Abstract
The mammary immune and physiological responses to distinct mammary-pathogenic E. coli (MPEC) strains were studied. One gland in each of ten cows were challenged intra-mammary and milk composition (lactose, fat, total protein, casein), biochemical (glucose, glucose-6-phosphate (Glu6P), oxalate, malate, lactate, pyruvate and citrate, malate and lactate dehydrogenases, lactate dehydrogenase (LDH), nitrite, lactic peroxidase, catalase, albumin, lactoferrin, immunoglobulin) and clotting parameters were followed for 35 days post-challenge. Challenge lead to clinical acute mastitis, with peak bacterial counts in milk at 16–24 h post-challenge. Biochemical and clotting parameters in milk reported were partially in accord with lipopolysaccharide-induced mastitis, but increased Glu6P and LDH activity and prolonged lactate dehydrogenase and Glu6P/Glu alterations were found. Some alterations measured in milk resolved within days after challenge, while others endured for above one month, regardless of bacterial clearance, and some reflected physiological responses to mastitis such as the balance between aerobic and anaerobic metabolism (citrate to lactate ratios). The results suggest that E. coli mastitis can be divided into two stages: an acute, clinical phase, as an immediate response to bacterial infection in the mammary gland, and a chronic phase, independent of bacteria clearance, in response to tissue damage caused during the acute phase.
Subject terms: Biochemistry, Inflammation, Acute inflammation, Chronic inflammation
Introduction
Escherichia coli is one of the major pathogen causing acute mastitis in cattle, a disease of significant economic and animal welfare impact in dairy production worldwide. Upon entry into the mammary gland, mammary pathogenic strains of E. coli (MPEC) rapidly grow using milk as a nutrient source, in a manner highly dependent on the expression of the ferric-dicitrate system by these bacteria1–3. The resulting infection leads to an acute inflammation that causes a sharp decrease in milk yield and deep changes of milk properties and constituents4. Even after clinical healing and infection clearance in the mammary gland, indicated by cessation of bacterial secretion in the milk, milk yield and quality might remain impaired for a long period in a significant number of affected cows. The degree and length of the post-acute inflammation effect on udder health and milk quality vary greatly and may depend on the health status of the individual animal, time of diagnosis and treatment, as well as the infecting E. coli strain5.
Much has been studied about the immune and inflammatory reaction to E. coli in the mammary gland, yet, less is known about the physiological and biochemical changes that occur in the gland and in the milk during and following intra-mammary infections by E. coli bacteria. In a previous study by our group6, it was shown that under acute mammary inflammation induced by intra-mammary challenge of cows with E. coli lipopolysaccharide (LPS), the passage of glucose-derived carbons shifts to the pentose phosphate pathway, leading to an epithelial cell metabolism shift to glycolysis at the expense of mitochondrial respiratory activity. A negative correlation between milk secretion and lactose, glucose and citrate concentrations and inflammation parameters was described. In parallel, the concentrations of glucose-6-phosphate (Glu6P), malate, oxaloacetate, lactate and pyruvate, and the activities of the respective enzymes glucose-6-phosphate dehydrogenase (Glu6PD), malate dehydrogenase (MDH) and lactate dehydrogenase (LDH) increased and, in general, positively correlated with inflammation parameters. A main difference between LPS and live bacteria intra-mammary challenge is the fact that bacteria represent a continuous stimulant of the mammary immune system due to bacteria replication in the gland, which may remain for days after the infection commence, whereas a single LPS injection would be a one-time stimulus. There is limited knowledge on the physiological and biochemical changes induced by live distinct mammary pathogenic E. coli and how these changes correlate to LPS-induced mastitis. Recently, an intra-mammary challenge experiment with MPEC strains (VL2874, VL2732 and P4) and with the non-mammary pathogenic E. coli strain K1 was conducted in order to study whether the dynamics and intensity of the immune response in bovine mammary glands is different and depends on the infecting E. coli strain4. These strains were chosen for representing distinct presentations of E. coli mastitis: per-acute (VL2874), persistent (VL2732) and clinical (P4), respectively4.
Cellular and chemokine responses and bacterial culture follow-up were performed for 35 days post-challenge (DPC). Cows challenged by any of the MPEC strains developed clinical acute mastitis, with peak bacterial counts in milk at 16–24 h post-challenge. During the 35 days of the study, differences related to the E. coli strains were found in the intensity and duration of the response in various of the parameters studied, namely the somatic cells count (SCC), leukocytes distribution, secreted cytokines (TNF-α, IL-6 and IL-17) and levels of membrane TLR4 on leukocytes in the milk4.
The objective of the present work was to study the physiological and biochemical responses measured in the milk and mammary gland tissue of cows challenged with the above mentioned three distinct mammary-pathogenic strains of E. coli.
Materials and Methods
Animals
Ten Israeli Holstein cows at the Agricultural Research Organization (A.R.O.) dairy farm at lactations 1–6, 133–442 days in lactation, and with milk yield of 22–38.4 L/day were used for intra-mammary challenge with mammary pathogenic strains of E. coli. The cows were milked thrice daily (05:00, 13:00 and 20:00) in a dairy parlor equipped with an on-line computerized AfiFarm Herd Management data acquisition system, including the AfiLab milk analyzer, which provides on-line data on gross milk composition - fat, protein and lactose (Afimilk, Afikim, Israel; http://www.afimilk.com). The average milk yield in the herd was >11,000 L during 305 days of lactation. Animals were fed a typical Israeli total mixed ration containing 65% concentrate and 35% forage (17% protein). Food was offered ad lib in mangers located in sheds. At the beginning of the study, all glands of the 10 cows were free of infection as tested by three consecutive bacteriological tests and had SCC <1 × 105 cell/mL milk.
Bacteria challenge
Three different E. coli strains were used for the intra-mammary challenge: VL2874 (isolated from per-acute mastitis), VL2732 (isolated from persistent mastitis) and P4 (widely used as model strain for mammary pathogenic E coli, isolated from clinical mastitis in the UK). Strains typing, phenotypic and genomic characteristics were extensively studied and described before2,7,8. Bacteria were recovered from frozen stocks (kept at −80 °C in a mixture of brain-heart infusion and 25% glycerol) on blood agar (Tryptose Blood Agar Base; Becton-Dickinson, Sparks, MD, USA, with 5% washed sheep erythrocytes) and incubated aerobically at 37 °C overnight. For inoculum preparation, bacteria were harvested and washed in pyrogenic-free saline (PFS), suspended in PFS and stored at 4 °C for 10 h. Aliquots were taken for colony forming units (CFU) count on blood agar. CFU number was adjusted with PFS right before challenge, aiming to an inoculum of ~50 CFU in 3 mL. Final CFU number in the inoculum ranged from 10–30 CFU/gland, as determined from aliquots separated just prior to challenge. The small CFU counts in the inoculum aimed to better represent a natural infection in which it is expected that only a small number of bacteria enter the mammary gland and initiate the infection.
Study layout
The 10 cows were challenged each in one gland, while all the other three glands served as control. Before challenge, milk yield, milk composition, SCC and leukocytes distribution were determined at −2 days and on day 0, separately for the intendent challenged gland and composite milk of the other three glands. At the day of challenge, after morning milking, a single gland (rear left or right in nine cows and front right in one cow) was infused via the teat canal with one of the E. coli strains (3–4 cows each). Clinical symptoms were assessed for the first 24 h, including every 4 h measurements of rectal temperature and presence of general signs of illness such as changes in respiratory rate, loss of appetite, diarrhea and dehydration. After challenge, milk yield was recorded separately for the challenged gland and the three control glands. Milk was sampled at: 4, 8, 12, and 16 h post-challenge, and then at 1, 2, 4, 7, 10, 14, 17, 21, 29, and 35 DPC. A week after the termination of the experiment (at day 45 PC) the cows were slaughtered in an abattoir. Post slaughter, teats were externally cleaned and disinfected with 70% alcohol, then the nipples and ~10 cm of parenchymal tissue above it were removed as sterile as possible, stored individually in sterile bags and placed on ice. Samples were transferred to the laboratory for histological and PCR analyses for the presence of the bacteria.
Histology
Samples for histological analysis were fixed in neutral buffered 4% formaldehyde and embedded in paraffin. Sections were cut (4 μm) and stained with hematoxylin and eosin (H&E). Slides were viewed using a light microscope (Nikon Eclipse E600, Melville, NY, USA), and images were captured using the MagnaFire Digital Camera (Optronics, Goleta, CA, USA) controlled by NIS-Elements F3.0 software (Nikon, Japan, https://www.microscope.healthcare.nikon.com/products/software).
Milk sample collection and analyses
For bacteriological tests, teats were cleaned, disinfected, foremilk was dispensed, and 3 mL samples of milk were aseptically collected in sterile assay tubes. For the other tests, the infected gland and then composite milk of the other three glands were milked separately into containers, milk volume was recorded and gently mixed and 0.5–1.0 L was taken for analysis as follows: SCC with the Fossomatic 360 (Foss Electric, Hillerød, Denmark) and gross milk composition - fat, protein, casein and lactose contents with the Milkoscan FT6000 (Foss Electric). These analyses were performed at the Israel Cattle Breeders Association Laboratory (Caesarea, Israel). Rennet clotting time (RCT) and curd firmness (CF) were tested using the Optigraph (Ysebaert, Frepillon, France) as previously described9. Fromase 15 TL (Gist-Brocades nv, Delft, The Netherlands) was diluted 1:100 from the stock solution and 0.5 mL/well were added to achieve clotting at about 900 s in non-infected milk. Tests were performed within 24 h after sample collection with milk stored at 4 °C. All the reagents for the below described procedures were purchased from Sigma (Rehovot, Israel). The concentrations of glucose, Glu6P, oxalate, malate, lactate, pyruvate and citrate were determined in skimmed milk samples10–12 by fluorometric methods in which the last stage was linked to formation of fluorochromophore as follow:
These fluorimetric procedures are insensitive to the disturbing effects of fat and casein micelles and the coupling to the formation of fluorochromophore increased their sensitivity. The limit of detection of these procedures was 10–50 µM and their sensitivity, residual standard deviation, was ± 2–5 µM.
The activity of malate dehydrogenase and lactate dehydrogenase in milk samples were performed. The final activity of diaphorase and the concentration of resazurin used for these enzyme-activity determinations were the same as used for the metabolite determinations. The basic reaction conditions for MDH activity in milk were as described by Silanikove et al.6. The differences in NADH concentration (between 10 and 1000 μM) at the linear stage of the reaction were divided by time and were converted to activity where 1 U of MDH will convert 1.0 μmol/L oxaloacetate and NADH to L-malate and NAD+ per minute at pH 7.5 at 25 °C. The reaction conditions for LDH activity were as described by Larsen13. One international unit of LDH activity was defined as the amount of enzyme that catalyzes the conversion of pyruvate into lactate to generate 1.0 μmol/L of NAD+ per minute at 37 °C.
Nitrite concentration was determined by the fluorometric assay with DAN reagent as described by Sonoda et al.14, after precipitating the casein by ultracentrifugation15. Lactic peroxidase (LPO) activity was assayed by the oxidation of 2,2 V-azino-bis (3-ethylbenzothiazoline-6-sulforic acid) diammonium salt, as described by Silanikove et al.15. Catalase activity was determined with the aid of fluorescent substrate as previously described16. Additional sub-sets of milk samples were defatted under cold conditions and analyzed according to previously described procedures for albumin, lactoferrin and immunoglobulin type G (IgG) by ELISA assays17–19.
Statistical analysis
Due to the low number of cows, and the lack of statistical differences among cows challenged with different bacteria strains in a preliminary statistical analysis (ANOVA, repeated measures), all cows were analyzed as a single group. The 10 cows were tested for 38 different variables during the time course of the experiment. Each cow was tested in 21 different time points, from day 0 to 35 days post-challenge during the study. The ProcMixed procedure of SAS (version 9.2, SAS Institute Inc., Cary, NC) for repeated measures in time, with cow controlled as random effect, was used for analysis with the general form: Dependent variable = time during the experiment + error. Where: time = 21 different time points from bacterial inoculation time to 35 days later. The dependent variables were: log SCC, milk (infected), milk (cow), fat, protein, % casein, lactose, RCT, CF, IgG, BSA, bovine lactoferrin, lactate, malate, lactate + malate, nitrite, LDH, glucose, Glu6P, Glu6PD, oxalate, lactoperoxidase, catalase, pyruvate and citrate. To compare levels within a variable, we ran the Bonferroni adjustment for multiple comparisons. Data are presented as means and SEM.
Ethics approval
Animal experimentation was approved by the Committee of Animal Experimentation of the A.R.O., the Volcani Center, Bet Dagan and followed the committee’s ethical guidelines (Permit no. 59315).
Results
Clinical symptoms, bacteriology, SCC and milk yield
Rectal temperatures increased after 8 h, peaked at 12–16 h (39.2–40.1 °C) and decreased to normal after 16–24 h. Local clinical swelling was observed only in the challenged glands from 12–16 h and it persisted for 1 d (1 cow), 2 d (4 cows), 7 d (1 cow) and 35 d (4 cows). Each gland was inoculated with 10–30 CFU. E. coli bacteria were detected in milk as early as 4–8 h post-challenge and peak bacterial counts were observed after 16–24 h, reaching on the average up to ~1 × 107 CFU per mL milk. Bacteria clearance was observed at 7 DPC, but in 50% of the glands (5/10 cows) bacteria were isolated from milk intermittently until the end of the study. Bacterial isolates from all cows at different time points were genotypically identical to the inoculated strains as tested by ERIC-PCR. Bacterial DNA was not detected in samples without bacterial growth in culture.
SCC in the infected glands increased significantly (P < 0.001) within ~12 h to over log 6 (1 × 106 cell/mL). SCC peaked on day 1 to log 7 and remained at that level in all challenged glands up to 7 d, after which it started to decrease to ~log 6 until the end of the study (35 d) with no differences if bacteria were isolated or not (Fig. 1). The increase of SCC consisted mainly of leukocytes (CD18+ marked cells), mostly polymorphonuclear (PMNs)4.
Figure 1.
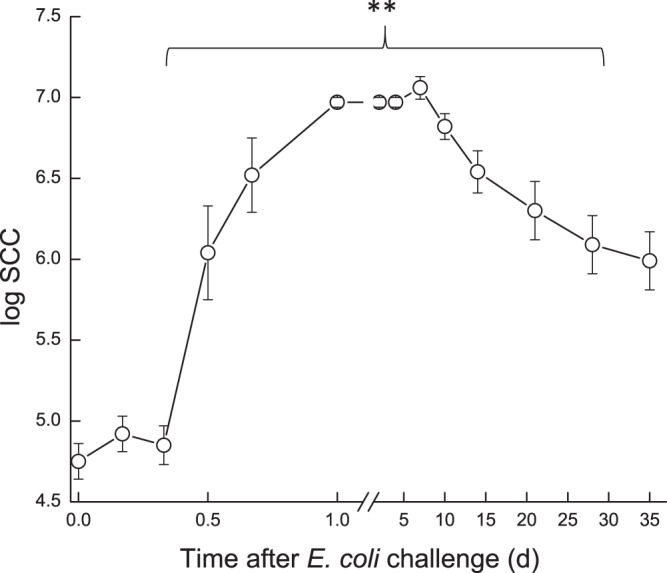
Somatic cell count (SCC) in milk of glands infected with pathogenic Escherichia coli. Mean and SE of somatic cell count in milk of the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. **significantly different from time 0 after challenge (P < 0.001).
Milk yield was calculated as percentage of yield on day 0 (22–38.4 L/d; 100%). Milk yield of challenged glands was compared to composite milk of the control glands of each cow and to the total cow’s milk (Fig. 2). During 1–3 DPC, milk yield dropped to 10–25% in the infected glands and to 30–50% in the control glands. None of the challenged glands fully recovered during the study period, showing approximately 40–60% loss of milk yield compared to ~20% loss in some control glands, while in others a compensation (gain in milk yield) was recorded. Overall, cow’s milk yield decreased by 10–30% at the end of the study (Fig. 2). The loss of milk yield per average cow during the 35 days of the study was 336 L (32%). Milk yield of infected glands significantly correlated with casein, lactose, coagulation properties, citrate, and significantly negatively correlated with SCC on the cow level.
Figure 2.
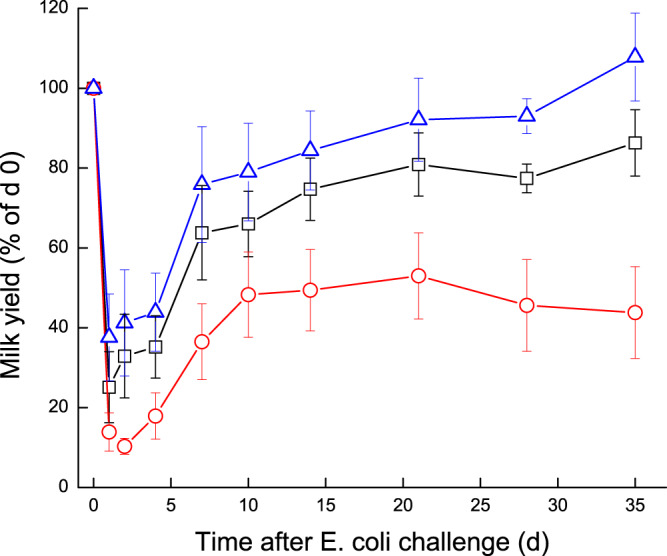
Milk yield of glands infected with pathogenic Escherichia coli. Mean and SE of milk yield measured separately for the infected glands ( ), commingled milk of the 3 uninfected control glands (
), commingled milk of the 3 uninfected control glands ( ) and the total cow’s milk yield (
) and the total cow’s milk yield ( ) of 10 cows challenged with different mammary pathogenic strains of E. coli. Milk yield was calculated as percentage of day 0 (100%).
) of 10 cows challenged with different mammary pathogenic strains of E. coli. Milk yield was calculated as percentage of day 0 (100%).
Biochemical response to E. coli challenge
No significant results were found between the distinct MPEC strains; therefore, the following results are presented and discussed for the three MPEC strains combined.
The statistical significance (P values) of the ANOVA results for the Day effect, R2 and % variance between cows from the overall variance in the trial are summarized in Table 1. Fat in milk decreased during the first 2 h and then significantly increased (P < 0.001) up to 7–10 DPC and gradually returned to pre-challenged level only at 35 DPC (Fig. 3). A significant decrease (P < 0.001) in total protein was already noticed at 6–8 h PC, after which total protein levels in milk increased up to a peak at 7–10 DPC, returning to pre-challenged levels at 35 DPC. In parallel, % casein decreased significantly from ~78% to ~68% between 8–24 h PC, then gradually increased afterwards but remained significantly lower than pre-challenged levels until the end of the study (Fig. 3).
Table 1.
The significance (P values) of the ANOVA results for the day effect and R2 of the variables tested.
| Variable | R-Square (day) | P[F] | Variance between cows |
|---|---|---|---|
| Log SCC | 0.749 | <0.001 | NS |
| Milk (infected gland) L/d | 0.403 | <0.001 | NS |
| Milk (cow) L/d | 0.121 | NS | 15.2% |
| Fat (g/L) | 0.170 | NS | 18.8% |
| Protein (g/L) | 0.509 | <0.001 | 29.3% |
| % Casein | 0.399 | 0.008 | 76.1% |
| Lactose (g/L) | 0.521 | <0.001 | 25.8% |
| RCT (sec) | 0.755 | <0.001 | NS |
| CF (V) | 0.630 | <0.001 | NS |
| IgG (mg/mL) | 0.335 | <0.001 | NS |
| BSA (µM/mL) | 0.499 | <0.001 | NS |
| Lactoferrin (µM/mL) | 0.551 | <0.001 | NS |
| Lactate (µM) | 0.588 | <0.001 | NS |
| Malate (µM) | 0.589 | <0.001 | NS |
| Nitrite (nM) | 0.528 | <0.001 | NS |
| LDH (U/mL) | 0.648 | <0.001 | NS |
| Glucose (µM) | 0.228 | 0.016 | NS |
| Glu6P (µM) | 0.392 | <0.001 | NS |
| Glu6PD (µM/mL) | 0.362 | <0.001 | NS |
| Oxalate (mM) | 0.215 | 0.06 | NS |
| Lactoperoxidase (U/mL) | 0.227 | 0.04 | NS |
| Catalase (U/mL) | 0.267 | 0.007 | NS |
| Pyruvate (µM) | 0.337 | 0.001 | NS |
| Citrate (mM) | 0.318 | 0.004 | NS |
Abbreviations used: SCC – somatic cell count; RCT – rennet clotting time; CF – curd firmness; IgG – immunoglobulin G; BSA – bovine serum albumin; LDH – lactate dehydrogenase; Glu6P – glucose 6 phosphate; Glu6PD - glucose 6 phosphate dehydrogenase;
NS = Variance between cows not significant (P > 0.05).
Figure 3.
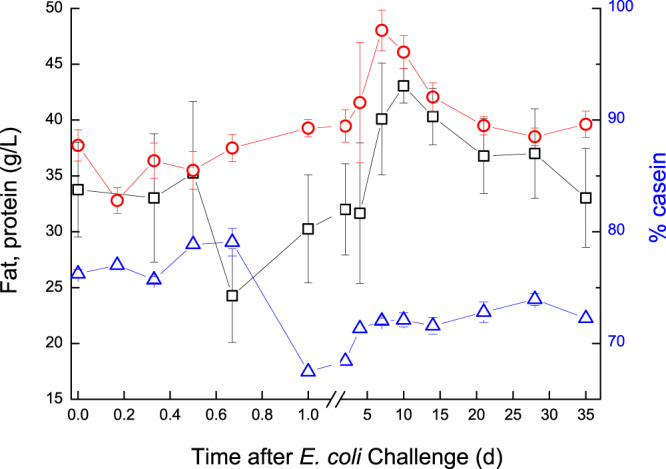
Fat, protein and % casein in milk of glands infected with pathogenic Escherichia coli. Mean and SE of fat ( ; g/L), protein (
; g/L), protein ( ; g/L) and % casein (
; g/L) and % casein ( ), measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. Fat decreased during the first 2 h and then increased (P < 0.001) up to 7–10 DPC and gradually returned to pre-challenged level only at 35 DPC. Total protein decreased (P < 0.001) at 6–8 h PC, after which it increased up to a peak at 7–10 DPC, returning to pre-challenged levels at 35 DPC. % casein decreased from ~78% to ~68% between 8–24 h PC, then gradually increased afterwards but remained lower (P < 0.05) than pre-challenged levels until the end of the study.
), measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. Fat decreased during the first 2 h and then increased (P < 0.001) up to 7–10 DPC and gradually returned to pre-challenged level only at 35 DPC. Total protein decreased (P < 0.001) at 6–8 h PC, after which it increased up to a peak at 7–10 DPC, returning to pre-challenged levels at 35 DPC. % casein decreased from ~78% to ~68% between 8–24 h PC, then gradually increased afterwards but remained lower (P < 0.05) than pre-challenged levels until the end of the study.
Milk coagulation properties assessed before challenge showed a mean RCT ~28 min and CF ~9 V. After challenge, milk coagulations properties were deeply affected and for the first 14 DPC, none of the milk samples coagulated. From day 21, milk from 4–5 challenged glands coagulated but formed a weak coagulum, while the other cows’ infected glands milk did not coagulate until day 35 (Fig. 4).
Figure 4.
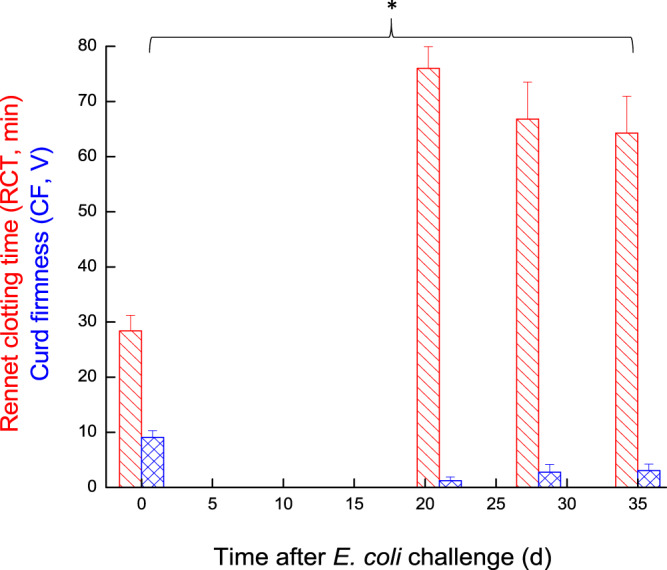
Rennet clotting time and curd firmness in milk of glands infected with pathogenic Escherichia coli. Mean and SE of Rennet clotting time (RCT; red slant lines fill) and Curd firmness (CF; blue mesh fill) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. *significantly different from time 0 for CF and RCT (P < 0.05).
Milk lactose of the infected glands decreased significantly (P < 0.05) at 12–16 h from ~50 g/L before E. coli inoculation to ~31 g/L. Only in 60% of the infected glands milk lactose increased gradually from 7–10 DPC up to pre-challenge level on days 28–35, while remaining low in the other glands (Fig. 5). The kinetics of changes in lactose concentration closely followed the kinetics of milk yield and the inflammatory response, as reflected by significant negative correlations with log SCC and the above-described inflammatory indices and oxidative-stress markers. A significant positive correlation was found between protein level and inflammation parameters, while a significant negative correlation was found between % casein and lactose and inflammation. Regarding clotting parameters, a significant negative correlation was found between CF to inflammation and RCT, and significant positive correlation was found between CF to lactose and % casein, meaning that these parameters were similarly affected by the inflammation process.
Figure 5.
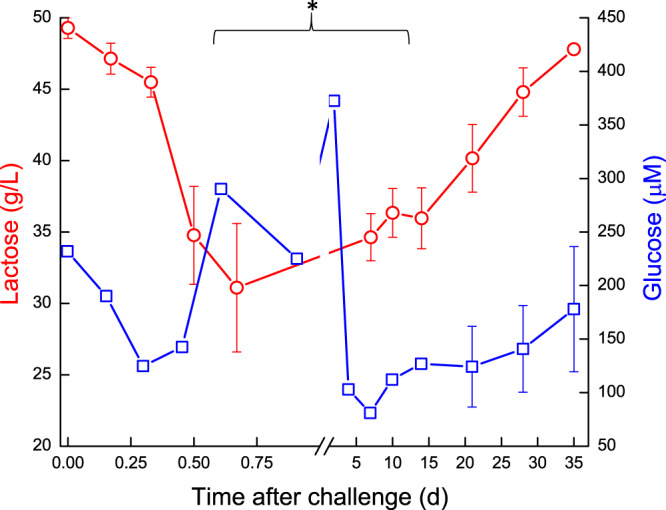
Lactose and glucose levels in milk of glands infected with pathogenic Escherichia coli. Mean and SE of lactose ( ; g/L) and glucose (
; g/L) and glucose ( ; μM) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. Glucose concentration increased significantly (P < 0.05) on day 2 followed by a sharp decrease on day 4 and gradually increase thereafter. *significantly different from time 0 for lactose between 0.5–10 days (P < 0.05).
; μM) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. Glucose concentration increased significantly (P < 0.05) on day 2 followed by a sharp decrease on day 4 and gradually increase thereafter. *significantly different from time 0 for lactose between 0.5–10 days (P < 0.05).
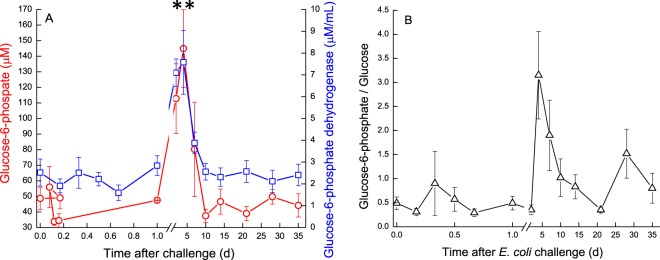
The pre-challenge glucose concentration in milk was ~289 μM. It increased significantly (P < 0.05) on day 2 to ~370 μM followed by a sharp decrease on day 4 and gradual increase thereafter (Fig. 5). Apart from these changes, milk glucose concentration exhibited kinetics that closely followed that of lactose. Glu6P in milk of the infected glands decreased at first on 12–16 h from ~49 μM before E. coli inoculation to ~33 μM, increased up to day 4. A sharp significant increase of Glu6P (P < 0.001) was observed on 4 DPC, similarly to a peak of Glu6PD, decrease of glucose and subsequent peak of Glu6P/Glu ratio at day 4. This peak decreased on 10 DPC and Glu6PD activity gradually returned to pre-challenge levels, as well as Glu6P and the Glu6P/glucose ratio, which then fluctuated until the end of the study (Fig. 6A,B).
Figure 6.
(A,B) Glucose derivatives and enzyme in milk of glands infected with pathogenic Escherichia coli. Mean and SE of glucose-6-phosphate ( ; μM) and glucose-6-phosphatedehydrogenase (
; μM) and glucose-6-phosphatedehydrogenase ( ; μM) (A) and glucose-6-hosphate/glucose (△) (B), measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. **significantly different from time 0 to days 2–4 for Glu6P and Glu6PD (P < 0.001).
; μM) (A) and glucose-6-hosphate/glucose (△) (B), measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. **significantly different from time 0 to days 2–4 for Glu6P and Glu6PD (P < 0.001).
Milk oxaloacetate levels increased in a two-step way. At first, a significant increase (P < 0.05) was observed at ~16 h, from ~0.8 mM to ~1.3 mM, then a second sharp increase (P < 0.001) was measured on 7 DPC to >1.75 mM, after which oxaloacetate levels decreased again to ~1.3 mM and remained higher than pre-challenge levels (Fig. 7). In the infected glands, lactate and malate concentrations displayed similar patterns. Their levels started to elevate at ~12 h, peaked on 2–4 DPC and declined thereafter (Fig. 8). A sharp increase of lactate was measured earlier, at ~12 h, whereas increase of malate was detected at 1 DPC. Lactate and malate levels remained about 6 and 3 folds higher than pre-challenge levels until the end of the experiment, respectively. Pyruvate behaved differently, compared to the previous parameters. Pyruvate levels fluctuated during the first day post-challenge; gradually increasing until a peak >1400 µM at 7 DPC, after which pyruvate levels sharply declined to ~750 µM at 10 DPC. By the end of the study, pyruvate levels were lower than pre-challenge levels (~600 µM and ~900 µM, respectively) (Fig. 9). Citrate concentration dynamics mirrored that of lactate and malate above. Citrate levels sharply decreased at 16 h from 8.9 mM to lowest levels (3.8 mM) at 2 DPC, then gradually increased but remained lower on 35 DPC compared to pre-challenge levels (Fig. 10). Consequently, the ratio citrate/lactate + malate dropped sharply, starting at 12 h from ~17 to <1 at 2 DPC and then started to rise to the pre-challenged level ratio at 28 DPC (Fig. 11).
Figure 7.
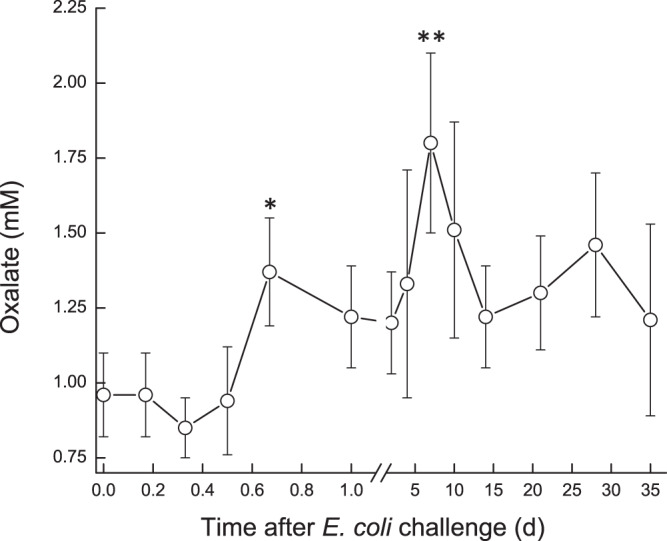
Oxalate in milk of glands infected with pathogenic Escherichia coli. Mean and SE of oxalate (mM) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli.*, **significantly different from time 0 to 0.6 d and on 7 DPC (P < 0.001).
Figure 8.
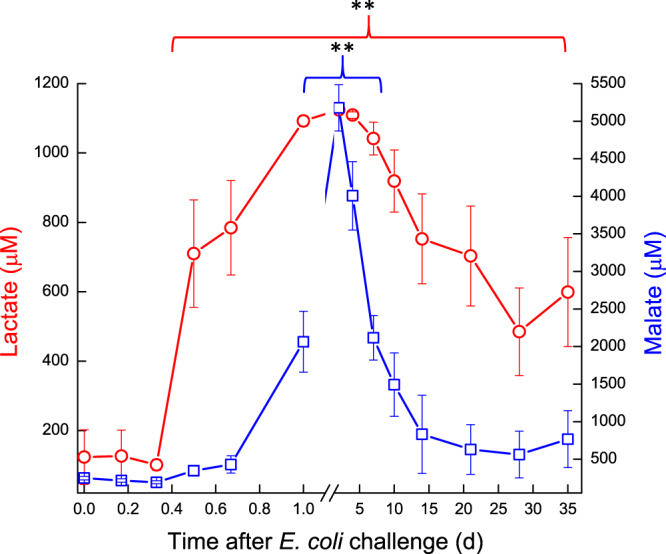
Lactate and malate in milk of glands infected with pathogenic Escherichia coli. Mean and SE of lactate ( ) and malate (
) and malate ( ) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli.
) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli.
Figure 9.
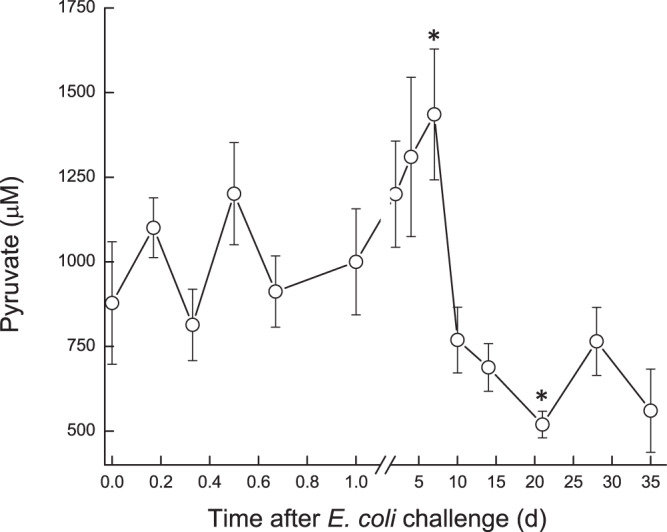
Pyruvate in milk of glands infected with pathogenic Escherichia coli. Mean and SE of pyruvate (μM) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. *significantly different from time 0 to 5 d and on 20 DPC (P < 0.05).
Figure 10.
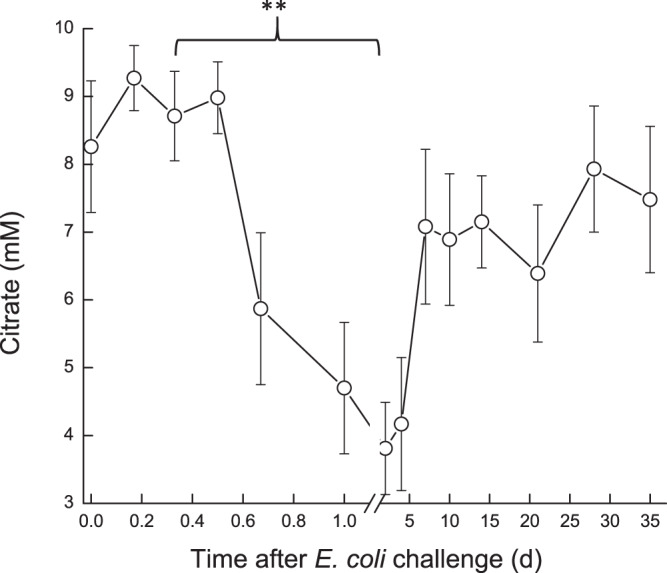
Citrate in milk of glands infected with pathogenic Escherichia coli. Mean and SE of citrate (mM) measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. **significantly different from time 0 to 0.8–5.0 DPC (P < 0.001).
Figure 11.
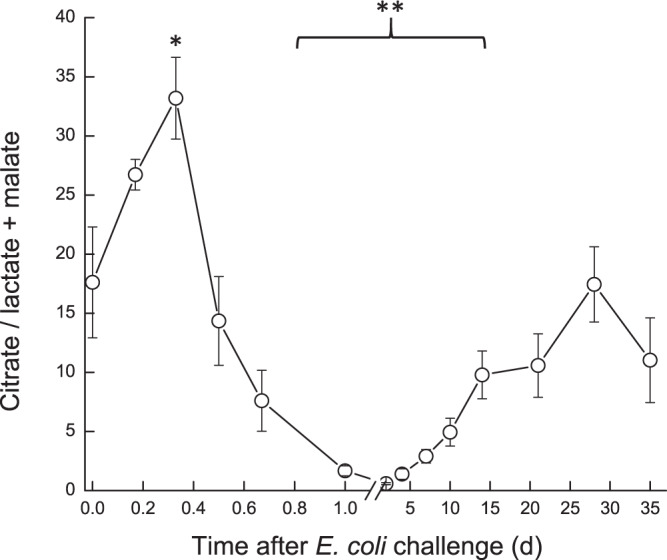
Citrate/lactate + malate in milk of glands infected with pathogenic Escherichia coli. Mean and SE of the ratio citrate/lactate + malate measured in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. *,**significantly different from time 0 to 0.4 d (P < 0.05) and from 0.8–15 DPC (P < 0.001).
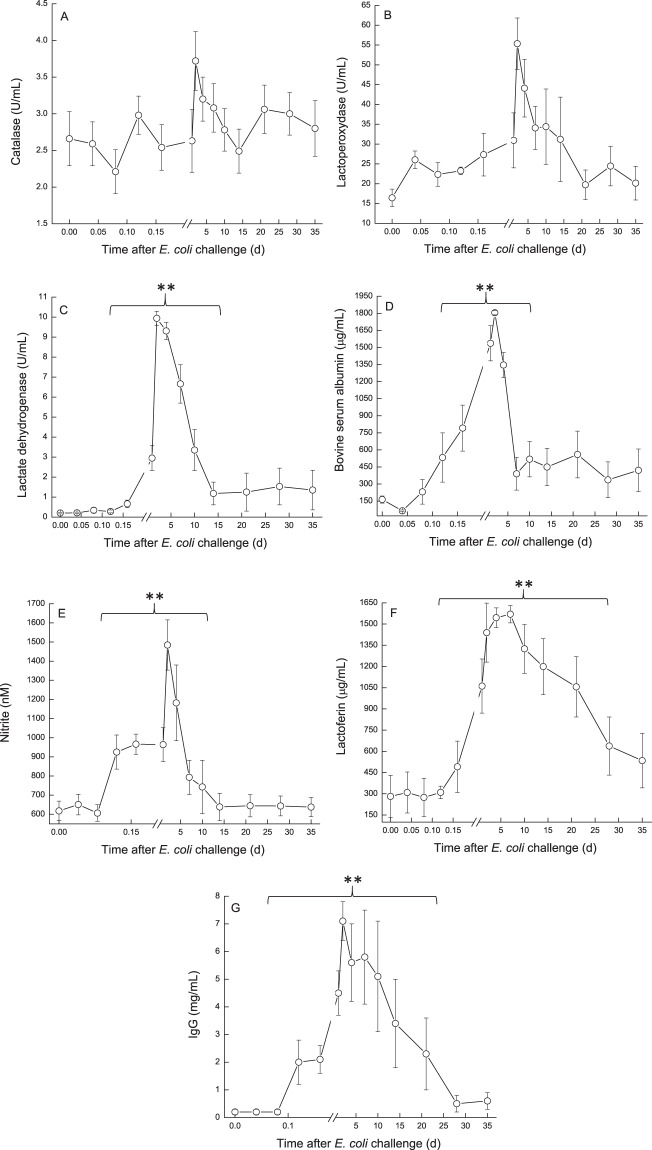
Catalase and lactoperoxidase activity, LDH, BSA and nitrite concentrations displayed similar patterns. Their levels started to elevate at ~12 h PC, peaked at 2–4 DPC and declined to about pre-challenged levels at the end of the study (Fig. 12A–E). These proteins, enzymes and ion which originate from blood, significantly positively correlated with SCC and milk protein, and significantly negatively correlated with infected glands and cow milk yield, lactose, % casein and CF. Lactoferrin concentration increased at 16–24 h from ~300 to 1,600 µg/mL, peaked at 7 DPC and declined to the end of the study, but remained ~2 fold higher from the pre-challenge level (Fig. 12F). IgG concentration increased within 12 h from 0.2 to >2 mg/mL, peaked at 3 DPC >7, remained in this level up to 21 DPC and only then declined, but also remained ~2 fold higher from the pre-challenge level at 35 DPC (Fig. 12G).
Figure 12.
(A–G) Physiological components in milk of glands infected with pathogenic Escherichia coli. Mean and SE of catalase (A), lactoperoxidase (B), lactate dehydrogenase (C), bovine serum albumin (D), nitrite (E), lactoferrin (F) and IgG (G) in the infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. Catalase and lactoperoxidase activity, LDH, BSA and nitrite concentrations displayed similar patterns. Their levels started to elevate at ~12 h PC, peaked at 2–4 DPC and declined to about pre-challenged levels at the end of the study (A–E). Lactoferrin concentration increased at 16–24 h, peaked at 7 DPC and declined to the end of the study, but remained ~2 fold higher from the pre-challenge level (F). IgG G concentration increased within 12 h, peaked at 3 DPC >7, remained in this level up to 21 DPC and only then declined, but also remained ~2 fold higher from the pre-challenged level at 35 DPC (G). **significantly different (P < 0.001).
Histology at 42 days post-challenge
Histological changes in infected and control glands were compared within each and among cows. Overall, the major differences in the control glands was the level of alveolar cuboidal epithelial structure in full lactating glands in the high yielding cows (Fig. 13A), and increased interlobular collagen rich areas with fibrous stroma and fat in low yielding cows (Fig. 13B). In challenged glands, regardless if bacteria were isolated on 35 DPC or not, increased interlobular collagen rich areas with fibrous stroma and fat were observed, and in the high yielding cows, normal alveolar structure. Moreover, in most challenged glands, leucocytes infiltration was observed, mainly inter-lobular mononuclear infiltration, and in two of the 10 glands PMN were observed within normal alveoli (Fig. 13C).
Figure 13.
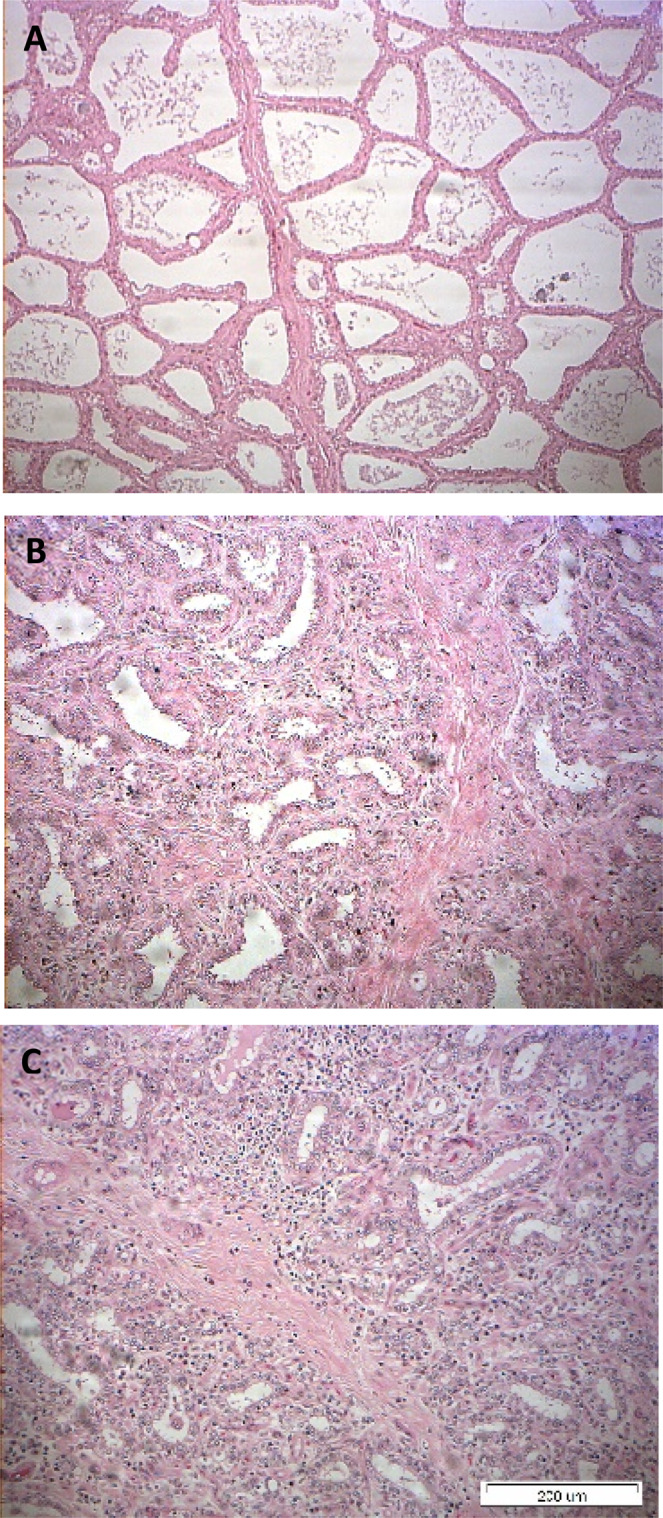
(A–C) Histological sections of udder tissues of glands infected with pathogenic Escherichia coli. Representative hematoxylin-eosin histological sections of control and infected glands of 10 cows challenged with different mammary pathogenic strains of E. coli. Note the level of alveolar cuboidal epithelial structure in full lactating glands in high yielding cows (A), and increased interlobular collagen rich fibrous stroma and fat in low yielding cows (B). In challenged glands, increased interlobular collagen rich areas with fibrous stroma and fat was observed, with mononuclear leucocytes among lobules and PMN within normal alveoli in two out of 10 glands (C). Images were captured using the MagnaFire Digital Camera controlled by NIS-Elements F3.0 software (https://www.microscope.healthcare.nikon.com/products/software).
Discussion
E. coli mastitis is characterized by acute inflammation, including local clinical signs of inflammation, such as udder swelling and pain, and sharp decrease in milk production along with significant changes in its composition, such as increased SCC and passage of blood elements into milk. Systemic signs, including increased body temperature, diarrhea and dehydration are sometimes also observed. E. coli growth in milk in the mammary gland is fast, and increased bacterial counts are detected as early as within 4 h from challenge4 even when a minimal inoculum is used to best represent natural infections (10–30 CFU in the present study). The present study focused on the physiological and biochemical changes in milk following challenge with three different MPEC strains isolated from distinct mastitis presentations (VL2874, VL2732 and P4)4. Although some significant differences were found in the inflammatory and immune responses in the mammary gland between these strains, the physiological and biochemical markers revealed only minor differences without statistical significance. Therefore, cows challenged with any of the three strains were combined in a single group for analysis.
Cows did not receive medication, thus bacteria clearing in milk was natural, starting on 7 DPC. In 50% of the glands, bacteria shedding was intermittent until the end of the study. Regardless of the clearing rate and bacteria numbers in milk, no significant correlation was found with all the parameters tested, suggesting that the recovery process depends mainly on the severity of the infection and the inflammatory response elicited, and possibly tissue damage, during the first hours or days after E. coli entry to the mammary gland.
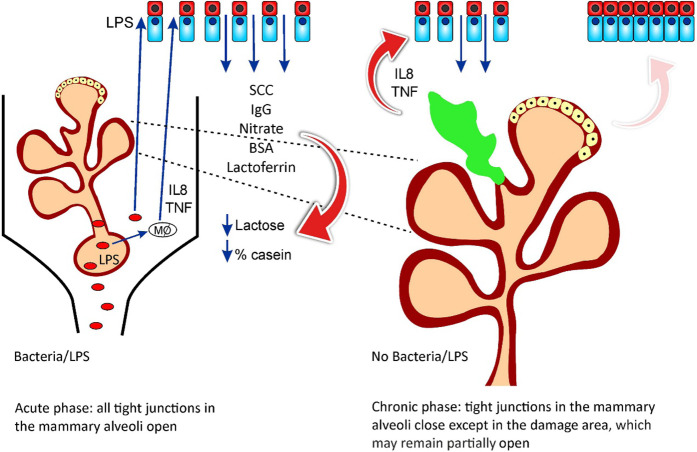
Two clear infection stages were observed: 1: Initiation and establishment of the infection and consecutive inflammation, which started at 4–10 h PC and ended by 7–15 DPC; 2: Resolution of infection, as expressed by most of the parameters tested recovering to pre-challenge levels, although with different dynamics. A schematic presentation of the two-phase proposal for the development of E. coli mastitis is presented in Fig. 14. However, of note, SCC and milk yield at the infected glands and at the cow level was not restored until the end of the study at 35 DPC, similar to our observations in natural E. coli mastitis5.
Figure 14.
A schematic presentation of a two-phase proposal for the development of Escherichia coli mastitis. Phase 1 - The acute phase: Bacterial infection in the mammary gland or lipopolysaccharide (LPS) induced mastitis, trigger an immediate immune and inflammatory responses in which the endothelial-mammary barriers are open, allowing for cellular and humoral infiltration from blood to milk. Phase 2 - The chronic phase: The tight junctions close and infiltration is limited to areas of deep damage in the gland, which may perpetuate alterations that are measured in milk regardless of bacterial clearance off the mammary gland.
Milk production in the mammary epithelial cells exists under osmotic balance between blood and the epithelial cells20. Acute signaling of E. coli LPS and immune cytokines like IL8, TNF and others occur within hours, starting a cascade of events, including infiltration of leucocytes, mainly PMN, and selectively opening of the tight junctions21 as has been showed in our earlier study4.
The shift of mammary epithelial cells metabolism to anaerobic glycolysis as a tradeoff between use of glucose to support lactose synthesis and liberation of glucose to support the immune system was thoroughly discussed by Silanikove et al.6, using E. coli LPS as a model for mammary gland infection. LPS is a major immunogenic determinant of E. coli and Gram-negative bacteria in general, but there are important differences between LPS inoculation vs. live E. coli bacteria challenge. First, a single inoculation of LPS leads to a single triggering event of the inflammatory response, whereas live bacteria replicate in the mammary gland and therefore represent a longer trigger for inflammation. Second, LPS by itself does not cause direct damage to the gland tissues whereas live bacteria have different virulence factors that can interact with and damage the mammary epithelial cells, leading to a longer recovery process. Third, the amount of LPS often used to induce mastitis is often higher than the amount of LPS found in a small bacterial inoculum, such as the one used here. Here we observed that the inflammation caused by E. coli could be divided into two stages: an acute phase, with clinical symptoms and a chronic phase, independent of bacteria clearance and in response to the tissue damage caused in the first phase (Fig. 14). The present results suggest that the tight junctions in the mammary alveoli were “open” at 12–24 h PC. This is expressed by infiltration of glucose, IgG, BSA, nitrite and LDH from blood into the milk for 2–3 d after LPS inoculation22 and 6–10 d after live bacteria inoculation. However, the free transfer of the immune cells (SCC) remained for weeks, suggesting active cell infiltration.
Overall, the dynamics of biochemical parameters evaluated was similar to that of LPS induced mastitis,6 but levels achieved were either higher (e.g. glucose-6-phosphate and lactate dehydrogenase activity) or changes remained longer (e.g. lactate dehydrogenase, Glu6P/glucose). The present results are in agreement with those published by Moyes et al.23 although most of the parameters tested in that study returned to the pre-challenge levels (~20–40 CFU of live E. coli - Danish field isolate k2bh2) within 2–4 d. For instance, peak glucose and GLU6P, drop in lactose and increase in fat measured following intra-mammary infusion of E. coli were quite similar to other findings23, but in the present study, fat increased after about 5–10 DPC, compared to 1–2 DPC23. The ratio citrate/lactate + malate, which represents the milk-reflected mitochondrial/cytosol metabolic index12,24, significantly decreased during the acute phase of inflammation as described in LPS induced mastitis. Here, however, this change remained for a longer period, and the ratio did not return to pre-challenge levels by the end of the study at 35 DPC. Cows tested 30 days after an episode of natural E. coli mastitis (“post E. coli”) showed similar results12.
Peak bacterial counts in milk were observed at 24 h4, following a steep decrease in citrate between 16 to 24 h after challenge and concomitant to a significant increase in lactoferrin in milk. The ability to assimilate iron from citrate is a key feature of MPEC3 and therefore it is possible that the exchange of citrate to lactoferrin as main iron chelator in milk contributes to reduce bacterial load in the mammary gland25.
Interestingly, regardless of bacteria presence in the challenged mammary glands, milk yield was lower than pre-challenge, the number of the immune cells remained high (>1 × 106 cell/mL) and the quality of the milk was low as expressed by impaired clotting parameters, even though fat and protein levels were actually higher than pre-challenge ones. This could be related to other milk constituents, but also to the quality of fat and protein fractions present in milk following inflammation, which deserve further investigation. Histology at 42 DPC allowed the observation of disrupted mammary gland tissues, affected alveolar cuboidal epithelial structure and increased interlobular collagen rich areas with fibrous stroma and fat. In most gland, leukocytes infiltration, mainly mononuclears among lobules and PMN within the normal alveoli was present. This correlates with the chronic, long-term effects reminiscent of mastitis caused by E. coli, indicating tissue damage and the process of cleaning of damaged tissue, and which result in affected milk quality and yield5. Overall, the present study adds information about the physiological and biochemical changes observed in milk during and following acute intra-mammary inflammation induced by infection by E. coli bacteria. Moreover, the present results highlight the shift between the acute phase and a chronic phase of E. coli mastitis, the latter being independent of bacterial shedding in milk; and is therefore independent from an ongoing infection. This chronic phase of inflammation is characterized by continuous changes in milk properties for a long period after clinical recovery, and the present results may explain the long-term effects on milk quality that have been previously described5. This study also emphasizes some differences between LPS and live bacteria-induced mastitis, which are important to be accounted when choosing the right model for mastitis research.
Conclusions
Escherichia coli challenge induces acute conversion of epithelial cells metabolism from principally mitochondrial-oxidative to principally cytosolic (glycolytic), which allows diversion of metabolic resources normally used to synthesize milk and to support the immune system. In turn, reduction in lactose concentration is consistent with the concept that it is part of the defense mechanism of the mammary gland. We suggest a two-phase mechanism for the development of E. coli mastitis. In phase 1, the acute phase, bacterial infection in the mammary gland or in LPS induced mastitis, triggers an immediate immune and inflammatory responses in which the endothelial-mammary barriers are open, allowing for cellular and humoral infiltration from the blood to the milk. In phase 2, the chronic phase, tight junctions close and infiltration is limited to areas of deep damage in the gland, which may perpetuate alterations which can be measured in the milk, regardless of bacterial clearance from the mammary gland.
Acknowledgements
This work was partly financially supported by the Israel Dairy Board.
Author contributions
S.B., D.H. and G.L. were responsible for performing the experiments, collecting data, experimental design, data analysis and preparing the manuscript draft; S.J. was responsible for animal husbandry and care; O.K. performed the laboratory work; Y.L. performed the statistical analyses; N.E. performed histological exams, provided histological pictures and descriptions; U.M., N.S. S.B. and G.L. analyzed the data and helped in writing and editing the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/2/2021
A Correction to this paper has been published: 10.1038/s41598-021-84933-z
References
- 1.Blum SE, et al. Identification of a bovine mastitis Escherichia coli subset. Vet Microbiol. 2008;132:135–148. doi: 10.1016/j.vetmic.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Blum SE, et al. Genomic and phenomic study of mammary pathogenic Escherichia coli. PLoS One. 2015;10:e0136387. doi: 10.1371/journal.pone.0136387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum SE, et al. Postgenomics characterization of an essential genetic determinant of mammary pathogenic Escherichia coli. MBio. 2018;9.2:e00423–18. doi: 10.1128/mBio.00423-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum SE, Heller ED, Jacoby S, Krifucks O, Leitner G. Comparison of the immune responses associated with experimental bovine mastitis caused by different strains of Escherichia coli. J. Dairy Res. 2017;84:190–197. doi: 10.1017/S0022029917000206. [DOI] [PubMed] [Google Scholar]
- 5.Blum SE, Heller ED, Leitner G. Long term effects of Escherichia coli mastitis. Vet. J. 2014;201:72–77. doi: 10.1016/j.tvjl.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Silanikove N, et al. Reduced use of glucose by normoxic cow’s mammary gland under acute inflammation: an example of homeostatic aerobic glycolysis. RSC Advances. 2016;6:114644–114657. doi: 10.1039/C6RA22934D. [DOI] [Google Scholar]
- 7.Blum SE, Leitner G. Genotyping and virulence factors assessment of bovine mastitis Escherichia coli. Vet. Microbiol. 2013;163:305–312. doi: 10.1016/j.vetmic.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Kempf F, Slugocki C, Blum SE, Leitner G, Germon P. Genomic comparative study of bovine mastitis Escherichia coli. PLoS One. 2016;11:e0147954. doi: 10.1371/journal.pone.0147954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merin U, et al. Subclinical udder infection with Streptococcus dysgalactiae impairs milk coagulation properties: the emerging role of proteose peptones. Dairy Sci. Technol. 2008;88:407–419. doi: 10.1051/dst:2008022. [DOI] [Google Scholar]
- 10.Shapiro F, Silanikove N. Rapid and accurate determination of D-and L-lactate, lactose and galactose by enzymatic reactions coupled to formation of a fluorochromophore: Applications in food quality control. Food Chem. 2010;119:829–833. doi: 10.1016/j.foodchem.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro F, Silanikove N. Rapid and accurate determination of malate, citrate, pyruvate and oxaloacetate by enzymatic reactions coupled to formation of a fluorochromophore: Application in colorful juices and fermentable food (yogurt, wine) analysis. Food Chem. 2011;129:608–613. doi: 10.1016/j.foodchem.2011.04.074. [DOI] [PubMed] [Google Scholar]
- 12.Silanikove N, Merin U, Shapiro F, Leitner G. Milk metabolites as indicators of mammary gland functions and milk quality. J. Dairy Res. 2014;81:358–363. doi: 10.1017/S0022029914000260. [DOI] [PubMed] [Google Scholar]
- 13.Larsen. T. Determination of lactate dehydrogenase (LDH) activity in milk by a fluorometric assay. J. Dairy Res. 2005;72:209–216,. doi: 10.1017/S0022029905000865. [DOI] [PubMed] [Google Scholar]
- 14.Sonoda M, et al. An assay method for nitric oxide-related compounds in whole blood. Anal. Biochem. 1997;247:417–427. doi: 10.1006/abio.1997.2056. [DOI] [PubMed] [Google Scholar]
- 15.Silanikove N, Shapiro F, Shamay A, Leitner G. Role of xanthine oxidase, lactoperoxidase, and NO in the innate immune system of mammary secretion during active involution in dairy cows: manipulation with casein hydrolyzates. Free Radic. Biol. Med. 2005;38:1139–1151. doi: 10.1016/j.freeradbiomed.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Silanikove N, Shapiro F, Silanikove M, Merin U, Leitner G. Hydrogen peroxide-dependent conversion of nitrite to nitrate as a crucial feature of bovine milk catalase. J. Agric. Food Chem. 2009;57:8018–8025. doi: 10.1021/jf900618w. [DOI] [PubMed] [Google Scholar]
- 17.Leitner G, et al. Changes in milk composition as affected by subclinical mastitis in sheep. J. Dairy Sci. 2004;87:46–52. doi: 10.3168/jds.S0022-0302(04)73140-9. [DOI] [PubMed] [Google Scholar]
- 18.Leitner G, Krifucks O, Jacoby S, Lavi Y, Silanikove N. Concentrations of ganglioside type M1 and immunoglobulin G in colostrum are inversely related to bacterial infection at early lactation in cows. J. Dairy Sci. 2008;91:3337–3342. doi: 10.3168/jds.2008-1010. [DOI] [PubMed] [Google Scholar]
- 19.Silanikove N, et al. Lipopolysaccharide challenge of the mammary gland in cows induces nitrosative stress that impairs milk oxidative stability. Animal. 2012;6:1451–1459. doi: 10.1017/S1751731112000201. [DOI] [PubMed] [Google Scholar]
- 20.Reece, W. O., Erickson, H. H., Goff, J. P. & Uemura, E. E. (Eds.). Dukes’ Physiology of Domestic Animal. 13th Edn. (Cornell: Wiley-Blackwell, 2015).
- 21.Ma JL, et al. Serum concentration and mRNA expression in milk somatic cells of toll-like receptor 2, toll-like receptor 4, and cytokines in dairy cows following intramammary inoculation with Escherichia coli. J. Dairy Sci. 2011;94:5903–5912. doi: 10.3168/jds.2011-4167. [DOI] [PubMed] [Google Scholar]
- 22.Silanikove N, et al. Lipopolysaccharide challenge of the mammary gland in bovine induced a transient glandular shift to anaerobic metabolism. J. Dairy Sci. 2011;94:4468–4475. doi: 10.3168/jds.2010-4092. [DOI] [PubMed] [Google Scholar]
- 23.Moyes KM, Larsen T, Sørensen P, Ingvartsen KL. Changes in various metabolic parameters in blood and milk during experimental Escherichia coli mastitis for primiparous Holstein dairy cows during early lactation. J. Anim. Sci. Biotechnol. 2014;5:47. doi: 10.1186/2049-1891-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyvönen P, et al. Concentrations of bovine lactoferrin and citrate in milk during experimental endotoxin mastitis in early-versus late-lactating dairy cows. J. Dairy Res. 2010;77:474–480. doi: 10.1017/S0022029910000579. [DOI] [PubMed] [Google Scholar]
- 25.Brock JH. The physiology of lactoferrin. Biochem. Cell Biol. 2002;80:1–6. doi: 10.1139/o01-212. [DOI] [PubMed] [Google Scholar]