Abstract
Background
Diabetes can complicate hypertension management by increasing the risk of cardiovascular disease (CVD) and all-cause mortality. Studies targeting diabetes detection in hypertensive individuals demonstrating an increased risk of diabetes are lacking. We aimed to assess the performance of hemoglobin A1c (HbA1c) and its cut-off point in detecting diabetes in the abovementioned population.
Methods
Data from 4,096 community-dwellers with hypertension but without known diabetes were obtained from the Study on Evaluation of iNnovated Screening tools and determInation of optimal diagnostic cut-off points for type 2 diaBetes in Chinese muLti-Ethnic (SENSIBLE) study; these data were randomly split into exploration (70% of the sample) and internal validation (the remaining 30%) datasets. The optimal HbA1c cut-off point was derived from the exploration dataset and externally validated using another dataset from 2,431 hypertensive individuals. The oral glucose tolerance test was considered the gold-standard for confirming diabetes.
Results
The areas under the ROC curves for HbA1c to detect diabetes were 0.842, 0.832, and 0.829 for the exploration, internal validation, and external validation datasets, respectively. An optimal HbA1c cut-off point of 5.8% (40 mmol/mol) yielded a sensitivity of 76.2% and a specificity of 74.5%. Individuals who were not diagnosed as having diabetes by HbA1c at 5.8% (40 mmol/mol) had a lower 10-year CVD risk score than those diagnosed as having diabetes (P=0.01). HbA1c≤5.1% (32 mmol/mol) and ≥6.4% (46 mmol/mol) could indicate the absence and presence of diabetes, respectively.
Conclusions
HbA1c could detect diabetes effectively in community-dwellers with hypertension.
Keywords: Hemoglobin A1c, Diabetes, Hypertension, Detection, Cut-off, Community-dwellers, Cardiovascular disease
INTRODUCTION
As a major risk factor for cardiovascular disease (CVD) and all-cause mortality, hypertension has become a global health challenge [1]. The latest national survey from China has shown that the prevalence of hypertension is approximately 45% in adults aged 35–75 years [2]. Diabetes is a well-recognized contributing factor for hypertension [3], and its presence can complicate hypertension management [4, 5]. This is partly reflected by data demonstrating that individuals with both hypertension and diabetes exhibit a markedly increased risk of CVD or all-cause mortality than those with only hypertension [6, 7]. Previous studies have shown that hypertension is associated with a higher risk of diabetes compared with normal blood pressure [8, 9]. Therefore, early detection of diabetes among hypertensive individuals is of clinical importance, as it would enable the implementation of efficient interventions in a timely manner. However, to date, no study has focused on detecting diabetes in this population.
Hemoglobin A1c (HbA1c) reflects the average blood glucose level in the preceding 8–12 weeks and can be measured without fasting; the American Diabetes Association (ADA) recommended using HbA1c to detect diabetes or individuals at risk for diabetes in 2010, after years of debate [10]. Several studies have investigated the performance of HbA1c in detecting undiagnosed diabetes across a spectrum of diverse populations such as general adults [11, 12], gestational women [13], and children [14]. However, its performance among hypertensive individuals and how to better facilitate its use in this population (e.g., how to rule out diabetes) remain largely unknown. Current ADA guidelines recommend an HbA1c cut-off point of 6.5% (48 mmol/mol) for diagnosing diabetes [10]; however, it is unclear whether this cut-off point is suitable for hypertensive individuals.
Therefore, we aimed to evaluate the performance of HbA1c in diabetes screening, as well as to identify its optimal cut-off point for community-dwellers with hypertension. We also compared the diagnostic efficacy of the newly derived HbA1c cut-off point with that of the recommended 6.5% (48 mmol/mol) cut-off point [10], as well as the differences in cardiometabolic risk profiles between individuals who were not diagnosed as having diabetes and those who were diagnosed as having diabetes using the newly derived HbA1c cut-off point.
MATERIALS AND METHODS
Study population
All study individuals were from the Study on Evaluation of iNnovated Screening tools and determInation of optimal diagnostic cut-off points for type 2 diaBetes in Chinese muLti-Ethnic (SENSIBLE) study [15] and the SENSIBLE-Addition study (see below). In the present study, which had a prospective cross-sectional design, individuals who were 20–70 years old and diagnosed as having hypertension, but free of known diabetes, were selected [15]. Eligible individuals from the SENSIBLE study were split randomly into two groups: one (70% of the sample) to assess the performance of HbA1c in detecting diabetes (the exploration dataset) and another (the remaining 30%) for internal validation (the internal validation dataset). The SENSIBLE-Addition study (the external validation dataset) was used for external validation. The protocols for the SENSIBLE and SENSIBLE-Addition studies were approved by the Ethical Review Committees of Zhongda Hospital, Southeast University, Nanjing, China and the other 16 participating hospitals/institutes in China. All individuals provided written informed consent prior to participation. This study followed the STARD guidelines [16].
The anthropometric and biochemical characteristics of individuals in the exploration (N=2,868), internal validation (N=1,228), and external validation (N=2,431) datasets are shown in Table 1. Compared with the exploration and internal validation datasets, individuals in the external validation dataset were younger, had higher body mass index (BMI) and waist circumference (WC) values, and showed lower systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-c) levels (all P<0.05). The prevalence of undiagnosed diabetes among these three cohorts were 14.2%, 14.5%, and 17.5%, respectively.
Table 1.
Characteristics of the individuals in the different study cohorts
| Exploration population (cohort 1) | Internal validation (cohort 2) | External validation (cohort 3) | P | |
|---|---|---|---|---|
| Individuals, N | 2,868 | 1,228 | 2,431* | |
| Male, N (%) | 1,120 (39.1) | 476 (38.8) | 1,078 (44.3) | <0.001 |
| Smoker, N (%) | 643 (22.4) | 258 (21.0) | 641 (26.4) | <0.001 |
| Known hypertension, N (%) | 1,254 (43.7) | 556 (45.3) | 1,284 (52.8) | <0.001 |
| Age (yr) | 57 (50, 63) | 58 (50, 64) | 55 (49, 61) | 0.0001 |
| BMI (kg/m2) | 25.6 (23.1, 28.1) | 25.6 (23.1, 28.0) | 26.3 (24.1, 28.4) | 0.0001 |
| WC (cm) | 85 (78, 92) | 84 (78, 92) | 88 (81, 93) | 0.0001 |
| SBP (mmHg) | 148 (140, 160) | 147 (140, 160) | 147 (140, 158) | 0.03 |
| DBP (mmHg) | 91 (84, 98) | 91 (83, 98) | 90 (84, 96) | 0.0007 |
| FPG (mmol/L) | 5.6 (5.2, 6.1) | 5.5 (5.1, 6.0) | 5.8 (5.5, 6.3) | 0.0001 |
| 2h-PG (mmol/L) | 7.1 (5.7, 8.9) | 7.0 (5.7, 8.7) | 7.4 (6.2, 9.1) | 0.0001 |
| HbA1c (%) | 5.5 (5.2, 5.9) | 5.4 (4.7, 6.2) | 5.5 (5.2, 5.8) | 0.0001 |
| HbA1c (mmol/mol) | 37 (33, 41) | 36 (28, 44) | 37 (33, 40) | 0.0001 |
| TC (mmol/L) | 5.4 (4.7, 6.2) | 5.4 (4.7, 6.2) | 4.9 (4.3, 5.5) | 0.0001 |
| TG (mmol/L) | 1.4 (1.0, 2.1) | 1.3 (0.9, 2.0) | 1.5 (1.0, 2.3) | 0.0001 |
| HDL-c (mmol/L) | 1.5 (1.3, 1.8) | 1.5 (1.3, 1.8) | 1.5 (1.3, 1.7) | 0.0001 |
| LDL-c (mmol/L) | 3.2 (2.6, 3.7) | 3.1 (2.6, 3.6) | 2.7 (2.3, 3.2) | 0.0001 |
| Cr (μmol/L) | 63 (53, 74) | 63 (53, 74) | 64 (55, 75) | 0.01 |
Data are presented as median (interquartile range) or, where stated, as number and percentage.
Three individuals did not provide DBP data.
Abbreviations: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; 2h-PG, 2-hr postprandial glucose; HbA1c, hemoglobin A1c; TC, total cholesterol; TG, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; Cr, creatine.
SENSIBLE study
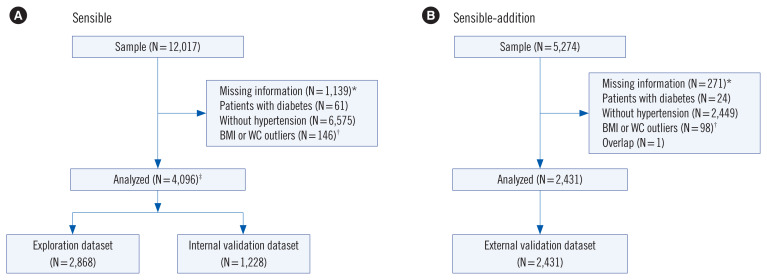
A cross-sectional survey was conducted in seven provinces in China from November 1st, 2016 to June 30th, 2017 [15]. An age- and sex-stratified, random sample of 13,620 community-dwellers who had lived ≥5 years in their current residence was invited, and 12,017 of the invited individuals participated in this study, with 4,096 eligible for analysis (Fig. 1A).
Fig. 1.
Participant selection process for (A) the SENSIBLE study and (B) the SENSIBLE-Addition study. *indicates missing information for age, BMI, WC, smoking, glycemic biomarkers, and lipid profiles; †Outliers indicate data >99th percentile or <1st percentile of the dataset; ‡Data for these individuals were randomly split into the exploration dataset (70% of the sample) and the internal validation dataset (the remaining 30%).
Abbreviations: SENSIBLE, Study on Evaluation of iNnovated Screening tools and determInation of optimal diagnostic cut-off points for type 2 diaBetes in Chinese muLti-Ethnic; BMI, body mass index; WC, waist circumference.
All individuals were asked to complete a questionnaire containing information regarding their sociodemographic characteristics, lifestyle factors, and medical history. Body weight, height, and WC were measured using standard methods, and BMI was calculated. Blood pressure was measured using an electronic sphygmomanometer (YE680E, Jiangsu Yuyue Medical Equipment Inc., Nanjing, Jiangsu, China) [15]. All individuals were asked to fast for at least 10 hours before receiving a 75 g standardized oral glucose tolerance test (OGTT), for which venous blood samples (5 mL EDTA tubes) were drawn before and two hrs post glucose loading. Blood samples were centrifuged at 1,000–1,200×g for 5 minutes at room temperature (around 22–25°C) within 30 minutes of collection and shipped at 4°C by air to Nanjing Adicon Clinical Laboratories, Nanjing, China. Fasting plasma glucose (FPG), 2-hours postprandial glucose (2h-PG), TC, triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), and LDL-c levels were measured using the automated analyzer, Synchron LX-20 (Beckman Coulter Inc., Fullerton, CA, USA). Specifically, the intra-assay and inter-assay coefficients of variation for blood glucose were both ≤3%. HbA1c was determined with a D-10 Hemoglobin Analyzer (Bio-Rad Inc., Hercules, CA, USA) using an HPLC-based method. This method was certified by the National Glycohemoglobin Standardization Program and standardized to the Diabetes Control and Complications Trial assay approach [17]. In this study, the intra-assay coefficient of variation for HbA1c was 0.81–1.67%, and the inter-assay coefficient of variation for HbA1c was 1.35–2.27%.
SENSIBLE-Addition study
This study had a design similar to that of the SENSIBLE study; however, it was conducted in the Jiangsu Province from April 15th, 2017 to July 31st, 2017, together with liver function measurements and acquisition of additional information regarding health care needs. In total, 5,274 individuals completed a questionnaire survey and underwent an OGTT. Of these, 2,431 were eligible for the external validation dataset (Fig. 1B). Laboratory markers, including FPG, 2h-PG, HbA1c, TC, TG, HDL-c, and LDL-c were analyzed using the same approaches as outlined for the SENSIBLE study.
Definitions
Hypertension was defined as SBP ≥140 mmHg or DBP ≥90 mmHg on average, a self-reported history of hypertension, or a self-reported use of antihypertensive drugs [2]. Diabetes was diagnosed based on the 1999 WHO criteria: FPG ≥7.0 mmol/L, 2h-PG ≥11.1 mmol/L, or both [18]. The 10-year risk score of CVD was calculated according to the Framingham Risk Score for predicting CVD [19]. True-positive cases were defined as individuals with confirmed diabetes based on the 1999 WHO criteria who could be identified as having diabetes by HbA1c at the derived cut-off point, while false-negative cases were defined as individuals with confirmed diabetes based on the 1999 WHO criteria who could not be identified as having diabetes by HbA1c at the derived cut-off point. Screen-positive cases were defined as individuals with HbA1c above the derived cut-off point, while screen-negative cases were defined as individuals with HbA1c below the derived cut-off point.
Statistical analysis
Statistical analyses were conducted using STATA version 14.0 (StataCorp LP, College Station, TX, USA). As the continuous variables were non-normally distributed (assessed by Shapiro-Wilk normality test), they are presented as the median (interquartile range). Categorical variables are expressed as a number (proportion). Differences in continuous and categorical variables were determined using the Kruskal-Wallis test (at least three groups) or Wilcoxon rank-sum test (two groups) and the Chi-squared test, respectively. The diagnostic efficacy of HbA1c in detecting diabetes was evaluated using the area under the ROC (AUROC) curve. The optimal HbA1c cut-off point was determined by minimizing the [(1−sensitivity)2+(1−specificity)2] score, which represents the maximum sum of the sensitivity and specificity [20]. Sensitivities, specificities, and AUROCs were compared using the methods proposed by Altman and Bland [21]. Moreover, based on the methods described by Lu, et al. [22], the HbA1c value at the 2.5th percentile was determined as the cut-off point for ruling out diabetes, while that at the 97.5th percentile was determined as the cut-off point for ruling in diabetes. Subgroup analyses of hypertension awareness (known vs unknown), age (<50, 50–60, vs 60–70 years), and exercise status (regular vs irregular) and sensitivity analyses, excluding individuals using statins and those with anemia, were performed to assess the robustness of the primary results. To increase the data analysis statistical power, a meta-analytical approach using a random-effects model, as well as a combination of all three cohorts at the individual patient level, was used. All statistical tests were two-sided, with P<0.05 considered statistically significant.
RESULTS
Performance of HbA1c in detecting diabetes
The performance of HbA1c in detecting diabetes among the exploration population is presented in Table 2. Increasing the HbA1c cut-off point resulted in decreased sensitivity and negative predictive values, but increased specificity and positive predictive values. An HbA1c cut-off point of 5.8% (40 mmol/mol) yielded the best trade-off for sensitivity and specificity, showing a sensitivity of 76.2% and a specificity of 74.5%. Using the derived 5.8% (40 mmol/mol) HbA1c cut-off point for detecting diabetes, the internal validation dataset showed sensitivity and specificity values similar to those of the exploration dataset (both P>0.70; Table 2). However, when the suggested diagnostic HbA1c cut-off point of 6.5% (48 mmol/mol) [10] was applied, the sensitivity was only 44.2% in the exploration dataset and further decreased to 39.9% in the internal validation dataset and 33.1% in the external validation dataset.
Table 2.
Performance of hemoglobin A1c in the different study cohorts*
| HbA1c cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Exploration dataset | ||||
| 5.6% (38 mmol/mol) | 87.0 (83.3–90.1) | 57.2 (55.2–59.2) | 25.2 (22.9–27.5) | 96.4 (95.3–97.3) |
| 5.7% (39 mmol/mol) | 83.1 (79.0–86.6) | 66.6 (64.7–68.5) | 29.1 (26.5–31.8) | 96.0 (94.9–96.8) |
| 5.8% (40 mmol/mol) | 76.2 (71.7–80.2) | 74.5 (72.7–76.2) | 33.0 (30.0–36.2) | 95.0 (93.9–95.9) |
| 5.9% (41 mmol/mol) | 71.0 (66.3–75.4) | 81.3 (79.7–82.8) | 38.5 (35.0–42.1) | 94.4 (93.4–95.4) |
| 6.0% (42 mmol/mol) | 66.6 (61.8–71.2) | 85.6 (84.1–86.9) | 43.3 (39.4–47.3) | 93.9 (92.9–94.9) |
| 6.1% (43 mmol/mol) | 60.2 (55.3–65.0) | 90.2 (89.0–91.4) | 50.4 (45.9–54.9) | 93.2 (92.1–94.2) |
| 6.5% (48 mmol/mol) | 44.2 (39.3–49.2) | 98.1 (97.4–98.6) | 78.9 (73.1–84.1) | 91.4 (90.3–92.4) |
|
| ||||
| Internal validation dataset | ||||
| 5.8% (48 mmol/mol) | 76.4 (69.5–82.4) | 75.1 (72.3–77.6) | 34.2 (29.5–39.1) | 94.9 (93.2–96.3) |
|
| ||||
| External validation dataset | ||||
|
| ||||
| 5.8% (48 mmol/mol) | 68.8 (64.1–73.2) | 81.2 (79.4–82.9) | 43.7 (39.9–47.6) | 92.5 (91.1–93.6) |
Data are presented as percentage (95% confidence interval).
The Study on Evaluation of iNnovated Screening tools and determInation of optimal diagnostic cut-off points for type 2 diaBetes in Chinese muLti-Ethnic (SENSIBLE) data were split randomly into the exploration dataset (70% of the sample) and the internal validation dataset (the remaining 30%).
Abbreviations: HbA1c, hemoglobin A1c; PPV, positive predictive value; NPV, negative predictive value.
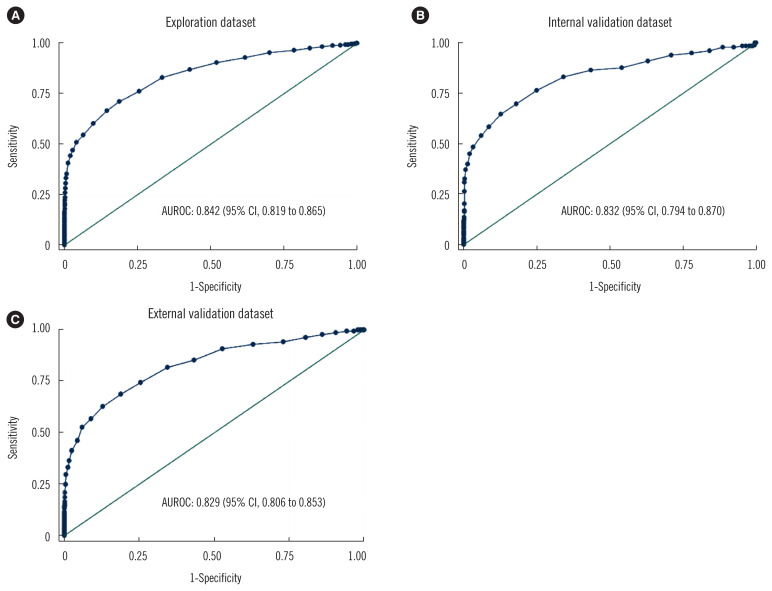
The AUROCs of HbA1c in detecting undiagnosed diabetes were 0.842 (95% confidence interval [CI], 0.819–0.865) in the exploration dataset (Fig. 2A), 0.832 (95% CI, 0.794–0.870) in the internal validation dataset (Fig. 2B), and 0.829 (95% CI, 0.806–0.853) in the external validation dataset (Fig. 2C). The AUROCs of both validation datasets were comparable to that of the exploration dataset (P=0.66 and 0.44, respectively). The HbA1c AUROC remained largely unchanged following the exclusion of individuals using statins or with anemia (data not shown).
Fig. 2.
ROC curves for hemoglobin A1c in the different study cohorts. (A) exploration dataset. (B) internal validation dataset. (C) external validation dataset.
Abbreviations: AUROC, area under the ROC curve; CI, confidence interval.
Subgroup analyses showed that the HbA1c AUROC was comparable among groups stratified by hypertension awareness, age, or exercise status in the exploration and validation datasets (all P>0.05; Table 3). To enhance the statistical power, the AUROCs from all three cohorts were pooled using a random-effects meta-analysis model. The results indicated that there was no significant difference between women and men (0.859 vs. 0.820, P=0.09) and between the other subgroups listed in Table 3 (all P>0.05).
Table 3.
AUROC curve of HbA1c by subgroup
| Subgroups | AUROC (95% CI) | ||
|---|---|---|---|
|
| |||
| Exploration dataset | Internal validation dataset | External validation dataset | |
| Sex-based groups | |||
| Male | 0.821 (0.782–0.859) | 0.784 (0.721–0.848) | 0.827 (0.792–0.862) |
| Female | 0.859 (0.831–0.887) | 0.881 (0.842–0.921) | 0.832 (0.800–0.864) |
|
| |||
| Hypertension awareness groups | |||
| Known | 0.838 (0.805–0.871) | 0.811 (0.758–0.864) | 0.838 (0.809–0.867) |
| Unknown | 0.844 (0.811–0.877) | 0.849 (0.792–0.905) | 0.810 (0.770–0.850) |
|
| |||
| Age-based groups | |||
| <50 yr | 0.849 (0.784–0.913) | 0.735 (0.608–0.863) | 0.865 (0.822–0.908) |
| 50–60 yr | 0.843 (0.803–0.883) | 0.852 (0.789–0.915) | 0.811 (0.773–0.850) |
| 60–70 yr | 0.825 (0.792–0.859) | 0.840 (0.791–0.889) | 0.823 (0.782–0.863) |
|
| |||
| Regular exercise groups* | |||
| Yes | 0.839 (0.811–0.876) | 0.854 (0.802–0.905) | 0.857 (0.818–0.896) |
| No | 0.839 (0.806–0.873) | 0.817 (0.763–0.871) | 0.816 (0.787–0.845) |
This information was obtained by asking “Do you undertake regular exercise every week?”
Abbreviations: AUROC, area under the ROC curve; HbA1c, hemoglobin A1c; CI, confidence interval.
Comparisons between individuals identified as true-positive and false-negative
As all the three cohorts used comparable survey procedures with overall identical information, they were combined to increase the statistical power for further comparisons between individuals identified as true-positive and false-negative using the derived HbA1c cut-off point of 5.8% (40 mmol/mol). The results suggested that individuals who were true-positive for diabetes had a more unfavorable cardiometabolic risk profile than those who were false-negative (Table 4). Moreover, true-positive individuals had a higher 10-year risk score of CVD than false-negative individuals (26.7% vs 24.2%, P=0.01). In addition, screen-positive individuals exhibited a higher 10-year risk score of CVD than screen-negative individuals (Table 4).
Table 4.
Characteristics of true-positive versus false-negative cases and screen-positive versus screen-negative cases in all three datasets
| Individuals with confirmed diabetes | Individuals who underwent diabetes screening* | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| True-positive† (N=684) | False-negative‡ (N=254) | P | Screen-positive§ (N=2,006) | Screen-negative|| (N=4,521) | P | |
| Age (yr) | 58 (52, 63) | 55 (50, 63) | 0.05 | 58 (52, 64) | 55 (49, 62) | <0.0001 |
|
| ||||||
| BMI (kg/m2) | 27.0 (24.9, 29.5) | 26.7 (23.8, 28.9) | 0.006 | 26.4 (24.3, 28.9) | 25.6 (23.2, 27.9) | <0.0001 |
|
| ||||||
| WC (cm) | 90 (83, 96) | 88 (80, 95) | 0.01 | 87 (80, 94) | 85 (79, 92) | <0.0001 |
|
| ||||||
| SBP (mmHg) | 149 (140, 161) | 151 (142, 163) | 0.26 | 148 (140, 160) | 147 (140, 158) | 0.002 |
|
| ||||||
| DBP (mmHg) | 91 (83, 97) | 92 (84, 98) | 0.12 | 90 (83, 96) | 91 (84, 97) | 0.005 |
|
| ||||||
| FPG (mmol/L) | 7.3 (6.5, 8.3) | 6.7 (5.9, 7.2) | <0.0001 | 6.1 (5.6, 6.9) | 5.5 (5.2, 5.9) | <0.0001 |
|
| ||||||
| PPG (mmol/L) | 13.3 (11.5, 16.5) | 11.5 (10.3, 12.6) | <0.0001 | 8.8 (6.8, 11.9) | 6.8 (5.6, 8.1) | <0.0001 |
|
| ||||||
| HbA1c (%) | 6.5 (6.1, 7.2) | 5.2 (4.5, 6.0) | <0.001 | 6.1 (5.9, 6.4) | 5.3 (5.1, 5.5) | <0.0001 |
|
| ||||||
| HbA1c (mmol/mol) | 48 (43, 55) | 33 (26, 42) | <0.0001 | 43 (41, 46) | 34 (32, 37) | <0.0001 |
|
| ||||||
| TC (mmol/L) | 5.5 (4.8, 6.3) | 5.2 (4.5, 6.0) | 0.0001 | 5.4 (4.7, 6.2) | 5.1 (4.4, 5.9) | <0.0001 |
|
| ||||||
| TG (mmol/L) | 1.9 (1.3, 2.8) | 1.8 (1.2, 2.7) | 0.16 | 1.6 (1.1, 2.4) | 1.4 (0.9, 2.1) | <0.0001 |
|
| ||||||
| HDL-c (mmol/L) | 1.5 (1.2, 1.7) | 1.5 (1.3, 1.8) | 0.03 | 1.5 (1.2, 1.7) | 1.5 (1.3, 1.8) | <0.0001 |
|
| ||||||
| LDL-c (mmol/L) | 3.2 (2.7, 3.8) | 2.8 (2.3, 3.5) | <0.0001 | 3.1 (2.6, 3.7) | 2.9 (2.4, 3.4) | <0.0001 |
|
| ||||||
| 10-yr CVD risk score (%)¶ | 26.7 (18.5, 39.8) | 24.2 (16.2, 36.1) | 0.01 | 21.1 (12.4, 35.9) | 14.3 (8.5, 24.5) | <0.0001 |
Data are presented as median (interquartile range).
Three individuals did not provide DBP data;
True-positive cases were defined as individuals with confirmed diabetes based on the 1999 WHO criteria that were detected by HbA1c at the derived cut-off point of 5.8% (40 mmol/mol);
False-negative cases were defined as individuals with confirmed diabetes based on the 1999 WHO criteria that were not detected by HbA1c at the derived cut-off point of 5.8% (40 mmol/mol);
Screen-positive cases were defined as individuals with an HbA1c ≥ the derived cut-off point of 5.8% (40 mmol/mol);
Screen-negative cases were defined as individuals with an HbA1c < the derived cut-off point of 5.8% (40 mmol/mol);
The 10-year risk score of CVD was calculated according to the Framingham Risk Score for predicting CVD [19].
Abbreviations: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; PPG, post-prandial plasma glucose; HbA1c, hemoglobin A1c; TC, total cholesterol; TG, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; CVD, cardiovascular disease.
Using HbA1c to rule out or rule in diabetes
Among individuals with undiagnosed diabetes (based on the 1999 WHO criteria) in the exploration dataset, the HbA1c value at the 2.5th percentile was approximately 5.0% (31 mmol/mol). Therefore, an HbA1c cut-off point of 5.1% (32 mmol/mol) was chosen to rule out diabetes. For individuals without diabetes, the HbA1c value at the 97.5th percentile was approximately 6.3% (45 mmol/mol) [22]; thus, an HbA1c cut-off point of 6.4% (46 mmol/mol) was selected to rule in diabetes.
When an HbA1c cut-off point of 6.4% (46 mmol/mol) was applied, 79.2% of the diabetes cases from the internal validation dataset and 82.9% of those from the external validation dataset could be ruled in. Using an HbA1c cut-off point of 5.1% (32 mmol/mol), only 4.0% of the diabetes cases in the internal validation dataset and 3.9% of those in the external validation dataset could be ruled out. Moreover, if an HbA1c cut-off point of 6.4% (46 mmol/mol) was selected for ruling in diabetes, approximately 25.4% of the hypertensive individuals with HbA1c ≥5.8% (40 mmol/mol) in the internal validation dataset and 27.9% in the external validation dataset would not require an OGTT for diabetes confirmation.
DISCUSSION
Our study, which comprised three cross-sectional cohorts, suggests that HbA1c at 5.8% (40 mmol/mol) might be the optimal cut-off point for diabetes screening among community-dwellers with hypertension. Individuals who were not diagnosed as having diabetes by HbA1c at 5.8% (false-negative individuals) had a lower 10-year CVD risk score and a more favorable cardiometabolic risk profile than those correctly identified as having diabetes (true-positive individuals). In addition, HbA1c ≤5.1% (32 mmol/mol) and ≥6.4% (46 mmol/mol) may assist in specifying the absence and presence of diabetes, respectively.
The performance of HbA1c in detecting diabetes has received substantial interest [11, 12, 23, 24]. In a systematic review of nine studies, Bennett, et al. [11] suggested that HbA1c at 6.1% (43 mmol/mol) could be employed as the optimal cut-off point for detecting diabetes but argued that population-specific cut-offs may vary by the population prevalence of diabetes. Partly in accordance with their argument, our study showed that an HbA1c cut-off point of 5.8% (40 mmol/mol) provides the best trade-off in screening for diabetes among community-dwellers with hypertension who have a higher prevalence of diabetes than among those without hypertension [2].
The choice of HbA1c at 5.8% (40 mmol/mol) as the optimal cut-off point for detecting diabetes in hypertensive individuals could be partly supported by the recent observation of Li, et al. [23] that high-risk populations with HbA1c values of 5.5% (37 mmol/mol)−6.1% (43 mmol/mol) exhibit an impaired β-cell function and an ameliorated cardiometabolic risk profile. Moreover, this choice corresponds well with the latest data indicating that the optimal cut-off point of HbA1c for detecting diabetes should be lower than that recommended by the ADA (6.5%; 48 mmol/mol), especially in Asian countries [24]. Franco, et al. [25] pointed out that the ADA-proposed HbA1c cut-off point was adequate for detecting diabetes in a high-risk population, presenting a sensitivity of 71.3% and a specificity of 90.5%. This contrasts with our results and those of another study, which showed that an HbA1c cut-off point of 6.5% (48 mmol/mol) would miss identifying a large proportion of diabetes cases (up to two-thirds) [26].
Our study showed that the accuracy of HbA1c in detecting undiagnosed diabetes would not be affected by sex. Moreover, we found that age may also not influence the accuracy of HbA1c in detecting diabetes, which is consistent with some results of Lee, et al. [27]. Furthermore, our study suggests that neither regular exercise, which reduces the risk of diabetes [28], nor medications, such as statins, which increase the risk of diabetes [29], weaken the performance of HbA1c in detecting diabetes.
Although hypertensive individuals with an HbA1c ≥5.8% (40 mmol/mol) exhibited a significantly higher 10-year risk score of CVD than those with an HbA1c <5.8% (40 mmol/mol), approximately 24–32% of the individuals with previously undiagnosed diabetes might be missed by employing the cut-off point of 5.8% for diabetes detection. This might be because these individuals (false-negative cases) showed a more favorable cardiometabolic risk profile and had a lower risk of CVD than true-positive individuals.
Furthermore, our study demonstrates that an HbA1c cut-off point of 5.1% (32 mmol/mol) could be applied to rule out diabetes and that the prevalence of diabetes begins to rise when HbA1c reaches 5.2% (33 mmol/mol)−5.7% (39 mmol/mol) (data not shown). Thus, hypertensive individuals with an HbA1c of 5.2% (33 mmol/mol)−5.7% (39 mmol/mol) may have an increased risk of developing diabetes and, therefore, need a regular check-up for diabetes and apply certain lifestyle interventions to prevent progression to diabetes [28]. Although HbA1c ≥5.8% (40 mmol/mol) showed robust sensitivity in detecting diabetes, this cut-off point could lead to a misdiagnosis rate of up to 25.5%. Thus, subsequent confirmatory testing using OGTT might be necessary for all individuals with an HbA1c ≥5.8% (40 mmol/mol). However, based on our results that HbA1c ≥6.4% (46 mmol/mol) has sufficient capability to indicate the presence of diabetes, it would be practical to advise that only hypertensive individuals with an HbA1c of 5.8% (40 mmol/mol)−6.3% (45 mmol/mol) undergo confirmatory testing.
The strengths of this study include the facts that it enrolled a large and representative sample and used internal and external validation datasets to test the results derived from the exploration population. Moreover, to the best of our knowledge, this is the first study that assessed the performance of HbA1c in community-dwellers with hypertension. However, this study has some limitations. First, diabetes was confirmed only based on a single OGTT. Second, blood pressure was measured only on a single day, while hypertension confirmation requires three blood-pressure measurements on separate days. However, it is somewhat impractical to measure blood pressure in triplicate on different days in a large-scale epidemiological study [30]. Finally, although our sensitivity analysis suggests that the presence of anemia did not significantly influence the performance of HbA1c, other conditions, including hemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency, may affect the relationship between HbA1c and diabetes detection [17].
In conclusion, this study suggests that HbA1c has reasonable diagnostic efficacy for detecting diabetes in community-dwellers with hypertension and that HbA1c ≥5.8% (40 mmol/mol) could be employed for screening for diabetes. HbA1c ≤5.1% (32 mmol/mol) and ≥6.4% (46 mmol/mol) may specify the absence and presence of diabetes in this population, respectively. Hypertensive individuals with HbA1c between 5.2% (33 mmol/mol)−5.7% (39 mmol/mol) would benefit from ongoing and regular check-ups for diabetes, while those with HbA1c between 5.8% (40 mmol/mol)−6.3% (45 mmol/mol) may require confirmatory testing.
ACKNOWLEDGEMENTS
None.
Footnotes
AUTHOR CONTRIBUTIONS
SQ, ZD, BW, HG, and ZS designed this study and drafted the manuscript. SQ, ZD, WL, and JC contributed to analytical method standardization. SQ, HW, JL, MC, BW, HG, and ZS acquired and analyzed the data. SQ, ZD, JC, HW, JL, MC, and HG carried out the statistical analysis. WL, JC, MC, BW, HG, and ZL reviewed and edited the manuscript. All authors approved the final manuscript.
CONFLICTS OF INTEREST
No potential conflicts of interest relevant to this paper were reported.
RESEARCH FUNDING
This work was supported by the National Key R&D Program of China (grant No. 2016YFC1305700), the National Key Scientific Instrument and Equipment Development Project of China (grant No. 51627808), the Nanjing Special Fund for Health Science and Technology Development (grant No. YKK18261), and the Excellence Project Funds of Southeast University (grant No. 119 0001801). The funders had no role in the design of the study; collection, analysis, and interpretation of data; or in writing or submitting this manuscript.
REFERENCES
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–58. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 3.Tsimihodimos V, Gonzalez-Villalpando C, Meigs JB, Ferrannini E. Hypertension and diabetes mellitus: coprediction and time trajectories. Hypertension. 2018;71:422–8. doi: 10.1161/HYPERTENSIONAHA.117.10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li T, Chen S, Guo X, Yang J, Sun Y. Impact of hypertension with or without diabetes on left ventricular remodeling in rural Chinese population: a cross-sectional study. BMC Cardiovasc Disord. 2017;17:206. doi: 10.1186/s12872-017-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10-Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J Hypertens. 2017;35:922–44. doi: 10.1097/HJH.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 6.Oh JY, Allison MA, Barrett-Connor E. Different impacts of hypertension and diabetes mellitus on all-cause and cardiovascular mortality in community-dwelling older adults: the Rancho Bernardo Study. J Hypertens. 2017;35:55–62. doi: 10.1097/HJH.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 7.Zafari N, Asgari S, Lotfaliany M, Hadaegh A, Azizi F, Hadaegh F. Impact of hypertension versus diabetes on cardiovascular and all-cause mortality in Iranian older adults: results of 14 years of follow-up. Sci Rep. 2017;7:14220. doi: 10.1038/s41598-017-14631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MJ, Lim NK, Choi SJ, Park HY. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertens Res. 2015;38:783–9. doi: 10.1038/hr.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsumi Y, Morimoto A, Asayama K, Sonoda N, Miyamatsu N, Ohno Y, et al. Risk of developing type 2 diabetes according to blood pressure levels and presence or absence of hypertensive treatment: the Saku study. Hypertens Res. 2019;42:105–13. doi: 10.1038/s41440-018-0121-6. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(S1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett CM, Guo M, Dharmage SC. HbA1c as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med. 2007;24:333–43. doi: 10.1111/j.1464-5491.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- 12.Bertran EA, Berlie HD, Taylor A, Divine G, Jaber LA. Diagnostic performance of HbA1c for diabetes in Arab vs. European populations: a systematic review and meta-analysis. Diabet Med. 2017;34:156–66. doi: 10.1111/dme.13118. [DOI] [PubMed] [Google Scholar]
- 13.Maesa JM, Fernandez-Riejos P, Gonzalez-Rodriguez C, Sanchez-Margalet V. Screening for gestational diabetes mellitus by measuring glycated hemoglobin can reduce the use of the glucose challenge test. Ann Lab Med. 2019;39:524–9. doi: 10.3343/alm.2019.39.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehehalt S, Wiegand S, Körner A, Schweizer R, Liesenkötter KP, Partsch CJ, et al. Diabetes screening in overweight and obese children and adolescents: choosing the right test. Eur J Pediatr. 2017;176:89–97. doi: 10.1007/s00431-016-2807-6. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Xie B, Qiu S, Huang X, Chen J, Wang X, et al. Non-lab and semi-lab algorithms for screening undiagnosed diabetes: a cross-sectional study. EBioMedicine. 2018;35:307–16. doi: 10.1016/j.ebiom.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33:393–400. doi: 10.3343/alm.2013.33.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23:1108–12. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 20.Safari S, Baratloo A, Elfil M, Negida A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emerg (Tehran) 2016;4:111–3. [PMC free article] [PubMed] [Google Scholar]
- 21.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu ZX, Walker KZ, O’Dea K, Sikaris KA, Shaw JE. A1C for screening and diagnosis of type 2 diabetes in routine clinical practice. Diabetes Care. 2010;33:817–9. doi: 10.2337/dc09-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G, Han L, Wang Y, Zhao Y, Li Y, Fu J, et al. Evaluation of ADA HbA1c criteria in the diagnosis of pre-diabetes and diabetes in a population of Chinese adolescents and young adults at high risk for diabetes: a cross-sectional study. BMJ Open. 2018;8:e020665. doi: 10.1136/bmjopen-2017-020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoyer A, Rathmann W, Kuss O. Utility of HbA1c and fasting plasma glucose for screening of Type 2 diabetes: a meta-analysis of full ROC curves. Diabet Med. 2018;35:317–22. doi: 10.1111/dme.13560. [DOI] [PubMed] [Google Scholar]
- 25.Franco LJ, Dal Fabbro AL, Martinez EZ, Sartorelli DS, Silva AS, Soares LP, et al. Performance of glycated haemoglobin (HbA1c) as a screening test for diabetes and impaired glucose tolerance (IGT) in a high risk population–the Brazilian Xavante Indians. Diabetes Res Clin Pract. 2014;106:337–42. doi: 10.1016/j.diabres.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Peter A, Fritsche A, Stefan N, Heni M, Häring HU, Schleicher E. Diagnostic value of hemoglobin A1c for type 2 diabetes mellitus in a population at risk. Exp Clin Endocrinol Diabetes. 2011;119:234–7. doi: 10.1055/s-0030-1270440. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Oh JY, Sung YA, Kim DJ, Kim SH, Kim SG, et al. Optimal hemoglobin A1C cutoff value for diagnosing type 2 diabetes mellitus in Korean adults. Diabetes Res Clin Pract. 2013;99:231–6. doi: 10.1016/j.diabres.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose–response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:2527–45. doi: 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casula M, Mozzanica F, Scotti L, Tragni E, Pirillo A, Corrao G, et al. Statin use and risk of new-onset diabetes: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2017;27:396–406. doi: 10.1016/j.numecd.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Di Bonito P, Valerio G, Pacifico L, Chiesa C, Invitti C, Morandi A, et al. A new index to simplify the screening of hypertension in overweight or obese youth. Nutr Metab Cardiovasc Dis. 2017;27:830–5. doi: 10.1016/j.numecd.2017.06.013. [DOI] [PubMed] [Google Scholar]