Abstract
Thyroid hormones (TH) control brown adipose tissue (BAT) activation and differentiation, but their subsequent homeostatic response following BAT activation remains obscure. This study aimed to investigate the relationship between cold- and capsinoids-induced BAT activation and TH changes between baseline and 2 hours post-intervention. Nineteen healthy subjects underwent 18F-fluorodeoxyglucose positron-emission tomography (18F-FDG PET) and whole-body calorimetry (WBC) after 2 hours of cold exposure (~14.5 °C) or capsinoids ingestion (12 mg) in a crossover design. Standardized uptake values (SUV-mean) of the region of interest and energy expenditure (EE) were measured. Plasma free triiodothyronine (FT3), free thyroxine (FT4) and thyroid stimulating hormone (TSH) were measured before and 2 hours after each intervention. Subjects were divided into groups based on the presence (n = 12) or absence (n = 7) of BAT after cold exposure. 12 of 19 subjects were classified as BAT-positive. Subjects with BAT had higher baseline FT3 concentration, baseline FT3/FT4 ratio compared with subjects without BAT. Controlling for body fat percentage, FT3 concentration at baseline was associated with EE change from baseline after cold exposure (P = 0.037) and capsinoids (P = 0.047). Plasma FT4 level significantly increased associated with reciprocal decline in TSH after acute cold exposure and capsinoids independently of subject and treatment status. Circulating FT3 was higher in BAT-positive subjects and was a stronger predictor of EE changes after cold exposure and capsinoids in healthy humans. BAT activation elevates plasma FT4 acutely and may contribute towards augmentation of thermogenesis via a positive feedback response.
Subject terms: Physiology, Endocrinology
Introduction
Thyroid hormones (TH) including triiodothyronine (T3) and thyroxine (T4) have long been well established to play an important role in energy expenditure (EE) and basal metabolic rate1. Regulating thermogenesis is one of the major tasks of TH in adult humans2. TH are important for heat generation during shivering and non-shivering cold adaptation. Furthermore, oxygen consumption and heat production at rest and during activities are sensitive to small alterations of TH3. The mechanisms for thyroid thermogenesis are complex4 and not fully elucidated, but one of its key thermogenic effectors operate through brown adipose tissue (BAT)5. With respect to thermoregulatory control, BAT is a critical organ that evolved primarily in mammals to defend against hypothermia through non-shivering thermogenesis (NST). The physiological link between BAT and TH is regulated by the sympathetic nervous system (SNS), while the molecular basis linking TH and SNS to BAT is now understood to operate via the 5’-flanking region of the uncoupling protein 1 (UCP1) gene under the control of both thyroid hormone response element (TRE) and cAMP response element (CRE)4,6,7. Effective adaptive thermogenesis in BAT requires TH8, adrenergic stimulation, and uncoupling protein-1 (UCP-1) expression9. TH mediation of adaptive thermogenesis involves both thyroid hormone receptor-α (TRα) and –β (TRβ). While the sympathetic response of BAT depends on TRα, Ribeiro et al.9 reported that TRβ is required for UCP-1 dependent adaptive thermogenesis in BAT of mice that underscored the importance of TH for BAT function. Lopez et al.10 reported that TH are able to up-regulate BAT via the brain as central administration of triiodothyronine (T3) resulted in BAT activation in rats. Adequate circulating concentrations of unbound, free T3 (FT3) derived from intrathyroidal and peripheral deiodination of T4 are necessary for recruitment of brown adipocytes and up-regulation of UCP-1. More recently, we have demonstrated that T3 also contributes to BAT activation via mitochondrial biogenesis and MTOR-mediated mitophagy11. T3 is thus one of the key essential hormones in the process of BAT activation and browning12–14.
Cold stress increases the expression and activity of tissue cAMP-dependent type 2 iodothyronine deiodinase (DIO2), a selenoenzyme which stimulates the conversion of T4 to active T315. TH has been extensively shown to play an important role in cold adaptation in rodents and humans16. As TH can influence BAT mass and activity with resulting augmentation of white adipose tissue (WAT) fat catabolism17,18, then TH probably exert a physiological role in mitigating against obesity and metabolic syndrome. Conversely, while NST to preserve core temperature is a primary function of BAT activation, it remains unknown if active BAT in turn affects thyroidal TH secretion through some form of homeostatic feedback. In our previous report, we found that EE was significantly increased after cold exposure and capsinoid ingestion in BAT-positive subjects assessed through 18F-FDG PET19. Although this can be attributed purely to BAT activation, might the thyroid also contributed to the persistence and maintenance of EE elevation via TH? To gain further insights regarding BAT activation effects on TH and vice versa in adult humans, this study evaluated TH changes after cold and capsinoids exposure in BAT-positive and BAT-negative healthy participants. The participants’ BAT status was classified by BAT activity via 18F-FDG PET/MR scan. The relationship between BAT and TH before and after cold and capsinoids exposure were evaluated.
Subjects and Methods
Subjects
Twenty-two healthy adults were recruited in this study. 2 subjects dropped out during the study due to unanticipated claustrophobia during PET/MR scan and one subject failed venous cannulation attempts before and after the whole body calorimetry (WBC) session and were thus excluded; 19 of them (age: 21–35 years; BMI: 18.5–25.9 kg/m2) completed all study procedures (Table 1). All participants were healthy and none had any personal or family history of either type 2 diabetes mellitus, thyroid or cardiovascular disease. None of the subjects smoked or used tobacco products, consumed special diets, or took medications known to alter BAT metabolism. All subjects were euthyroid with normal serum free thyroxine (FT4), FT3 and TSH concentrations. The present study was conducted according to the ethical guidelines of the Declaration of Helsinki, and all procedures were approved by the Domain-Specific Review Board of National Healthcare Group, Singapore (DSRB approval reference: 2015/00715), and registered with ClinicalTrials.gov (NCT02964442). Written informed consent was obtained from all subjects before participation.
Table 1.
Characteristics of total participants, participants with and without BAT after cold exposure.
| Total (19) | BAT-positive (12) | BAT-negative (7) | P value | |
|---|---|---|---|---|
| Age (years) | 26.0 ± 1.0 | 26.9 ± 1.2 | 24.4 ± 1.4 | 0.22 |
| BMI (kg/m2) | 21.7 ± 0.6 | 21.7 ± 0.8 | 21.8 ± 0.9 | 0.95 |
| Body fat (%) | 29.7 ± 1.8 | 29.4 ± 2.8 | 30.1 ± 1.5 | 0.85 |
| Fat mass (kg) | 18.6 ± 1.8 | 19.2 ± 2.7 | 17.6 ± 1.0 | 0.67 |
| Lean body mass (kg) | 40.9 ± 2.1 | 42.0 ± 2.7 | 39.2 ± 3.5 | 0.53 |
| RMR (kcal/day) | 1569 ± 75 | 1574 ± 88 | 1561 ± 145 | 0.94 |
| Triglyceride (mmol/L) | 0.9 ± 0.06 | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.04 |
| TSH (mIU/L) | 2.1 ± 0.2 | 2.3 ± 0.3 | 1.7 ± 0.2 | 0.24 |
| FT4 (pmol/L) | 16.9 ± 0.6 | 17.1 ± 0.8 | 16.6 ± 0.7 | 0.68 |
| FT3 (pmol/L) | 4.8 ± 0.1 | 5.0 ± 0.1 | 4.3 ± 0.2 | 0.008 |
| Free T3/Free T4 ratio | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.26 ± 0.01 | 0.047 |
| EE 2 h (kcal/day) | 1778 ± 82 | 1841 ± 101 | 1673 ± 139 | 0.34 |
| ∆ EE (kcal/day) | 210 ± 27 | 267 ± 29 | 111 ± 24 | 0.002 |
| PET SUV | 2.1 ± 0.3 | 2.9 ± 0.2 | 0.8 ± 0.1 | <0.001 |
Data presented as mean ± SEM. P values represents Student’s t-test between BAT- positive and BAT- negative participants. BAT, brown adipose tissue; BMI, body mass index, was calculated as body weight (kg) divided by the square of height (m); RMR, resting metabolic rate; TSH, thyroid-stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; EE, energy expenditure; PET, positron emission tomography; SUV, standardized uptake value.
Study design
The subjects attended two separate visits for PET/MR and two separate visits for whole body calorimetry /infrared thermal imaging (WBC/IRT), to assess the effects of cold exposure and capsinoids ingestion. Visits were separated by a minimum of 48 hours washout period in order to minimize carryover effects. Capsinoids capsules (12 mg, 8 gel capsules) were provided by Ajinomoto Inc. (Tokyo, Japan). The composition details were described previously20. Cold exposure was achieved using a cooling vest with a fixed temperature of 14.5 °C (Cool 58, Polar Products, Ohio, US). The participants were asked to refrain from caffeine, alcohol intake and any vigorous physical activity on the day before the study. The study protocol and flow chart were described previously19.
18F-FDG PET/MR Imaging
After fasting for 10–12 hours, the participants arrived at the Clinical Imaging Research Centre. An indwelling cannula was inserted into a forearm vein by a registered nurse after a 10-min rest, and fasting blood glucose was measured. At the first visit, subjects were instructed to ingest capsinoids 30 minutes before the PET/MR scan. During the second visit, subjects donned on the cold vest upon arrival at the research centre. For both visits, subjects were administered an intravenous injection of 18F-FDG (3 mCi), 20 min before the start of the scans. The scans were performed on a hybrid PET-MR system (Biograph mMR, Siemens Healthcare, Erlangen, Germany) for 80 minutes. One experienced researcher blinded to the allocated intervention assessed FDG uptake on both sides of neck and supraclavicular regions excluding bone, muscle and blood vessels. BAT activity was quantified by calculating SUV mean, SUV max and BAT volume. The cut-off value of SUV mean for dividing subjects into BAT-positive and BAT-negative groups was 2.0. Detectable BAT volume was calculated as the sum of pixels meeting this classification criteria multiplied by the pixel volume. The imaging analysis was described as previously21.
Whole body calorimetry
After an overnight fast of 10–12 hours, EE was assessed through gaseous exchanges using a dual-chamber whole body calorimeter (WBC) facility located at the Clinical Nutrition Research Centre. The WBC chambers are open-circuit air-tight indirect calorimeters and provide EE, fat oxidation and respiratory quotient (RQ) measurement based on oxygen consumption and carbon dioxide production. The WBC has been described in detail in our previous published paper22. During each test session, after 45 minutes of resting metabolic rate (RMR) measurement, the subjects were asked to wear the cooling vest or ingest 12 mg capsinoids inside the WBC room for up to 2 hours.
Blood analysis
Screening blood samples were sent to the National University Hospital referral laboratory for biochemical assessment of renal, liver and thyroid function. Fasting and 2 hours post-intervention blood samples during the WBC session was collected from venous samples to measure TH. Plasma concentration of free TH (FT3, FT4) and TSH were measured using immunoassay analyser (Cobas e411, Roche). The intra-assay coefficient of variation (intra-assay CV) of the Cobas immunoassays for the analytes was less than 3%.
Data and statistical analysis
This was a secondary analysis of data acquired from participants in a protocol that investigated BAT activation and energy expenditure by cold and capsinoids stimulation (www.Clinical Trials.gov identifier NCT 02964442). Details on power calculations were shown in our previous publication23. EE was calculated based on volume of O2 consumption (VO2) and CO2 production (VCO2) using the Weir equation24. RMR was defined as the average 45 minutes EE during the 45 min measurement period. The change in EE and fat oxidation (FOX) in 2 hours was calculated as average 2 hours EE and FOX minus RMR. Paired-t tests were used to compare the differences between baseline and 2 hours post-intervention in the concentrations of free, unbound circulating TH. The data were checked for normality using Shapiro-Wilk test and visually using histogram and Q-Q-plot. The data analyzed by parametric statistics were normally distributed. Pearson correlations were performed to identify relations between variables. Data were presented as means ± SEM, unless otherwise stated. A P-value ≤ 0.05 was considered statistically significant. Statistical analysis was performed by using SPSS software version 23 (IBM SPSS Inc.).
Results
Clinical characteristics
The baseline characteristics of the 19 subjects are summarized in Table 1. Cold exposure increased the activity and volume of detectable BAT in 12 subjects. BAT was found in the cervical and supraclavicular adipose depots, with an estimated average SUV mean of 2.9, SUV max of 7.9 and average BAT volume of 75 cm3 after cold exposure (Table 1). Following the 18F-FDG uptake post-cold exposure measured by calculating mean SUV, the subjects were divided into two groups: those with no detectable 18F-FDG uptake (BAT-negative, n = 7) and those with detectable 18F-FDG uptake with mean SUV ≥ 2 (BAT-positive; n = 12). No significant differences in anthropometric measurements were found between the two groups (Table 1). However, we found fasting baseline FT3 level significantly higher in BAT-positive subjects (5.0 ± 0.1) compared with BAT-negative subjects (4.3 ± 0.2) (P = 0.008). The ratio of fasting baseline FT3/FT4 was significantly higher in BAT-positive subjects as compared with BAT-negative subjects (P = 0.047). No significant difference was found for TSH (P = 0.24) and FT4 (P = 0.68) levels between BAT-positive subjects and BAT-negative subjects in fasting basal state.
Relationship of thyroid hormones, blood lipids and body composition
Fasting FT3 concentration was positively associated with lean body mass (r = 0.49, P = 0.04), REE (r = 0.55, P = 0.01) and triglyceride level (r = 0.56, P = 0.01) but not with other baseline measurements (Table 2). FT4 concentration was negatively associated with % fat mass (r = −0.56, P = 0.01) and positively associated with fasting glucose level (r = 0.46, P = 0.047) but not with other baseline metabolic measurements including lean body mass (P = 0.20) and triglycerides (P = 0.59). TSH was not associated with baseline characteristics we have measured in Table 2.
Table 2.
Correlations between fasting thyroid hormone levels, body composition, blood markers and resting energy expenditure in total participants.
| Measure | FT3 (pmol/L) | FT4 (pmol/L) | TSH (uIU/mL) | |||
|---|---|---|---|---|---|---|
| Coefficient (r) | p-value | Coefficient (r) | p-value | Coefficient (r) | p-value | |
| BMI (kg/m2) | 0.32 | 0.18 | −0.04 | 0.87 | −0.21 | 0.4 |
| Lean body mass (kg) | 0.49 | 0.04 | 0.31 | 0.20 | 0.06 | 0.82 |
| % fat mass | −0.21 | 0.38 | −0.56 | 0.01 | −0.29 | 0.24 |
| Visceral fat | 0.47 | 0.07 | −0.05 | 0.85 | −0.14 | 0.60 |
| REE (kcal/day) | 0.55 | 0.01 | 0.26 | 0.28 | −0.02 | 0.92 |
| Glucose (mmol/L) | 0.32 | 0.19 | 0.46 | 0.047 | −0.24 | 0.33 |
| Triglyceride (mmol/L) | 0.56 | 0.01 | −0.13 | 0.59 | 0.15 | 0.55 |
N = 19; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3: free triiodothyronine; BMI, body mass index; REE, resting energy expenditure.
Cold and capsinoids-induced thyroid hormones changes
In all of the participants, both cold exposure and capsinoids ingestion significantly increased FT4 concentrations coupled with decreased TSH concentrations compared with baseline (Table 3). There were similarities between the effects of cold exposure and capsinoids ingestion on FT4 and TSH. The increase of FT3 was significantly higher after cold exposure compared with capsinoids ingestion (Table 3). However, when compared with baseline, 2 hours of cold exposure did not result in any significant change in FT3 level in all subjects which means BAT only affects baseline FT3 concentration with only small changes following BAT activation by cold within the same subjects (Table 3). By contrast, FT4 concentration was significantly increased after 2 hours of cold or capsinoids exposure regardless of BAT status (Table 4). TSH concentration was significantly decreased after 2 hours of cold exposure in all participants independent of BAT status (Table 4).
Table 3.
Thyroid hormones concentrations at baseline and 2 h post intervention and change from baseline after 2 treatments1.
| Capsinoids | Cold exposure | |||||
|---|---|---|---|---|---|---|
| baseline | 120 min | ∆ change | baseline | 120 min | ∆ change | |
| TSH (uIU/mL) | 2.2 ± 0.3 | 1.8 ± 0.22 | −0.4 ± 0.1 | 2.1 ± 0.2 | 1.6 ± 0.22 | −0.5 ± 0.1 |
| FT4 (pmol/L) | 16.4 ± 0.6 | 16.9 ± 0.62 | 0.5 ± 0.1 | 16.9 ± 0.6 | 17.2 ± 0.62 | 0.3 ± 0.1 |
| FT3 (pmol/L) | 4.7 ± 0.2 | 4.7 ± 0.2 | −0.04 ± 0.04 | 4.8 ± 0.2 | 4.8 ± 0.2 | 0.05 ± 0.043 |
1Values are means ± SEMs; n = 19. TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3: free triiodothyronine.
2Different from baseline within the same treatment group, P < 0.05. 3Different from after capsinoids ingestion, P < 0.05.
Table 4.
Summary of outcomes of thyroid hormones after capsinoids ingestion and cold exposure in BAT positive and BAT-negative subjects1.
| BAT-positive subjects | BAT-negative subjects | |||||||
|---|---|---|---|---|---|---|---|---|
| Capsinoids ingestion | Cold exposure | Capsinoids ingestion | Cold exposure | |||||
| baseline | 120 min | baseline | 120 min | baseline | 120 min | baseline | 120 min | |
| TSH (uIU/mL) | 2.4 ± 0.4 | 2.0 ± 0.32 | 2.3 ± 0.3 | 1.8 ± 0.32 | 1.9 ± 0.2 | 1.5 ± 0.32 | 1.7 ± 0.2 | 1.3 ± 0.12 |
| FT4 (pmol/L) | 16.7 ± 0.9 | 17.2 ± 0.92 | 17.1 ± 0.8 | 17.5 ± 0.82 | 15.9 ± 0.7 | 16.4 ± 0.72 | 16.6 ± 0.7 | 16.8 ± 0.72 |
| FT3 (pmol/L) | 5.1 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.1 | 5.1 ± 0.1 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.3 ± 0.2 | 4.4 ± 0.3 |
1Values are mean ± SEMs; all values calculated from 19 subjects (12 BAT-positive subjects vs. 7 BAT-negative subjects); 2Different from baseline within the same treatment group, P < 0.05; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3: free triiodothyronine.
Relationship of BAT, EE and thyroid hormones
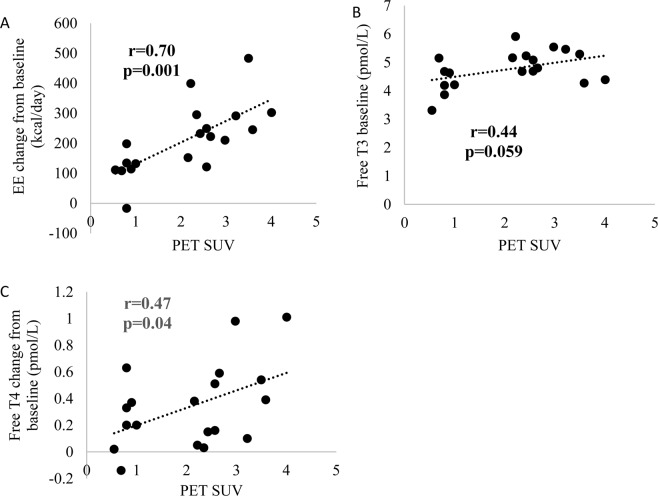
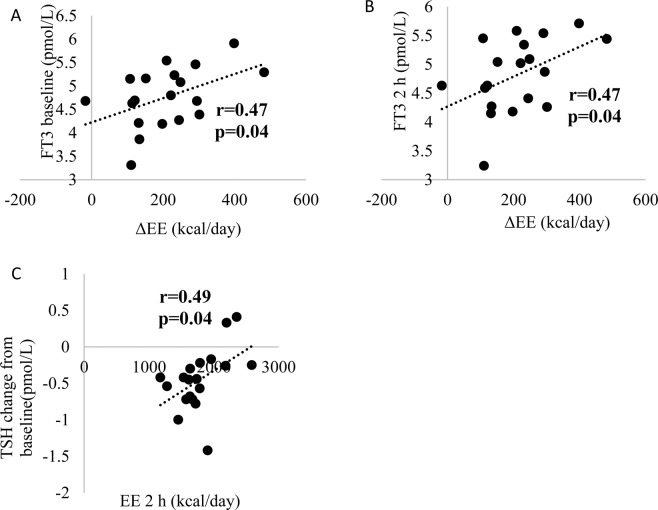
Cold-induced BAT activity was measured via PET/MR as previous reported25. PET SUV was positively associated with EE change from baseline after cold exposure (r = 0.7, P = 0.001). There was a trend towards a positive association between FT3 concentration at baseline and PET SUV (r = 0.44, P = 0.06). Also, FT3 at baseline is correlated to EE change post-cold and post-capsinoids (Table 5). FT4 change from baseline after cold exposure was positively associated with PET SUV (r = 0.47, 0.04) (Fig. 1). But FT4 at baseline was not associated with EE change post-cold and post-capsinoids (data not shown). FT3 concentration at fasting and 2 hours after cold exposure were positively associated with EE change from baseline (both r = 0.47, P = 0.04). TSH change from baseline was positively associated with EE after 2 hours cold exposure (Fig. 2).
Table 5.
Regression results of EE change from baseline with thyroid hormones at baseline and 2 hours after capsinoids ingestion and cold exposure.
| baseline | ∆EE after capsinoids ingestion | Coefficient | ∆ EE after cold exposure | |||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P value | 95% CI | P value | ||
| FT3 (pmol/L) | 44.1 | 0.7, 87.5 | 0.04 | 73.4 | −8.7, 155.5 | 0.04 |
| FT4 (pmol/L) | 1.5 | −14.1, 17.1 | 0.8 | −4.2 | −31.1, 22.8 | 0.75 |
| TSH (uIU/mL) | 12.5 | −17.7, 42.6 | 0.4 | −6.2 | −65.0, 52.5 | 0.83 |
| 120 min FT3 (pmol/L) | −4.6 | −167.6, 158.4 | 0.95 | −32.1 | −387.9, 323.7 | 0.85 |
| FT4 (pmol/L) | −38.7 | −124.1, 46.9 | 0.35 | 60.0 | −126.4, 246.3 | 0.50 |
| TSH (uIU/mL) | 10.2 | −66.5, 86.8 | 0.78 | 94.2 | −106.5, 294.9 | 0.33 |
All values calculated from 19 subjects. The baseline analysis was adjusted with body fat percentage (%). The 120 min analysis was adjusted with body fat percentage (%) and thyroid hormones baseline value. TSH, TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3: free triiodothyronine.
Figure 1.
Correlations between PET SUV and EE change from baseline after cold exposure (A), FT3 at baseline (B) and FT4 concentration change from baseline after cold exposure (C) (n = 19). PET, positron emission tomography; FT3, free triiodothyronine; FT4, free thyroxine.
Figure 2.
Correlations between EE change from baseline (ΔEE) and FT3 at baseline (A) and 2 hours post-cold exposure (B), TSH change from baseline and EE 2 hours after cold exposure (C) (n = 19). EE, energy expenditure; FT3, free Triiodothyronine; TSH, thyroid stimulating hormone.
Discussion
The present findings revealed very interesting biological phenomena that have never previously been reported anywhere thus far in the extant literature. Essentially, we can better comprehend these TH changes when we scrutinize the results above at four levels in terms of group comparisons based on: (1) BAT status, (2) modality of BAT stimulation (ie. cold versus capsinoids), (3) pre-stimulated versus post-stimulated time points, and (4) FT3 versus FT4.
Firstly, two potential physiological processes may account for the observation of baseline TH levels (especially FT3) being significantly higher among BAT-positive compared to BAT-negative subjects. One hypothesis is that the presence of BAT within an individual accentuates TH secretion by the thyroid gland via some yet undefined positive feedback interactions and mechanisms. The other possibility is that a higher TH output by the thyroid results in greater brown adipocyte differentiation and BAT activation, which in turn increases the probability of higher TH being found among those who are BAT-positive. Both processes are not mutually exclusive and may co-exist in any given individual to produce this finding. It would require dynamic experiments using appropriately labelled tracers to decipher the kinetics involved and which mechanism predominates. Secondly, while both cold and capsinoids stimulation evoke changes in circulating TH levels regardless of BAT status, the magnitude of increase in TH was greater in FT4 compared to FT3. Because T4 is specifically produced by the thyroid whereas T3 can be derived from both the thyroid and peripheral tissues possessing 5’-deiodinase activity, it is likely that cold and capsinoids directly act on the SNS which then triggers the secretion of thyroidal secretion of TH, with T4 predominating over T3 as per established thyroid physiology26. As well, the TH elevations observed were consistently associated with significant reciprocal TSH reductions, which implies that the primary ‘driver’ of the TH secretion resides within the thyroid gland itself rather than being mediated at the central, hypothalamus-pituitary level.
Given that TSH is an exquisitely sensitive biomarker and regulator of ambient TH levels, the fact that TSH declined inversely to the TH escalations was itself remarkable as it suggested that these “quantitatively miniscule” changes in TH are physiologically significant. This crucial result implied, as a necessary corollary, that TSH should not have altered to any detectable extent had the magnitude of those TH changes been clinically irrelevant. Notably, the pattern of TSH change was similar in both cold and capsinoids stimulation of BAT, and TSH varied inversely with all these TH elevations.
Apparently, FT4 rose much more so than FT3 as a result of cold stimulation, associated with a decreased TSH. This is consistent with the fact that at any time, the secretory rate of T4 by the thyroid is nearly 20-fold higher than that of T327. Apart from differences in thyroidal biosynthetic rate of T4 and T3, it is also known that the thyroid gland harbours a reservoir pool of preformed TH consisting of far more T4 and T328. Hence, it is conceivable that the FT4 level would escalate far more than FT3 in response to any stimulus that increases net thyroid glandular secretory output of TH. Importantly, we observe that the increase in FT3 in the circulation evoked by cold and capsinoids regardless of BAT status is miniscule compared to FT4 despite the documented 10- to 50-fold upregulation of type 2 iodothyronine deiodinase (D2) selenoenzyme during cold stress in BAT15. This is probably because the accentuation of D2 activity led to locally generated T3 that is mainly intracellular within BAT adipocytes with much lesser T3 being secreted into the blood. On the other hand, as the activity of T3 is 3–8-folds higher than T429, this could explain the strong correlation of FT3 changes to EE whereas FT4 changes showed no obvious correlation to EE.
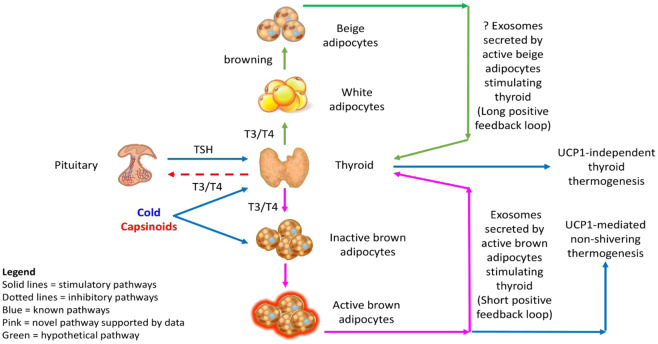
Interestingly, the fact that cold and capsinoids were also capable of inducing changes in TH and TSH regardless of BAT status supports yet another important notion, namely that such external stimuli might also directly act on the thyroid gland to release TH independently of BAT as well. As sympathetic innervation occurs within the thyroid follicles and norepinephrine released by intrathyroidal adrenergic nerves stimulates secretion of TH, it is not surprising that both cold and capsinoids which act via the SNS can stimulate an increase in TH with reciprocal reduction in TSH secretion even among those without demonstrable BAT30. Nevertheless, the finding that the increase in FT4 is 2.3-folds greater in BAT-positive relative to BAT-negative subjects seemingly support the presence of a tentative undefined feedforward factor emerging from activated BAT which has a capacity for stimulating T4 secretion. Finally, as the changes in TH and TSH were all significantly correlated to both PET-SUV and EE, our discovery lends credence to the hypothesis that the positive feedback loop bridging between the thyroid gland and BAT is not trivial but reveals an intriguing aspect of homeostatic regulation that probably plays a definite role in normal thermoregulation biology and in the maintenance of metabolic health. This shows that both BAT and the thyroid, being organs hardwired for thermogenesis, can be simultaneously activated independently by appropriate external stimuli with ensuing TH/TSH changes that reflected the existence of an intrinsically positive homeostatic feedback loop between the two organs (Fig. 3).
Figure 3.
The positive feedback loops that amplify thermogenesis within the framework of a novel BAT-thyroid axis. BAT, brown adipose tissue; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone; UCP1, uncoupling protein 1.
The mechanisms behind how BAT communicate with the thyroid likely consist of signalling pathways orchestrated via “batokines” (ie. BAT-derived adipokines) delivered to the thyroid packaged in exosomes. The recent literature has also shown that BAT exosomes contain non-coding RNA such as microRNAs and long non-coding RNAs which can exert gene regulation effects on distant targets, including the thyroid31–35. BAT-derived exosomes are also cellular cargoes for a wide range of proteins characterizing the BAT secretome. This BAT proteome can act as hormones and serve as biochemical mediators forming one limb of the feedforward path of the BAT-thyroid axis to generate an increased thyroidal output of TH. In this connection, it has been shown that neuregulin-4 (NRG4) is a novel adipokine secreted by both classical brown and beige adipocytes36,37. Plasma NRG4 levels have also been shown to be inversely related to the risk of obesity, metabolic syndrome and type 2 diabetes36,38–40. Interestingly, serum NRG4 has been demonstrated recently to be elevated in hyperthyroidism, a clinical state of heightened fat catabolism41. Hence, BAT exosomal NRG4 may potentially be one of several critical links between BAT and the thyroid gland42. Our own research also revealed the presence of selenoprotein P within cold-stimulated BAT exosomes (unpublished data). This finding adds further evidence that suggests BAT may regulate thyroid hormone synthesis because selenoprotein biosynthesis defects were shown to cause abnormal thyroid hormone production43–45. Such exosomal batokines may augment TH output via the observed short (acute) BAT-thyroid feedback loop mediated by pre-existing brown fat, and possibly a long (chronic) positive feedback loop mediated by beige fat derived from browning of WAT (Fig. 3). The consequent amplification in TH in this schema constitutes the other positive feedback path of the BAT-thyroid axis to reinforce BAT mass and activation. Conversely, while still hypothetical, the thyroid may equally serve as a source of exosomes that carry molecular cargoes critical for BAT activation and browning of WAT so as to accentuate thermogenesis. We have demonstrated previously that lnc-dPrdm16 is one such long non-coding RNA that mediates brown adipocyte differentiation46. It is thus conceivable that exosomal lnc-dPrdm16 from metabolically active tissues such as the thyroid could also regulate WAT browning and contribute to thermogenesis.
It may be argued that this novel axis may seem functionally negligible in humans living in persistently warm climates. However, we have elegantly shown here19,25,47 that a substantial number of people living within the tropics/equatorial belt with ambient temperatures up to 35 degrees Celsius can still be demonstrated to possess much activatable BAT by cold or capsinoids. Hence, we surmise that this BAT-thyroid axis is physiologically critical in maintaining normal metabolism in addition to defending against hypothermia. While speculative at this point, it is possible that a defect in this BAT-thyroid axis could be medically impactful and predispose the affected individuals to metabolic disorders, such as obesity and type 2 diabetes mellitus. No additional PET-MR scan at ambient temperature was performed on any subjects as a control condition due to ethical constraints and the need to abide by the ALARA principle of keeping ionization radiation exposure to the minimum while fulfilling any clinical/research objective for which we hereby acknowledge as a limitation of our study.
In summary, we postulate that part of the purpose for activated BAT triggering an increase in TH output by the thyroid gland is an evolutionary adaptation as such a biological response should translate, in accordance to fundamental control systems engineering principles, to a far more efficient and optimized thermogenic system if the resulting higher TH output in turn augments BAT activity and development (classic brown fat and beige fat via browning of WAT). We therefore predict that a similar ‘reflex’ feedback loop ought to occur in other mammalian vertebrates of comparable biology, a hypothesis awaiting further proof by future research. This positive feedback cycle within a BAT-thyroid axis favours the successful sustenance of thermogenesis and metabolic homeostasis more robustly than either organ operating independently without any linkage. Therefore, such a BAT-thyroid interaction conceivably exists to confer a biological survival advantage.
Clinical trial registration
The trial was registered at clinicaltrials.gov as NCT02964442.
Acknowledgements
Ajinomoto Co., Inc. (Tokyo, Japan) provided the capsinoids but was not involved in the conception, design or conduct of the study and did not influence the writing of this manuscript. We thank the volunteers who participated in this trial. The study was funded by the Singapore Ministry of Health’s National Medical Research Council (NMRC) Clinician Scientist Award (grant ID NMRC/CSA-INV/0003/2015) awarded to MKSL and also partly supported by the Agency for Science, Technology and Research (A*STAR). The study was supported by the National Medical Research Council Award grant. Grant number: NMRC/CSA-INV/0003/2015.
Author contributions
The authors’ responsibilities were as follows: L.J.S. conducted the study, analysed the data, interpreted results and wrote the manuscript; H.J.G. and P.G. conducted the study; L.S. provided intellectual inputs and critically reviewed the manuscript; C.J.H. provided intellectual inputs and critically reviewed the manuscript; M.K.S.L. conceived and designed the study, interpreted the data, provided intellectual inputs, illustrated the feedback diagram, co-wrote, edited and critically reviewed the manuscript. All authors proofread, edited and approved the final manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 2.Silva JE. The multiple contributions of thyroid hormone to heat production. J Clin Invest. 2001;108:35–37. doi: 10.1172/JCI13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82:1118–1125. doi: 10.1210/jcem.82.4.3873. [DOI] [PubMed] [Google Scholar]
- 4.Rabelo R, Schifman A, Rubio A, Sheng X, Silva JE. Delineation of thyroid hormone-responsive sequences within a critical enhancer in the rat uncoupling protein gene. Endocrinology. 1995;136:1003–1013. doi: 10.1210/endo.136.3.7867554. [DOI] [PubMed] [Google Scholar]
- 5.Weiner J, Hankir M, Heiker JT, Fenske W, Krause K. Thyroid hormones and browning of adipose tissue. Mol Cell Endocrinol. 2017;458:156–159. doi: 10.1016/j.mce.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- 7.Rabelo R, Reyes C, Schifman A, Silva JE. Interactions among receptors, thyroid hormone response elements, and ligands in the regulation of the rat uncoupling protein gene expression by thyroid hormone. Endocrinology. 1996;137:3478–3487. doi: 10.1210/endo.137.8.8754777. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro MO, et al. Evidence of UCP1-independent regulation of norepinephrine-induced thermogenesis in brown fat. Am J Physiol Endocrinol Metab. 2000;279:E314–322. doi: 10.1152/ajpendo.2000.279.2.E314. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro MO, et al. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-beta isoform specific and required for adaptive thermogenesis. Endocrinology. 2010;151:432–440. doi: 10.1210/en.2009-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez M, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yau WW, et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy. 2019;15:131–150. doi: 10.1080/15548627.2018.1511263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 13.Golozoubova V, et al. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol. 2004;18:384–401. doi: 10.1210/me.2003-0267. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, et al. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol. 2012;302:C463–472. doi: 10.1152/ajpcell.00010.2011. [DOI] [PubMed] [Google Scholar]
- 15.de Jesus LA, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurberg P, Andersen S, Karmisholt J. Cold adaptation and thyroid hormone metabolism. Horm Metab Res. 2005;37:545–549. doi: 10.1055/s-2005-870420. [DOI] [PubMed] [Google Scholar]
- 17.Viguerie N, et al. Regulation of human adipocyte gene expression by thyroid hormone. J Clin Endocrinol Metab. 2002;87:630–634. doi: 10.1210/jcem.87.2.8200. [DOI] [PubMed] [Google Scholar]
- 18.Lopez M, Alvarez CV, Nogueiras R, Dieguez C. Energy balance regulation by thyroid hormones at central level. Trends Mol Med. 2013;19:418–427. doi: 10.1016/j.molmed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, et al. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan. The American Journal of Clinical Nutrition. 2018;107:62–70. doi: 10.1093/ajcn/nqx025. [DOI] [PubMed] [Google Scholar]
- 20.Inoue N, Matsunaga Y, Satoh H, Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids) Biosci Biotechnol Biochem. 2007;71:380–389. doi: 10.1271/bbb.60341. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, et al. Brown Adipose Tissue: Multimodality Evaluation by PET, MRI, Infrared Thermography, and Whole-Body Calorimetry (TACTICAL-II) Obesity (Silver Spring) 2019 doi: 10.1002/oby.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh HJ, Govindharajulu P, Camps SG, Tan SY, Henry CJ. Gross and relative energy cost of domestic household activities in Asian men. Eur J Clin Nutr. 2016;70:1414–1419. doi: 10.1038/ejcn.2016.134. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, et al. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan. Am J Clin Nutr. 2018;107:62–70. doi: 10.1093/ajcn/nqx025. [DOI] [PubMed] [Google Scholar]
- 24.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, et al. Brown Adipose Tissue: Multimodality Evaluation by PET, MRI, Infrared Thermography, and Whole-Body Calorimetry (TACTICAL-II) Obesity (Silver Spring) 2019;27:1434–1442. doi: 10.1002/oby.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichihara K, et al. New indices for thyroid functional status, hormone binding, and peripheral hormone metabolism. Principal component analysis of 24,000 clinical data. Am J Clin Pathol. 1986;85:469–478. doi: 10.1093/ajcp/85.4.469. [DOI] [PubMed] [Google Scholar]
- 27.Larsen PR, Zavacki AM. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur Thyroid J. 2012;1:232–242. doi: 10.1159/000343922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirahanchi, Y. & Jialal, I. in StatPearls (2019).
- 29.Sandler B, et al. Thyroxine-thyroid hormone receptor interactions. J Biol Chem. 2004;279:55801–55808. doi: 10.1074/jbc.M410124200. [DOI] [PubMed] [Google Scholar]
- 30.Melander A, Westgren U, Ericson LE, Sundler F. Influence of the sympathetic nervous system on the secretion and metabolism of thyroid hormone. Endocrinology. 1977;101:1228–1237. doi: 10.1210/endo-101-4-1228. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun. 2016;7:11420. doi: 10.1038/ncomms11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Pfeifer A. Brown Fat-Derived Exosomes: Small Vesicles with Big Impact. Cell metabolism. 2017;25:759–760. doi: 10.1016/j.cmet.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Lee, M. W., Lee, M. & Oh, K. J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J Clin Med810.3390/jcm8060854 (2019). [DOI] [PMC free article] [PubMed]
- 34.Goody D, Pfeifer A. BAT Exosomes: Metabolic Crosstalk with Other Organs and Biomarkers for BAT Activity. Handb Exp Pharmacol. 2019;251:337–346. doi: 10.1007/164_2018_114. [DOI] [PubMed] [Google Scholar]
- 35.Chen XW, Li S, Lin JD. The Micro-Managing Fat: Exosomes as a New Messenger. Trends Endocrinol Metab. 2017;28:541–542. doi: 10.1016/j.tem.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang GX, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab. 2015;26:231–237. doi: 10.1016/j.tem.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan P, et al. Plasma Neuregulin 4 Levels Are Associated with Metabolic Syndrome in Patients Newly Diagnosed with Type 2 Diabetes Mellitus. Dis Markers. 2018;2018:6974191. doi: 10.1155/2018/6974191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Gao M, Liu D. Preventing High Fat Diet-induced Obesity and Improving Insulin Sensitivity through Neuregulin 4 Gene Transfer. Sci Rep. 2016;6:26242. doi: 10.1038/srep26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol Metab. 2017;6:863–872. doi: 10.1016/j.molmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M, et al. Elevated serum neuregulin 4 levels in patients with hyperthyroidism. Endocr Connect. 2019;8:728–735. doi: 10.1530/EC-19-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosell M, et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab. 2014;306:E945–964. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumitrescu AM, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 44.Kohrle J. Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes. 2015;22:392–401. doi: 10.1097/MED.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 45.Kohrle J. Selenium and the control of thyroid hormone metabolism. Thyroid. 2005;15:841–853. doi: 10.1089/thy.2005.15.841. [DOI] [PubMed] [Google Scholar]
- 46.Ding C, et al. De novo reconstruction of human adipose transcriptome reveals conserved lncRNAs as regulators of brown adipogenesis. Nat Commun. 2018;9:1329. doi: 10.1038/s41467-018-03754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ang QY, et al. A new method of infrared thermography for quantification of brown adipose tissue activation in healthy adults (TACTICAL): a randomized trial. J Physiol Sci. 2017;67:395–406. doi: 10.1007/s12576-016-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]