0.090 | 0.450 | Yes | 2.984 |
| MD05G1210000–MD10G1196300 | MdGSTU33–MdGSTU24 | Segmental | 0.047 | 0.204 | 0.232 | Yes | 6.797 |
| MD05G1210100–MD05G1210200 | MdGSTU30–MdGSTU31 | Tendem | 0.038 | 0.047 | 0.808 | Yes | 1.575 |
| MD05G1210600–MD05G1210700 | MdGSTU29–MdGSTU25 | Tendem | 0.031 | 0.104 | 0.296 | Yes | 3.474 |
| MD08G1244100–MD15G1438400 | MdDHAR2–MdDHAR1 | Segmental | 0.052 | 0.206 | 0.253 | Yes | 6.866 |
| MD09G1147400–MD17G1133800 | MdGSTF4–MdGSTF1 | Segmental | 0.151 | 0.300 | 0.503 | Yes | 9.997 |
| MD09G1279700–MD17G1286500 | MdGSTU27–MdGSTU23 | Segmental | 0.084 | 0.185 | 0.453 | Yes | 6.158 |
| MD10G1172200–MD10G1172300 | MdGSTU2–MdGSTU4 | Tendem | 0.102 | 0.331 | 0.309 | Yes | 11.023 |
| MD13G1081900–MD13G1082000 | MdGSTU17–MdGSTU18 | Tendem | 0.136 | 0.708 | 0.192 | Yes | 23.612 |
| MD13G1081900–MD16G1081100 | MdGSTU17–MdGSTU11 | Segmental | 0.118 | 0.810 | 0.146 | Yes | 27.006 |
| MD17G1134200–MD17G1134300 | MdGSTF5–MdGSTF14 | Tendem | 0.107 | 0.208 | 0.514 | Yes | 6.922 |
The non-synonymous (Ka), synonymous (Ks) and Ka/Ks ratio were calculated to determine the selective forces that may have led to the duplication events. A Ka and Ks ratio below one represents purifying or negative selection, a Ka and Ks ratio equal to one represents neutral selection, and a Ka and Ks ratio larger than one represents positive selection. In apple, the Ka/Ks ratios for the 16 duplicated pairs were < 1, and most were approximately 0.15, indicating that they experienced strong purifying selection. The duplicated pair MdGSTU15–MdGSTU16 had a Ka/Ks ratio of 3.39, which indicated that these genes were under positive selection (Table 2). The results suggest that purifying selection was the primary influence on the apple GST family genes.
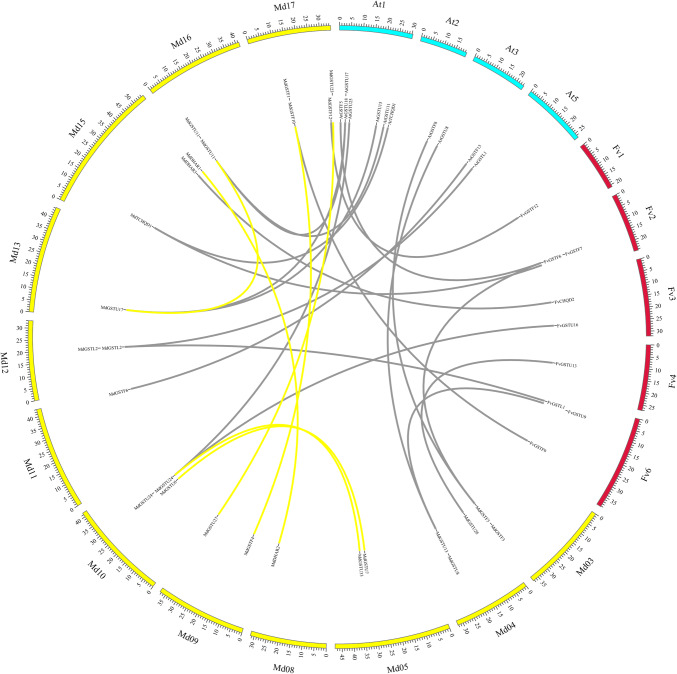
In addition, microsynteny analysis of one monocotyledon (rice) and three dicotyledons (apple, strawberry, rice and Arabidopsis) were performed to clarify the relationship of GSTs between monocots and eudicots. Interspecies microsynteny can be used to identify the location of orthologous genes. A total of 11 orthologous gene pairs between apple and strawberry, and 10 orthologous gene pairs between apple and Arabidopsis were found, whereas only one ortholog was identified between strawberry and Arabidopsis (Table 3 and Fig. 4). This indicates that many of the apple and strawberry GSTs and their Arabidopsis counterparts appeared to be derived from a common ancestor. Interestingly, no orthologous gene pairs were found between apple and rice, strawberry and rice, or Arabidopsis and rice, which may be due to the closer relationship of apple/strawberry to Arabidopsis compared to rice. Additionally, all the Ka/Ks ratios from the orthologous GST pairs were lower than 0.5, suggesting that these genes underwent intensive purifying selection.
Table 3.
Ka/Ks analysis between the duplicated apple, strawberry, Arabidopsis, and rice GSTs homologues
| Duplicate pair | Gene name | Ka | Ks | Ka/Ks | Purifying selection |
|---|---|---|---|---|---|
| AT1G02940–mrna10551 | AtGSTF5–FvGSTF7 | 0.4201 | 2.0486 | 0.2051 | Yes |
| AT1G10360–MD16G1081100 | AtGSTU18–MdGSTU11 | 0.267 | 3.800 | 0.070 | Yes |
| AT1G10370–MD13G1081900 | AtGSTU17–MdGSTU17 | 0.240 | 3.816 | 0.063 | Yes |
| AT1G17180–MD10G1197100 | AtGSTU25–MdGSTU20 | 0.181 | 3.578 | 0.050 | Yes |
| AT1G59670–MD13G1081900 | AtGSTU15–MdGSTU17 | 0.402 | 1.786 | 0.225 | Yes |
| AT1G69930–MD16G1081100 | AtGSTU11–MdGSTU11 | 0.372 | 1.925 | 0.193 | Yes |
| AT1G77290–MD15G1133600 | AtTCHQD1–MdTCHQD1 | 0.233 | 2.036 | 0.114 | Yes |
| AT2G47730–MD03G1282700 | AtGSTF8–MdGSTF3 | 0.259 | 3.768 | 0.069 | Yes |
| AT3G09270–MD04G1139500 | AtGSTU8–MdGSTU8 | 0.350 | 1.753 | 0.200 | Yes |
| AT3G62760–MD11G1303600 | AtGSTF13–MdGSTF6 | 0.363 | 1.564 | 0.232 | Yes |
| AT5G02780–MD12G1129400 | AtGSTL1–MdGSTL2 | 0.187 | 4.023 | 0.046 | Yes |
| mrna10383–MD10G1196300 | FvGSTU16–MdGSTU24 | 0.109 | 0.652 | 0.167 | Yes |
| mrna10550–MD03G1282700 | FvGSTF6–MdGSTF3 | 0.252 | 0.622 | 0.406 | Yes |
| mrna11271–MD15G1133600 | FvCHQD1–MdTCHQD1 | 0.152 | 0.692 | 0.219 | Yes |
| mrna18167–MD12G1129400 | FvGSTL1–MdGSTL2 | 0.099 | 0.670 | 0.148 | Yes |
| mrna18731–MD04G1139400 | FvGSTU9–MdGSTU13 | 0.283 | 0.988 | 0.287 | Yes |
| mrna22483–MD04G1033800 | FvGSTU13–MdGSTU28 | 0.155 | 0.903 | 0.171 | Yes |
| mrna25077–MD15G1403200 | FvCHQD2–MdDHAR3 | 0.057 | 0.833 | 0.068 | Yes |
| mrna28763–MD17G1133600 | FvGSTF9–MdGSTF10 | 0.068 | 0.735 | 0.093 | Yes |
| mrna31672–MD17G1272100 | FvGSTF12–MdGSTF12 | 0.134 | 1.001 | 0.134 | Yes |
Fig. 4.
Microsynteny analysis of GSTs among apple, strawberry, rice, and Arabidopsis chromosomes. Grey bars denote microsyntenic regions between apple, strawberry, rice and Arabidopsis GST genes. Yellow bars denote microsyntenic regions within apple. The chromosome number is indicated at the top of each chromosome. Scale bars marked on each chromosome indicates chromosome length (Mb)
Effects of ALA treatments on anthocyanin content and expression profiles of GSTs
ALA promotes anthocyanin accumulation in fruits. We applied 50 mg/L ALA on apple calli, and the ALA-treated calli had significantly higher anthocyanin content compared to the untreated control (Fig. 5). Control apple calli exposed to light for 72 h had 3.74 times more anthocyanin content, while ALA-treated calli had 13.56 times higher anthocyanin content. Moreover, based on RNA-seq transcriptomic data (unpublished), most structural and regulator genes involved in anthocyanin biosynthesis were up-regulated after ALA-treatment (Supplemental Fig. 7).
Fig. 5.

Effects of 5-ALA on coloration level and anthocyanin content in apple calli. A and B: Color (a) and anthocyanin content (b) in apple calli. Letters over bars indicate significant differences (P < 0.05; t test) between control and treatment. The blue bar indicates control treatment and red bar indicates ALA treatment
GSTs are flavonoid-binding proteins that transport anthocyanins in plants. However, little is known about the functions of GSTs in ALA-induced anthocyanin accumulation. Thus, we conducted RNA-seq transcriptomic analysis to investigate the expression pattern of GSTs in apple (Fig. 6). ALA treatment led to the divergent expression of 16 GSTs. Among these 16 genes, only MdGSTF12 was induced in all time points after ALA treatment. To verify the expression pattern of GSTs, a comprehensive qRT-PCR analysis of all GSTs was performed, except eight genes for which we could not design appropriate primers. The expression profiles of MdGSTs were classified into three sets. The first set was up-regulated after ALA treatment at three time points, and this group included the 12 members of the Tau class (Fig. 7a). The second set of genes were down-regulated at the three time points after ALA treatment, and included four, six, one and one members from Phi, Tau, TCHQD and Lambda classes, respectively (Fig. 7b). The last set included seven members of Phi, ten members of Tau, and three members of DHAR, and they displayed a variable response to ALA at different time points (Fig. 7c). These results imply that there is a complex GST expression network in response to ALA in apple. Interestingly, we noticed that MdGSTF12 was the GST that was strongly up-regulated in both RNA-seq and qRT-PCR experiments, indicating that MdGSTF12 may be important for ALA-regulated anthocyanin accumulation.
Fig. 6.

Heatmap of significantly expression level of GST genes of apple calli at three time point. And the expression values mapped to a color gradient from low (green) to high expression (red) are shown at the right of the figure. Log (RPKM) is the log of the gene expression value (RPKM) in each sample
Fig. 7.
RT-qPCR results of GSTs in apple callis at different times after ALA treatment. a–c Three groups of MdGSTs based on the expression profile. MdGST genes were up-regulated after ALA treatment (a). MdGST genes were down-regulated after ALA treatment (b). MdGST genes were varied after ALA treatment (c). The blue bar indicates control treatment and red bar indicates ALA treatment in both the panel. Data represent the mean ± SD (standard deviation) of three independent biological replicates
Discussion
Increasing evidence indicates that GSTs play vital roles in plant development, biotic and abiotic stress tolerance, and primary and secondary metabolism (Moons 2005; Yousuf et al. 2012; Dixon et al. 2010). In recent years, GSTs have been identified in many plant species, and the number and composition of GST family members are different in various plants. However, no genome-wide identification of the GST gene family has been reported in Rosaceae species, including apple, peach and strawberry. Here, 52, 42, 50 and 29 GST genes were identified in apple, pear, peach, and strawberry, respectively (Table 1 and Supplemental Tables 1–3). The strawberry had the fewest GSTs, and this may have caused by the loss of repetitive genes in strawberry compared to other plants, or GSTs may have expanded slower. Recently, 46 GSTs were reported in the pear genome (Wang et al. 2018). The difference between our results may be due to the different methods used.
Bioinformatic analysis of GST genes
It is generally believed that the function of a protein is related to its subcellular localization (Dönnes and Höglund 2004). In the present study, the bioinformatics analysis indicated that most of GSTs were located on cytoplasm (Table 1 and Supplemental Tables 2–4), and this was consistent with previous studies (Dong et al. 2016; Kayum et al. 2018a; Islam et al. 2017a). However, the GSTs that are important for flavonoid transport were also associated with membranes, such as the endoplasmic reticulum (ER) and the vacuole (Zhao 2015; Conn et al. 2008; Gomez et al. 2011; Kitamura et al. 2010). This discrepancy may be due to the program limitation and the complexity of protein localization (Xiong et al. 2016). Phylogenetic analyses revealed that GSTs in the four studied species were divided into Phi, Tau, DHAR, TCHQD, Lambda, and Theta classes (Fig. 1 and Supplemental Figs. 1–3). Similar to other plant species (Csiszár et al. 2014; Islam et al. 2018; Wang et al. 2018), the Tau family was most gene-rich. Theta and Zeta GSTs were the most ancestral classes, and were absent in the four Rosacea species, suggesting that they may have been lost in apple, pear, and strawberry during evolution. Usually, it is thought that the major driving force for generating novel genes and gene family expansion occur through whole-genome duplication (WGD), segmental duplication and tandem duplication events (Rensing 2014). Indeed, we found that tandem duplication was more frequent than segmental duplication in apple (Table 2). A similar pattern was also observed in rice (Jain et al. 2010), G. raimondii and G. arboreum (Dong et al. 2016), Capsella rubella (He et al. 2016), and poplar (Lan et al. 2009). Gene duplication not only expands genome content, but is also important for generating novel gene functions, which can allow for organisms to adapt to complex environments (Freeling 2009). The Ka/Ks ratios of all apple GST pairs were lower than 1, except MdGSTU15–MdGSTU16, suggesting that most gene pairs underwent strong purifying selection in apple. The mean age for duplication events in apple was 12.75 Mya, and the most recent tandem and segmental duplication event was estimated to have occurred 0.20 Mya and 4.17Mya, respectively. This is much later than the recent apple whole genome duplication, which happened between 30 and 45 Mya (Velasco et al. 2010). Both multiple duplication events and the ages of genome duplication events suggest that the evolutionary history of GSTs occurred through a complicated process.
The gain or loss of an exon or intron plays an important role in the diversification of multi-gene families. This event may arise from the rearrangement and fusions of different chromosome fragments (Xu et al. 2012). We found that most of the same subfamilies shared similar gene structures in apple. However, we also noticed that some GSTs had different intron–exon structure within the same subfamily (Fig. 2). The gene structure characteristics of GSTs were conserved in potato, sweet potato and populus (Islam et al. 2018; Lan et al. 2009; Ding et al. 2017; Vaish et al. 2018).
In this study, strong microsynteny was detected between apple and Arabidopsis/strawberry. In contrast, no microsynteny was detected between apple/strawberry/Arabidopsis and rice (Fig. 4), which may be due to the evolutionary distance of dicots and monocots. We conclude that monocot and dicot GSTs exhibited differences in their evolutionary history and there was likely a lineage specific expansion and diversification of GSTs (Chi et al. 2010).
ALA promoted anthocyanin accumulation through GST pathway
Exogenous application of ALA at a suitable concentration can be a powerful tool to increase the anthocyanin content of plants (Feng et al. 2016, 2015; Ye et al. 2017; Xie et al. 2013). The molecular mechanisms underlying ALA-induced anthocyanin accumulation remain poorly understood. Previous studies revealed that ALA up-regulates anthocyanin biosynthetic structural genes, such as PAL, CHS, LDOX and UFGT, and regulatory genes like MYB, bHLH, and WD40 (Zheng et al. 2017; Ye et al. 2017; Xie et al. 2013). Moreover, the transcription factor MdMADS1 is involved in ALA induced anthocyanin accumulation in apple calli (Feng et al. 2016). However, to the best of our knowledge, there have not been any investigations that reveal the function of transport proteins in response to ALA treatment. To gain insight into the expression profiles of GSTs in response to ALA treatment, transcriptomic data was analyzed and validated using qRT-PCR (Figs. 6, 7). Clear divergence in expression patterns was observed among apple GSTs in response to ALA treatments.
Based on qRT-PCR analysis, 12 Tau members responded rapidly to ALA treatment and for a longer time. Tau GSTs are involved in detoxification of xenobiotics, signaling, stress tolerance, and transporting anthocyanins (Marrs et al. 1995; Loyall et al. 2000; Thom et al. 2002; Jha et al. 2011). Heterologous expression of a rice Tau class GST (OsGSTU4) in Arabidopsis improved tolerance to salinity and oxidative stresses (Sharma et al. 2014). It is well known that ALA improves plant tolerance to various stress (Balestrasse et al. 2010; Naeem et al. 2010, 2011). Therefore, our results suggest that ALA may up-regulate Tau class GSTs, improving the stress resistance of apple. Further studies are need to determine the detailed function of the specific MdGST.
MdGSTF12 was the only GST that was strongly up-regulated by ALA. Recently, several transcriptomic studies demonstrated that MdGSTF12 may be involved in anthocyanin accumulation. MdGSTF12 has been shown to be the most suppressed gene in a yellow-skin somatic mutant apple line with corresponding reductions in anthocyanin content (El-Sharkawy et al. 2015). In the flavonoid biosynthetic processes of red-fleshed apples, MdGSTF12 was strongly up-regulated (Wang et al. 2015). Applying NAA and 2,4-D to apple calli at higher concentrations inhibits anthocyanin and flavonoid transport into the vacuole and down-regulates MdGSTF12 (Ji et al. 2015). Interestingly, MdGSTF12 falls within the same clade as genes that have been previously characterized as anthocyanin transporters, such as AtGSTF12/TT19 and Phi class GSTs, e.g., VviGST4 (Pérez-Díaz et al. 2016) and LcGST4 (Hu et al. 2016) (Supplemental Fig. 8). VviGST4 and LcGST4 are considered the ortholog of TT19 from Arabidopsis. In a complementation experiment, both VViGST4 and LcGST4 rescued the anthocyanin-less phenotype of the Arabidopsis tt19 mutant (Hu et al. 2016; Pérez-Díaz et al. 2016). In Litchi, Hu et al. (2016) found that the transcription factor of anthocyanin biosynthesis, LcMYB1, could regulate the expression of LcGST4. Taken together, these findings highlight the important role of MdGSTF12 in anthocyanin accumulation, and further functional analyses are required to shed light into how MdGSTF12 regulates anthocyanin accumulation.
Although the three other species were not treated with ALA, existing studies have found that overexpression of the GST gene can cause anthocyanin accumulation. Reduced anthocyanin content in the petioles (rap) mutant, a white-fruited variety of wild strawberry, was caused by a premature stop codon of GST. Transient knock-down of RAP leads to reduced fruit coloration in strawberry (Luo et al. 2018). During the development of peach fruits, ppa11307 and ppa00812, which encode GST, play an important role in anthocyanin transport in peach flesh (Cao et al. 2018).
Conclusions
We identified 52, 42, 50 and 29 GSTs from apple, pear, peach and strawberry, respectively. A detailed bioinformatics analysis on the phylogenetic relationships, gene structure, chromosomal location, and gene duplication of GSTs was performed. The expressions of MdGSTs was affected by ALA treatment, indicating that GSTs may be involved in ALA-induced anthocyanin accumulation. This is the first systematic isolation and analysis of apple GSTs, and provides insight into the functional analysis of MdGSTF12.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceived and designed the experiments: XF, LFSG, and JLW. Analyzed the data: XF and JZ. ALA treatment in apple calli observation: XF and JZ. RT-qPCR measuring: XF. Wrote and revise the paper: XF, YYA, and JLW.
Funding
This work was supported by the National Natural Science Foundation of China (31772253 and 31772283), and Key R&D projects in Jiangsu Province (BE2018389).
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
Not applicable.
References
- Akram NA, Ashraf M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul. 2013;32(3):663–679. [Google Scholar]
- Alfenito MR, Souer E, Goodman CD, Buell R, Mol J, Koes R, Walbot V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell. 1998;10(7):1135–1149. doi: 10.1105/tpc.10.7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrasse KB, Tomaro ML, Batlle A, Noriega GO. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry. 2010;71(17–18):2038–2045. doi: 10.1016/j.phytochem.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3(1):5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4(1):10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Ding T, Mao D, Zhu G, Fang W, Chen C, Wang X, Wang LJPP. Transcriptome analysis reveals novel genes involved in anthocyanin biosynthesis in the flesh of peach. Biochemistry. 2018;123:94–102. doi: 10.1016/j.plaphy.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol. 1999;70(1):1–9. [Google Scholar]
- Changcheng X, Shaoling Z, Hongju H. Effects of bagging and exogenous 5-aminolevulinic acid treatment on coloration of ‘Yunhongli 2’. J Nanjing Agric Univ. 2012;35(6):25–29. [Google Scholar]
- Chi Y, Cheng Y, Vanitha J, Kumar N, Ramamoorthy R, Ramachandran S, Jiang S-Y. Expansion mechanisms and functional divergence of the glutathione S-transferase family in sorghum and other higher plants. DNA Res. 2010;18(1):1–16. doi: 10.1093/dnares/dsq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn S, Curtin C, Bézier A, Franco C, Zhang W. Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins. J Exp Bot. 2008;59(13):3621–3634. doi: 10.1093/jxb/ern217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszár J, Horváth E, Váry Z, Gallé Á, Bela K, Brunner S, Tari I. Glutathione transferase supergene family in tomato: salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol Biochem. 2014;78:15–26. doi: 10.1016/j.plaphy.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Ding N, Wang A, Zhang X, Wu Y, Wang R, Cui H, Huang R, Luo Y. Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol. 2017;17(1):225. doi: 10.1186/s12870-017-1179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Edwards R. Roles for stress-inducible lambda glutathione transferases in flavonoid metabolism in plants as identified by ligand fishing. J Biol Chem. 2010;285(47):36322–36329. doi: 10.1074/jbc.M110.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Skipsey M, Edwards R. Roles for glutathione transferases in plant secondary metabolism. Phytochemistry. 2010;71(4):338–350. doi: 10.1016/j.phytochem.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Dong Y, Li C, Zhang Y, He Q, Daud MK, Chen J, Zhu S. Glutathione S-transferase gene family in Gossypium raimondii and G. arboreum: comparative genomic study and their expression under salt stress. Front Plant Sci. 2016;7:139. doi: 10.3389/fpls.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dönnes P, Höglund A. Predicting protein subcellular localization: past, present, and future. Genom Proteom Bioinform. 2004;2(4):209–215. doi: 10.1016/S1672-0229(04)02027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Dixon DP, Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;5(5):193–198. doi: 10.1016/s1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy I, Liang D, Xu K. Transcriptome analysis of an apple (Malus× domestica) yellow fruit somatic mutation identifies a gene network module highly associated with anthocyanin and epigenetic regulation. J Exp Bot. 2015;66(22):7359–7376. doi: 10.1093/jxb/erv433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Li M-f, Wu F, Li W-l, Li S-p. 5-Aminolevulinic acid affects fruit coloration, growth, and nutrition quality of Litchi chinensis Sonn. cv. Feizixiao in Hainan, tropical China. Sci Hortic. 2015;193:188–194. [Google Scholar]
- Feng X, An Y, Zheng J, Sun M, Wang L. Proteomics and SSH analyses of ALA-promoted fruit coloration and evidence for the involvement of a MADS-box gene, MdMADS1. Front Plant Sci. 2016;7:1615. doi: 10.3389/fpls.2016.01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frear D, Swanson H. Biosynthesis of S-(4-ethylamino-6-isopropylamino-2-s-triazino) glutathione: Partial purification and properties of a glutathione S-transferase from corn. Phytochemistry. 1970;9(10):2123–2132. [Google Scholar]
- Freeling M. Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annu Rev Plant Biol. 2009;60:433–453. doi: 10.1146/annurev.arplant.043008.092122. [DOI] [PubMed] [Google Scholar]
- Gomez C, Conejero G, Torregrosa L, Cheynier V, Terrier N, Ageorges A. In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST. Plant J. 2011;67(6):960–970. doi: 10.1111/j.1365-313X.2011.04648.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Cai Z, Zhang B, Xu J, Song H, Ma R. The mechanism analysis of anthocyanin accumulation in peach accelerated by ALA. Acta Hortic Sin. 2013;40:1043–1050. [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- He G, Guan C-N, Chen Q-X, Gou X-J, Liu W, Zeng Q-Y, Lan T. Genome-wide analysis of the glutathione S-transferase gene family in Capsella rubella: identification, expression, and biochemical functions. Front Plant Sci. 2016;7:1325. doi: 10.3389/fpls.2016.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Zhao J, Lai B, Qin Y, Wang H, Hu G. LcGST4 is an anthocyanin-related glutathione S-transferase gene in Litchi chinensis Sonn. Plant Cell Rep. 2016;35(4):831–843. doi: 10.1007/s00299-015-1924-4. [DOI] [PubMed] [Google Scholar]
- Islam MS, Choudhury M, Majlish A-NK, Islam T, Ghosh A. Comprehensive genome-wide analysis of Glutathione S-transferase gene family in potato (Solanum tuberosum L.) and their expression profiling in various anatomical tissues and perturbation conditions. Gene. 2018;639:149–162. doi: 10.1016/j.gene.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Islam S, Rahman IA, Islam T, Ghosh A. Genome-wide identification and expression analysis of glutathione S-transferase gene family in tomato: gaining an insight to their physiological and stress-specific roles. PLoS ONE. 2017;12(11):e0187504. doi: 10.1371/journal.pone.0187504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Sajib SD, Jui ZS, Arabia S, Islam T, Ghosh AJSr. Genome-wide identification of glutathione S-transferase gene family in pepper, its classification, and expression profiling under different anatomical and environmental conditions. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-45320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Ghanashyam C, Bhattacharjee A. Comprehensive expression analysis suggests overlapping and specific roles of rice glutathione S-transferase genes during development and stress responses. BMC Genom. 2010;11(1):73. doi: 10.1186/1471-2164-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha B, Sharma A, Mishra A. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol Biol Rep. 2011;38(7):4823–4832. doi: 10.1007/s11033-010-0625-x. [DOI] [PubMed] [Google Scholar]
- Ji X-H, Zhang R, Wang N, Yang L, Chen X-S. Transcriptome profiling reveals auxin suppressed anthocyanin biosynthesis in red-fleshed apple callus (Malus sieversii f. niedzwetzkyana) Plant Cell Tissue Organ Culture. 2015;123(2):389–404. [Google Scholar]
- Kayum A, Nath UK, Park J-I, Biswas MK, Choi EK, Song J-Y, Kim H-T, Nou I-S. Genome-wide identification, characterization, and expression profiling of glutathione S-transferase (GST) family in pumpkin reveals likely role in cold-stress tolerance. Genes. 2018;9(2):84. doi: 10.3390/genes9020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Akita Y, Ishizaka H, Narumi I, Tanaka A. Molecular characterization of an anthocyanin-related glutathione S-transferase gene in cyclamen. J Plant Physiol. 2012;169(6):636–642. doi: 10.1016/j.jplph.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Matsuda F, Tohge T, Yonekura-Sakakibara K, Yamazaki M, Saito K, Narumi I. Metabolic profiling and cytological analysis of proanthocyanidins in immature seeds of Arabidopsis thaliana flavonoid accumulation mutants. Plant J. 2010;62(4):549–559. doi: 10.1111/j.1365-313X.2010.04174.x. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Korkmaz Y, Demirkıran AR. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ Exp Bot. 2010;67(3):495–501. [Google Scholar]
- Lan T, Yang Z-L, Yang X, Liu Y-J, Wang X-R, Zeng Q-Y. Extensive functional diversification of the Populus glutathione S-transferase supergene family. Plant Cell. 2009;21(12):3749–3766. doi: 10.1105/tpc.109.070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen E, Alfenito M, Briggs W, Walbot V. A carnation anthocyanin mutant is complemented by the glutathione S-transferases encoded by maize Bz2 and petunia An9. Plant Cell Rep. 2003;21(9):900–904. doi: 10.1007/s00299-002-0545-x. [DOI] [PubMed] [Google Scholar]
- Licciardello C, D’Agostino N, Traini A, Recupero GR, Frusciante L, Chiusano ML. Characterization of the glutathione S-transferase gene family through ESTs and expression analyses within common and pigmented cultivars of Citrus sinensis (L.) Osbeck. BMC Plant Biol. 2014;14(1):39. doi: 10.1186/1471-2229-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loyall L, Uchida K, Braun S, Furuya M, Frohnmeyer H. Glutathione and a UV light–induced glutathione S-transferase are involved in signaling to chalcone synthase in cell cultures. Plant Cell. 2000;12(10):1939–1950. doi: 10.1105/tpc.12.10.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Dai C, Li Y, Feng J, Liu Z, Kang C, Joeb J. Reduced anthocyanins in petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J Exp Bot. 2018;69(10):2595–2608. doi: 10.1093/jxb/ery096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Biol. 1996;47(1):127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- Marrs KA, Alfenito MR, Lloyd AM, Walbot V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature. 1995;375(6530):397. doi: 10.1038/375397a0. [DOI] [PubMed] [Google Scholar]
- McGonigle B, Keeler SJ, Lau S-MC, Koeppe MK, O'Keefe DP. A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 2000;124(3):1105–1120. doi: 10.1104/pp.124.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon SA, Hou X, Wang L, Li Y. Promotive effect of 5-aminolevulinic acid on chlorophyll, antioxidative enzymes and photosynthesis of Pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) Acta Physiol Plant. 2009;31(1):51. [Google Scholar]
- Moons A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs) Vitam Horm. 2005;72:155–202. doi: 10.1016/S0083-6729(05)72005-7. [DOI] [PubMed] [Google Scholar]
- Mueller LA, Goodman CD, Silady RA, Walbot V. AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol. 2000;123(4):1561–1570. doi: 10.1104/pp.123.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem M, Jin Z, Wan G, Liu D, Liu H, Yoneyama K, Zhou W. 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.) Plant Soil. 2010;332(1–2):405–415. [Google Scholar]
- Naeem M, Rasheed M, Liu D, Jin Z, Ming D, Yoneyama K, Takeuchi Y, Zhou W. 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant. 2011;33(2):517–528. [Google Scholar]
- Nguyen H, Kim H-S, Jung S. Altered tetrapyrrole metabolism and transcriptome during growth-promoting actions in rice plants treated with 5-aminolevulinic acid. Plant Growth Regul. 2016;78(1):133–144. [Google Scholar]
- Pérez-Díaz R, Madrid-Espinoza J, Salinas-Cornejo J, González-Villanueva E, Ruiz-Lara S. Differential roles for VviGST1, VviGST3, and VviGST4 in proanthocyanidin and anthocyanin transport in Vitis vinífera. Front Plant Sci. 2016;7:1166. doi: 10.3389/fpls.2016.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA. Gene duplication as a driver of plant morphogenetic evolution. Curr Opin Plant Biol. 2014;17:43–48. doi: 10.1016/j.pbi.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol. 2000;41(11):1229–1234. doi: 10.1093/pcp/pcd051. [DOI] [PubMed] [Google Scholar]
- Sappl PG, Carroll AJ, Clifton R, Lister R, Whelan J, Harvey Millar A, Singh KB. The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J. 2009;58(1):53–68. doi: 10.1111/j.1365-313X.2008.03761.x. [DOI] [PubMed] [Google Scholar]
- Sarma AD, Sharma R. Anthocyanin-DNA copigmentation complex: mutual protection against oxidative damage. Phytochemistry. 1999;52(7):1313–1318. [Google Scholar]
- Sharma R, Sahoo A, Devendran R, Jain M. Over-expression of a rice tau class glutathione s-transferase gene improves tolerance to salinity and oxidative stresses in Arabidopsis. PLoS ONE. 2014;9(3):e92900. doi: 10.1371/journal.pone.0092900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Meade G, Foley VM. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360(1):1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soranzo N, Gorla MS, Mizzi L, De Toma G, Frova C. Organisation and structural evolution of the rice glutathione S-transferase gene family. Mol Genet Genom. 2004;271(5):511–521. doi: 10.1007/s00438-004-1006-8. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li H, Huang J-R. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol Plant. 2012;5(2):387–400. doi: 10.1093/mp/ssr110. [DOI] [PubMed] [Google Scholar]
- Thom R, Cummins I, Dixon DP, Edwards R, Cole DJ, Lapthorn AJ. Structure of a tau class glutathione S-transferase from wheat active in herbicide detoxification. Biochemistry. 2002;41(22):7008–7020. doi: 10.1021/bi015964x. [DOI] [PubMed] [Google Scholar]
- Ubi BE, Honda C, Bessho H, Kondo S, Wada M, Kobayashi S, Moriguchi T. Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci. 2006;170(3):571–578. [Google Scholar]
- Vaish S, Awasthi P, Tiwari S, Tiwari SK, Gupta D, Basantani MK. In silico genome-wide identification and characterization of the glutathione S-transferase gene family in Vigna radiata. Genome. 2018;61(5):311–322. doi: 10.1139/gen-2017-0192. [DOI] [PubMed] [Google Scholar]
- Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D. The genome of the domesticated apple (Malus × domestica Borkh) Nat Genet. 2010;42(10):833. doi: 10.1038/ng.654. [DOI] [PubMed] [Google Scholar]
- Vogt T, Pollak P, Tarlyn N, Taylor LP. Pollination-or wound-induced kaempferol accumulation in petunia stigmas enhances seed production. Plant Cell. 1994;6(1):11–23. doi: 10.1105/tpc.6.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Qian M, Wang R, Wang L, Zhang S. Characterization of the glutathione S-transferase (GST) gene family in Pyrus bretschneideri and their expression pattern upon superficial scald development. Plant Growth Regul. 2018;86:1–12. [Google Scholar]
- Wang N, Zheng Y, Duan N, Zhang Z, Ji X, Jiang S, Sun S, Yang L, Bai Y, Fei Z. Comparative transcriptomes analysis of red-and white-fleshed apples in an F1 population of malus sieversii f. niedzwetzkyana crossed with M. domestica ‘Fuji’. PLoS ONE. 2015;10(7):e0133468. doi: 10.1371/journal.pone.0133468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, Lee T-h, Jin H, Marler B, Guo H. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49–e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Wang Z, Cheng X, Gao J, Zhang Z, Wang L. 5-Aminolevulinic acid promotes anthocyanin accumulation in Fuji apples. Plant Growth Regul. 2013;69(3):295–303. [Google Scholar]
- Xiong E, Zheng C, Wu X, Wang W. Protein subcellular location: the gap between prediction and experimentation. Plant Mol Biol Rep. 2016;34(1):52–61. [Google Scholar]
- Xu G, Guo C, Shan H, Kong H. Divergence of duplicate genes in exon–intron structure. Proc Natl Acad Sci. 2012;109(4):1187–1192. doi: 10.1073/pnas.1109047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Yang X, Chen Q, Xu F, Wang G. Promotive effects of 5-aminolevulinic acid on fruit quality and coloration of Prunus persica (L.) Batsch. Sci Hortic. 2017;217:266–275. [Google Scholar]
- Youssef T, Awad MA. Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J Plant Growth Regul. 2008;27(1):1. [Google Scholar]
- Yousuf PY, Hakeem KUR, Chandna R, Ahmad P. Abiotic stress responses in plants. New York: Springer; 2012. Role of glutathione reductase in plant abiotic stress; pp. 149–158. [Google Scholar]
- Zhang Z, Xiao J, Wu J, Zhang H, Liu G, Wang X, Dai L. ParaAT: a parallel tool for constructing multiple protein-coding DNA alignments. Biochem Biophys Res Commun. 2012;419(4):779–781. doi: 10.1016/j.bbrc.2012.02.101. [DOI] [PubMed] [Google Scholar]
- Zhao J. Flavonoid transport mechanisms: how to go, and with whom. Trends Plant Sci. 2015;20(9):576–585. doi: 10.1016/j.tplants.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Zhao J, Dixon RA. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010;15(2):72–80. doi: 10.1016/j.tplants.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Zheng J, An Y-y, Feng X-x, Wang L-j. Rhizospheric application with 5-aminolevulinic acid improves coloration and quality in ‘Fuji’ apples. Sci Hortic. 2017;224:74–83. [Google Scholar]
- Zhu J-H, Li H-L, Guo D, Wang Y, Dai H-F, Mei W-L, Peng S-Q. Transcriptome-wide identification and expression analysis of glutathione s-transferase genes involved in flavonoids accumulation in Dracaena cambodiana. Plant Physiol Biochem. 2016;104:304–311. doi: 10.1016/j.plaphy.2016.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.