Abstract
Context
Use of continuous glucose monitoring (CGM) is increasing for insulin-requiring patients with diabetes. Although data on glycemic profiles of healthy, nondiabetic individuals exist for older sensors, assessment of glycemic metrics with new-generation CGM devices is lacking.
Objective
To establish reference sensor glucose ranges in healthy, nondiabetic individuals across different age groups using a current generation CGM sensor.
Design
Multicenter, prospective study.
Setting
Twelve centers within the T1D Exchange Clinic Network.
Patients or Participants
Nonpregnant, healthy, nondiabetic children and adults (age ≥6 years) with nonobese body mass index.
Intervention
Each participant wore a blinded Dexcom G6 CGM, with once-daily calibration, for up to 10 days.
Main Outcome Measures
CGM metrics of mean glucose, hyperglycemia, hypoglycemia, and glycemic variability.
Results
A total of 153 participants (age 7 to 80 years) were included in the analyses. Mean average glucose was 98 to 99 mg/dL (5.4 to 5.5 mmol/L) for all age groups except those over 60 years, in whom mean average glucose was 104 mg/dL (5.8 mmol/L). The median time between 70 to 140 mg/dL (3.9 to 7.8 mmol/L) was 96% (interquartile range, 93 to 98). Mean within-individual coefficient of variation was 17 ± 3%. Median time spent with glucose levels >140 mg/dL was 2.1% (30 min/d), and median time spent with glucose levels <70 mg/dL (3.9 mmol/L) was 1.1% (15 min/d).
Conclusion
By assessing across age groups in a healthy, nondiabetic population, normative sensor glucose data have been derived and will be useful as a benchmark for future research studies.
This study provides normative sensor glucose data in a healthy, nondiabetic population of children and adults.
Since the publication of the Diabetes Control and Complication Trial results in 1993, glycated Hb (HbA1c) has been the gold standard to assess outcomes of diabetes management. This is due to the fact that HbA1c is a reflection of overall glycemic control from the prior 60-90 days, as well as clear evidence from the DCCT establishing it as a surrogate marker for diabetes microvascular complications risk (1). However, HbA1c does not capture clinically relevant hyperglycemic and hypoglycemic patterns, which limits its utility in personalizing insulin-dosing decisions.
Continuous glucose monitoring (CGM) provides information on trends for hypoglycemia, hyperglycemia, and glycemic variability that helps patients and providers optimize glycemic control without increasing the risk for hypoglycemia. With currently available accurate CGM devices, management of patients with type 1 diabetes (T1D) has increasingly depended on CGM for day-to-day adjustments of diabetes treatment (2). Moreover, CGM metrics are being used to examine and compare the relative effectiveness and safety of new therapeutic agents and modern technologies in clinical diabetes research.
Understanding CGM metrics in healthy participants without diabetes is needed to serve as a benchmark for research studies to define impaired glycemic status and for future technologies aiming to help individuals achieve near-normal glycemic profiles. However, there are limited data on CGM-measured glucose concentrations in individuals without diabetes. Most studies of CGM profiles in healthy, nondiabetic individuals have had small sample sizes or used early-generation CGM systems, which were less accurate than current devices (3–7). This study was undertaken to provide an updated set of normative CGM sensor glucose data in a large group of healthy, nondiabetic children, adolescents, and adults.
Methods
Participants
The study was conducted at 12 diabetes centers within the T1D Exchange Clinic Network after approval by institutional review boards (8). Study participants provided written informed consent prior to study participation. Participants were healthy, nondiabetic children and adults, primarily recruited from family, friends, and neighbors of patients seen in the diabetes clinics. Major eligibility criteria were: age ≥6 years; body mass index <30.0 kg/m2 for participants ≥18 years old and between the 5th and 85th percentile, inclusive for age and sex, for participants <18 years old; no chronic illness or medications that might affect glucose metabolism; and point-of-care HbA1c <5.7% (39 mmol/mol). Female participants pregnant at the time of study enrollment were not eligible.
At the initial visit, blood samples were obtained and sent to a central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA) to measure HbA1c and pancreatic autoantibodies (antiglutamic acid decarboxylase, anti-islet antigen 2, anti-insulin antibodies, and zinc transporter-8 autoantibodies). Only participants with no islet autoantibodies and a central laboratory HbA1c level <5.7% were included in the analysis.
An early-generation Dexcom G6 CGM (Dexcom, Inc., San Diego, CA) in blinded mode (i.e., the participant was unable to see the glucose values) was worn for up to 10 days with one daily calibration using a study-provided blood glucose meter (Contour Next EZ; Ascensia Diabetes Care, Parsippany, NJ). The Dexcom G6 CGM sensor measures interstitial glucose concentrations every 5 minutes. The reported mean absolute relative difference (MARD) for the Dexcom G6 in accuracy studies comparing sensor glucose values with reference blood glucose values is 9.0% (9). Participants completed a daily log indicating times and intensity of any exercise, start times of meals and snacks, and sleep and wake times. Approximately 10 days after the blinded CGM sensor was placed, the participant returned to have the sensor removed, and the CGM and blood glucose meter data were downloaded. Participants who had <72 hours of CGM data were given an opportunity to wear another sensor for up to 10 additional days. Only participants with ≥72 total hours of CGM data (with at least 24 hours overnight) were included in the analysis.
Statistical methods
All glucose outcomes are reported as means with SD or as medians with interquartile ranges (IQR), depending on variable distribution. Each CGM glucose reading is counted as a data point and is summarized in the glucose outcomes on either a participant or event level. Percent time spent within a threshold (e.g., % time below 70 mg/dL, or 3.9 mmol/L) was calculated as the number of CGM glucose readings that fell within the threshold divided by the total number of CGM glucose readings from the participant, represented as a percentage. A hypoglycemic event was defined as at least two sensor values <54 mg/dL (3.0 mmol/L) that were ≥15 minutes apart with no intervening values >54 mg/dL. At least two sensor values >70 mg/dL that were ≥15 minutes apart with no intervening values <70 mg/dL were required to end a hypoglycemic event. Glucose outcomes were assessed overall and by age group (6 to <12, 12 to <18, 18 to <25, 25 to <60, and ≥60 years of age). For diurnal variations of glucose metrics, daytime was defined as 6:00 am to 11:59 pm and nighttime as 12:00 am to 5:59 am.
In viewing the data, the ≥60 years age group appeared to be an outlier compared with the other age groups, so a post hoc analysis was performed. Differences in mean glucose and percentage of time in range of 70 to 140 mg/dL between participants aged ≥60 years vs participants aged <60 years were assessed though two-sample Student t tests. The association between mean glucose and HbA1c was assessed using a Pearson correlation coefficient, and 95% confidence intervals were computed. To calculate MARD, each calibration reading was paired with the closest CGM reading within 10 minutes after the calibration reading.
A sensitivity analysis was performed to assess glucose outcomes after removing implausible values. In this analysis, implausible data were defined as the following: any low CGM readings <50 mg/dL (2.8 mmol/L) during sleep time as defined by logs (in which case the participant was likely to be lying on the CGM sensor), any low CGM readings <50 mg/dL on the first day of sensor wear for each participant, any strings (three or more consecutive readings) of CGM-measured lows <50 mg/dL flanked by CGM readings ≥80 mg/dL (4.4 mmol/L) within 10 minutes before and after the string, any strings (three or more consecutive readings) of CGM-measured lows <50 mg/dL immediately followed by calibration readings of ≥80 mg/dL, any strings in which readings of >200 mg/dL (11.1 mmol/L) were interspersed with readings of <70 mg/dL, and any CGM data between an incorrect calibration (wrong entry of meter blood glucose) and the next correct calibration. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
A total of 201 participants were screened for the study. Thirty participants failed screening, two participants withdrew from the study before sensor data were collected, and 16 subjects were excluded from the analysis due to various reasons as shown in the online repository (10). Therefore, 153 participants were included in the analyses, ranging from 7 to 80 years (mean, 31 years). The baseline characteristics of the study participants are shown in Table 1. Mean CGM use by the participants was 192 hours (8 days), and MARD comparing the CGM and blood glucose meter glucose measurements was 6.0% (n = 1555 paired measurements).
Table 1.
Participant Characteristics (n = 153)
| Overall (n = 153) | Age <18 y (n = 57) | Age ≥18 y (n = 96) | |
|---|---|---|---|
| Age, y (mean ± SD) | 31 ± 21 | 12 ± 3 | 42 ± 19 |
| 6 to <12, n (%) | 27 (18) | 27 (47) | NA |
| 12 to <18, n (%) | 30 (20) | 30 (53) | NA |
| 18 to <25, n (%) | 29 (19) | NA | 29 (30) |
| 25 to <60, n (%) | 41 (27) | NA | 41 (43) |
| ≥60, n (%) | 26 (17) | NA | 26 (27) |
| Range | 7–80 | 7–17 | 18–80 |
| Male, n (%) | 52 (34) | 27 (47) | 25 (26) |
| Ethnicity, n (%) | |||
| Hispanic | 15 (10) | 3 (5) | 12 (13) |
| Not Hispanic or Latino | 137 (90) | 53 (93) | 84 (88) |
| Unknown | 1 (<1) | 1 (2) | 0 (0) |
| Race, n (%) | |||
| White | 142 (93) | 56 (98) | 86 (90) |
| Black/African American | 3 (2) | 0 (0) | 3 (3) |
| Asian | 3 (2) | 1 (2) | 2 (2) |
| More than one race | 5 (3) | 0 (0) | 5 (5) |
| BMI, kg/m2 (mean ± SD) | NA | NA | 24.4 ± 3.2 |
| BMI percentile | NA | 52 ± 2 | NA |
| BMI category,a n (%) | |||
| Underweight | 3 (2) | 0 (0) | 3 (3) |
| Normal weight | 106 (69) | 55 (96) | 51 (53) |
| Overweight | 44 (29) | 2 (4)a | 42 (44) |
| HbA1c, % (mean ± SD) | 5.1 ± 0.3 | 5.1 ± 0.2 | 5.1 ± 0.3 |
| Range | 4.2–5.6 | 4.5–5.5 | 4.2–5.6 |
| Has first-degree biological family member with T1Db | |||
| n/total (%) | 49/150 (33) | 29/57 (51) | 20/93 (22) |
| Parent with T1D | 10 (7) | 5 (9) | 5 (5) |
| Sibling with T1D | 26 (17) | 25 (44) | 1 (1) |
| Child with T1D | 15 (10) | 0 (0) | 15 (16) |
Abbreviations: BMI, body mass index.
The underweight, normal weight, and overweight BMI categories for participants aged ≥18 years are <18.5, 18.5 to <24.9, and ≥24.9, respectively. The underweight, normal weight, and overweight BMI percentile categories for participants aged <18 years are <5th percentile, 5th to <85th percentile, and ≥85th percentile, respectively (calculated using the 2000 CDC growth charts). The two pediatric participants in the overweight category had BMI percentile of <86%.
Three participants indicated unknown for having a first-degree family member with T1D and were excluded from the denominator.
Overall, the mean of the individual average 24-hour glucose was 99 ± 7 mg/dL (5.5 ± 0.4 mmol/L), and the mean of the within-individual coefficient of variation (CV), a metric of glucose variability, was 17 ± 3% (Table 2). Overall, median percentage of time spent between 70 and 140 mg/dL (3.9 to 7.8 mmol/L) was 96% (IQR, 93 to 98). The mean average glucose level was 98 to 99 mg/dL (5.4 to 5.5 mmol/L) for all age groups except those ≥60 years old in whom mean average glucose was 104 mg/dL (5.8 mmol/L; P < 0.001 comparing those ≥60 years old with those younger). Similarly, median time spent in the glucose range of 70 to 140 mg/dL was lowest at 93% in adults ≥60 years of age (P < 0.001). Overall, median time spent with glucose levels >140 mg/dL was 2.1% (30 min/d) and <70 mg/dL was 1.1% (15 min/d). Sensor glucose values >180 mg/dL (10.0 mmol/L) and <54 mg/dL (3.0 mmol/L) were uncommon. However, 28% of participants had at least one hypoglycemic event.
Table 2.
Summary of Glucose Metrics Over a 24-h Period (n = 153)
| All Participants | Age Group | |||||
|---|---|---|---|---|---|---|
| 6 to <12 y | 12 to <18 y | 18 to <25 y | 25 to <60 y | ≥60 y | ||
| n | 153 | 27 | 30 | 29 | 41 | 26 |
| CGM use, h (mean ± SD) [range] | 192 ± 31 [84–245] | 180 ± 35 [84–233] | 181 ± 28 [111–233] | 192 ± 28 [92–223] | 207 ± 25 [136–245] | 195 ± 33 [124–236] |
| Overall glucose distribution and variability | ||||||
| Mean, mg/dL (mean ± SD) | 99 ± 7 | 99 ± 7 | 98 ± 6 | 98 ± 6 | 99 ± 6 | 104 ± 9 |
| SD, mg/dL (mean ± SD) | 17 ± 3 | 16 ± 3 | 15 ± 2 | 18 ± 3 | 16 ± 3 | 18 ± 5 |
| CV, % (mean ± SD) | 17 ± 3 | 16 ± 3 | 15 ± 2 | 18 ± 3 | 16 ± 3 | 17 ± 4 |
| Percentage of glucose sensor values, median (IQR) | ||||||
| >180 mg/dL | 0.0 (0.0–0.2) | 0.0 (0.0–0.1) | 0.0 (0.0–0.0) | 0.0 (0.0–0.4) | 0.0 (0.0–0.2) | 0.1 (0.0–0.5) |
| >160 mg/dL | 0.3 (0.1–0.9) | 0.2 (0.1–0.8) | 0.2 (0.0–0.2) | 0.4 (0.2–1.1) | 0.4 (0.2–0.9) | 0.6 (0.1–2.9) |
| >140 mg/dL | 2.1 (0.9–3.9) | 1.7 (0.8–2.9) | 1.2 (0.3–2.0) | 2.4 (1.3–4.4) | 2.1 (1.1–3.1) | 4.1 (1.3–8.6) |
| 70–140 mg/dL | 96 (93–98) | 97 (94–97) | 97 (95–98) | 95 (91–97) | 97 (94–98) | 93 (89–96) |
| 70–120 mg/dL | 89 (82–92) | 90 (83–92) | 92 (86–93) | 87 (82–90) | 89 (86–91) | 81 (71–86) |
| <70 mg/dL | 1.1 (0.3–2.9) | 1.1 (0.3–3.3) | 1.7 (0.6–2.6) | 1.3 (0.5–3.6) | 1.0 (0.3–2.3) | 1.4 (0.2–3.4) |
| <60 mg/dL | 0.2 (0.0–0.6) | 0.2 (0.0–0.3) | 0.2 (0.0–0.8) | 0.2 (0.0–0.7) | 0.2 (0.0–0.4) | 0.3 (0.0–0.7) |
| <54 mg/dL | 0.0 (0.0–0.2) | 0.0 (0.0–0.2) | 0.0 (0.0–0.4) | 0.1 (0.0–0.4) | 0.0 (0.0–0.2) | 0.1 (0.0–0.2) |
| Percentage of participants with ≥1 hypoglycemic eventa | 28 | 19 | 27 | 41 | 24 | 31 |
| Duration of hypoglycemic events for participants with ≥1 hypoglycemic event (min)a,b | ||||||
| n | 70 | 9 | 10 | 23 | 17 | 11 |
| Median (IQR) | 58 (40–100) | 60 (35–165) | 53 (40–85) | 50 (40–75) | 65 (40–100) | 80 (50–120) |
A hypo event is defined as at least two sensor values <54 mg/dL that are ≥15 min apart with no intervening values >54 mg/dL. At least two sensor values >70 mg/dL that are ≥15 min apart with no intervening values <70 mg/dL are required to end a hypo event.
Based on an event level.
The CGM glucose distributions during daytime and nighttime, overall and by age groups, are presented in Table 3. Overall, mean average glucose during the daytime was 100 ± 7 mg/dL (5.6 ± 0.4 mmol/L), and mean average glucose during nighttime was 98 ± 9 mg/dL (5.4 ± 0.5 mmol/L). Median sensor glucose between 70 and 140 mg/dL during the daytime and nighttime was 96% (IQR, 92 to 97%) and 99% (IQR, 95 to 100), respectively, and mean glycemic variability measured by CV during the daytime and nighttime was 17% ± 3% and 13% ± 4%. Glucose concentrations >140 mg/dL and <70 mg/dL were uncommon overnight, with median percentages of values of 0% (IQR, 0.0 to 1.0) and 0.4% (IQR, 0.0 to 2.5), respectively. Yet, 14% of participants had a hypoglycemic event overnight (Table 3).
Table 3.
Summary of Glucose Metrics by Daytime and Nighttime (n = 153)
| All Participants | Age Group | |||||
|---|---|---|---|---|---|---|
| 6 to <12 y | 12 to <18 y | 18 to <25 y | 25 to <60 y | ≥60 y | ||
| N | 153 | 27 | 30 | 29 | 41 | 26 |
| Daytime (6:00 am to 11:59 pm) | ||||||
| CGM use, h (mean ± SD) [range] | 142 ± 24 | 133 ± 27 | 133 ± 21 | 143 ± 21 | 154 ± 18 | 145 ± 26 |
| [54–179] | [54–173] | [87 to 173] | [68–169] | [104–179] | [96–176] | |
| Overall glucose distribution and variability | ||||||
| Mean, mg/dL (mean ± SD) | 100 ± 7 | 99 ± 6 | 99 ± 6 | 99 ± 6 | 100 ± 6 | 104 ± 9 |
| SD, mg/dL (mean ± SD) | 17 ± 3 | 17 ± 2 | 15 ± 3 | 19 ± 3 | 17 ± 3 | 19 ± 5 |
| CV, % (mean ± SD) | 17 ± 3 | 17 ± 3 | 16 ± 2 | 19 ± 3 | 17 ± 3 | 18 ± 4 |
| Percentage of glucose sensor values, median (IQR) | ||||||
| >180 mg/dL | 0.0 (0.0–0.2) | 0.0 (0.0–0.2) | 0.0 (0.0–0.0) | 0.0 (0.0–0.5) | 0.0 (0.0–0.2) | 0.2 (0.0–0.3) |
| >160 mg/dL | 0.4 (0.1–1.1) | 0.3 (0.1–0.8) | 0.2 (0.0–0.3) | 0.6 (0.2–1.5) | 0.5 (0.2–1.1) | 0.7 (0.1–3.0) |
| >140 mg/dL | 2.4 (1.2–4.5) | 2.1 (1.0–3.4) | 1.4 (0.4–2.2) | 3.0 (1.6–5.1) | 2.7 (1.5–3.8) | 5.2 (1.6–9.0) |
| 70–140 mg/dL | 96 (92–97) | 96 (94–97) | 97 (95–98) | 95 (91–96) | 96 (93–97) | 92 (88–97) |
| 70–120 mg/dL | 87 (80–91) | 87 (81–91) | 90 (84–92) | 86 (81–89) | 87 (84–90) | 78 (70–87) |
| <70 mg/dL | 1.4 (0.4–2.7) | 0.9 (0.5–2.8) | 1.4 (0.6–2.4) | 1.4 (0.5–4.9) | 1.3 (0.3–2.5) | 1.4 (0.3–3.6) |
| <60 mg/dL | 0.2 (0.0–0.5) | 0.2 (0.0–0.4) | 0.2 (0.0–0.4) | 0.2 (0.0–0.7) | 0.2 (0.0–0.4) | 0.2 (0.0–0.6) |
| <54 mg/dL | 0.0 (0.0–0.2) | 0.0 (0.0–0.1) | 0.0 (0.0–0.2) | 0.0 (0.0–0.4) | 0.0 (0.0–0.2) | 0.0 (0.0–0.1) |
| Percentage of participants with ≥1 hypoglycemic eventa | 18 | 4 | 13 | 31 | 20 | 19 |
| Duration of hypoglycemic events for participants with ≥1 hypoglycemic event, mina,b | ||||||
| n | 37 | 1 | 4 | 14 | 10 | 8 |
| Median (IQR) | 60 (45–100) | 165 | 63 (40–120) | 53 (45–75) | 83 (35–100) | 78 (52–117) |
| Nighttime (12:00 am to 5:59 am) | ||||||
| CGM use, h (mean ± SD) [range] | 50 ± 8 | 48 ± 9 | 48 ± 8 | 49 ± 8 | 53 ± 8 | 50 ± 8 |
| [24–66] | [27–60] | [24–60] | [24–60] | [30–66] | [26–60] | |
| Overall glucose distribution and variability | ||||||
| Mean, mg/dL (mean ± SD) | 98 ± 9 | 96 ± 10 | 96 ± 8 | 97 ± 7 | 98 ± 8 | 103 ± 12 |
| SD, mg/dL (mean ± SD) | 12 ± 4 | 12 ± 4 | 11 ± 2 | 14 ± 4 | 11 ± 4 | 13 ± 5 |
| CV, % (mean ± SD) | 13 ± 4 | 12 ± 4 | 12 ± 2 | 15 ± 4 | 12 ± 4 | 12 ± 4 |
| Percentage of glucose sensor values median (IQR) | ||||||
| >180 mg/dL | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| >160 mg/dL | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.3) |
| >140 mg/dL | 0.0 (0.0–1.0) | 0.0 (0.0–0.6) | 0.0 (0.0–0.6) | 0.6 (0.0–2.4) | 0.0 (0.0–0.9) | 0.0 (0.0–2.5) |
| 70–140 mg/dL | 99 (95–100) | 99 (96–100) | 98 (96–100) | 97 (93–99) | 99 (98–100) | 98 (94–100) |
| 70–120 mg/dL | 94 (88–97) | 95 (89–98) | 95 (91–97) | 91 (87–94) | 96 (87–98) | 92 (84–96) |
| <70 mg/dL | 0.4 (0.0–2.5) | 0.3 (0.0–2.3) | 0.9 (0.0–3.2) | 0.9 (0.0–3.1) | 0.4 (0.0–1.2) | 0.4 (0.0–2.4) |
| <60 mg/dL | 0.0 (0.0–0.6) | 0.0 (0.0–0.6) | 0.1 (0.0–1.0) | 0.0 (0.0–0.8) | 0.0 (0.0–0.3) | 0.0 (0.0–0.6) |
| <54 mg/dL | 0.0 (0.0–0.3) | 0.0 (0.0–0.3) | 0.0 (0.0–0.5) | 0.0 (0.0–0.3) | 0.0 (0.0–0.0) | 0.0 (0.0–0.3) |
| Percentage of participants with ≥1 hypoglycemic eventa | 14 | 19 | 20 | 17 | 7 | 8 |
| Duration of hypoglycemic events for participants with ≥1 hypoglycemic event (min)a,b | ||||||
| n | 33 | 8 | 6 | 9 | 7 | 3 |
| Median (IQR) | 50 (40–85) | 52 (33–152) | 52 (35–85) | 45 (40–75) | 55 (40–120) | 80 (40–125) |
A hypo event is defined as at least two sensor values <54 mg/dL that are ≥15 min apart with no intervening values >54 mg/dL. At least two sensor values >70 mg/dL that are ≥15 min apart with no intervening values <70 mg/dL are required to end a hypo event
Based on an event level.
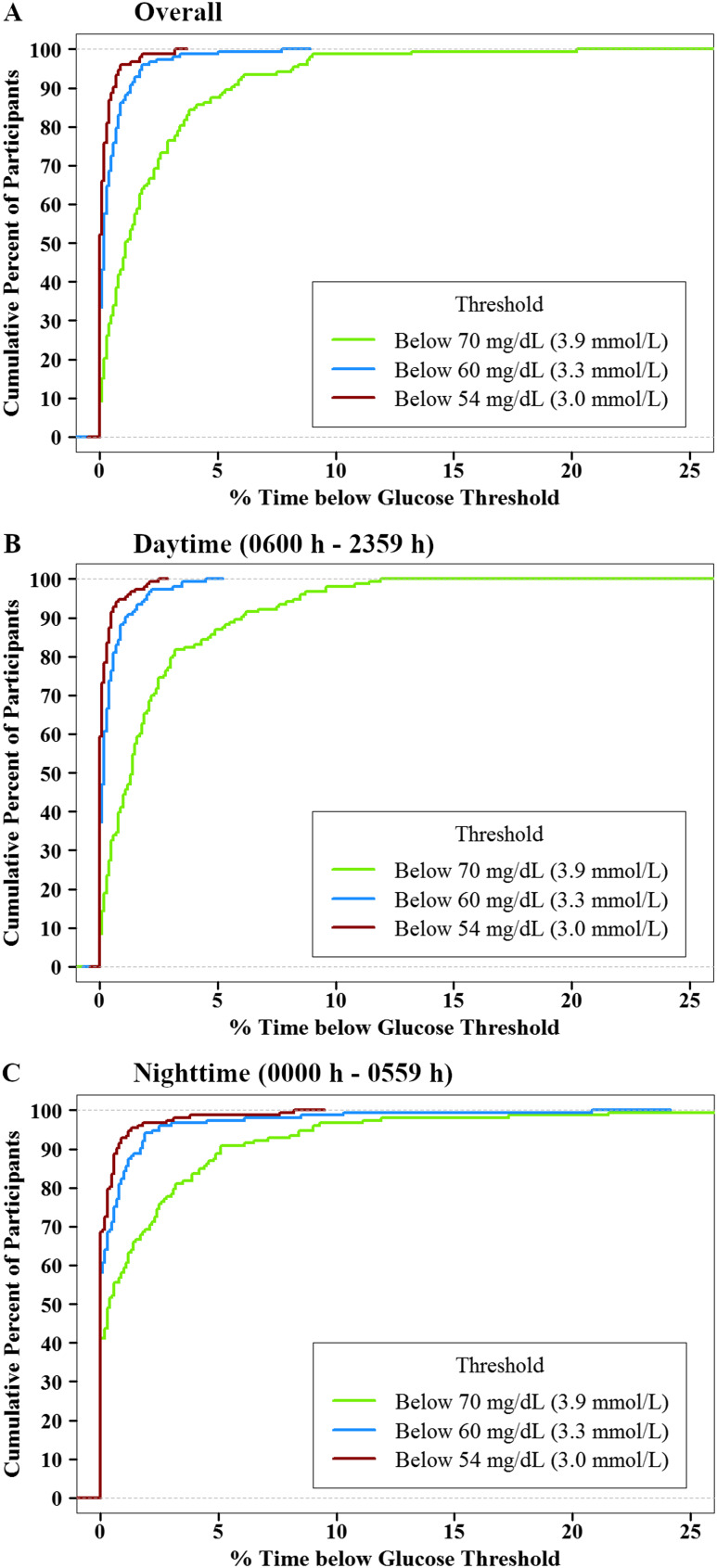
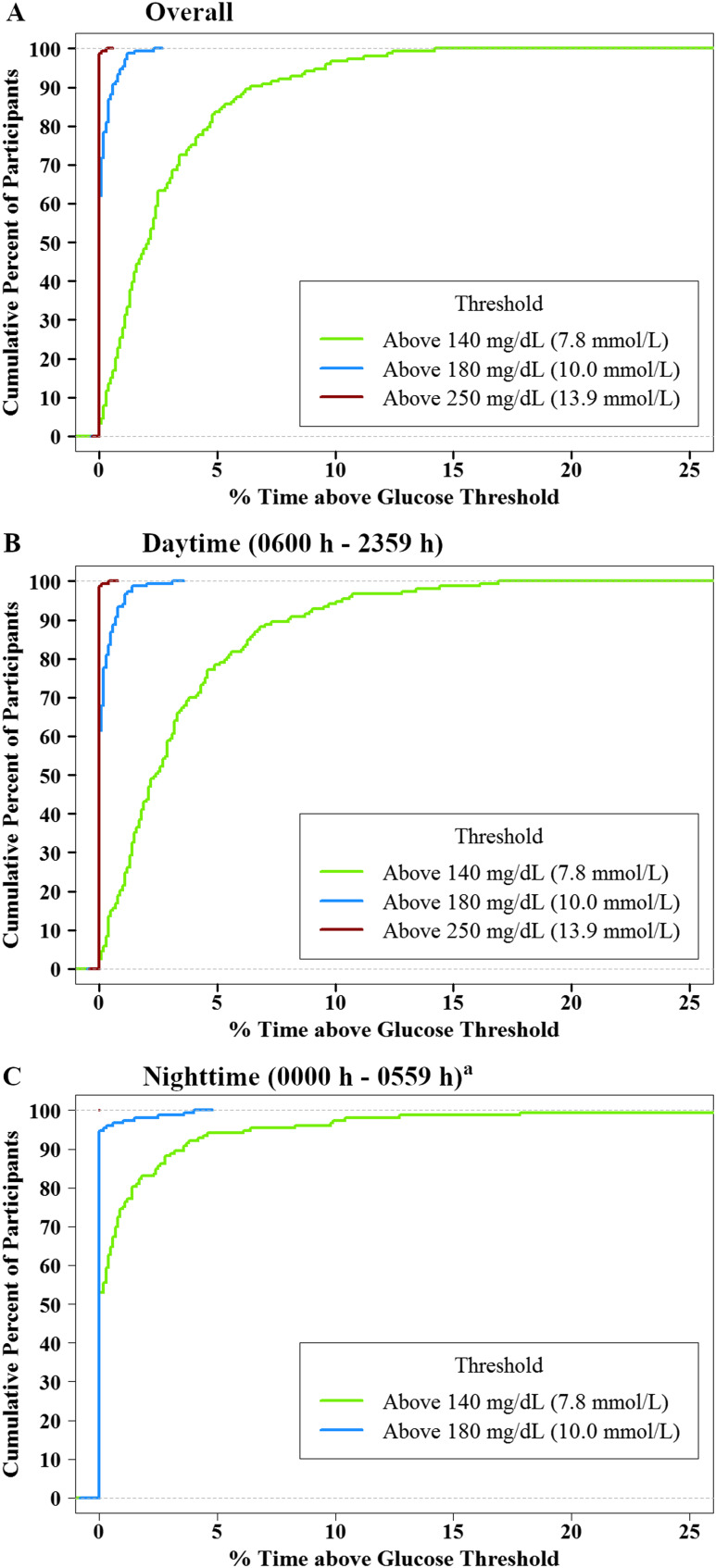
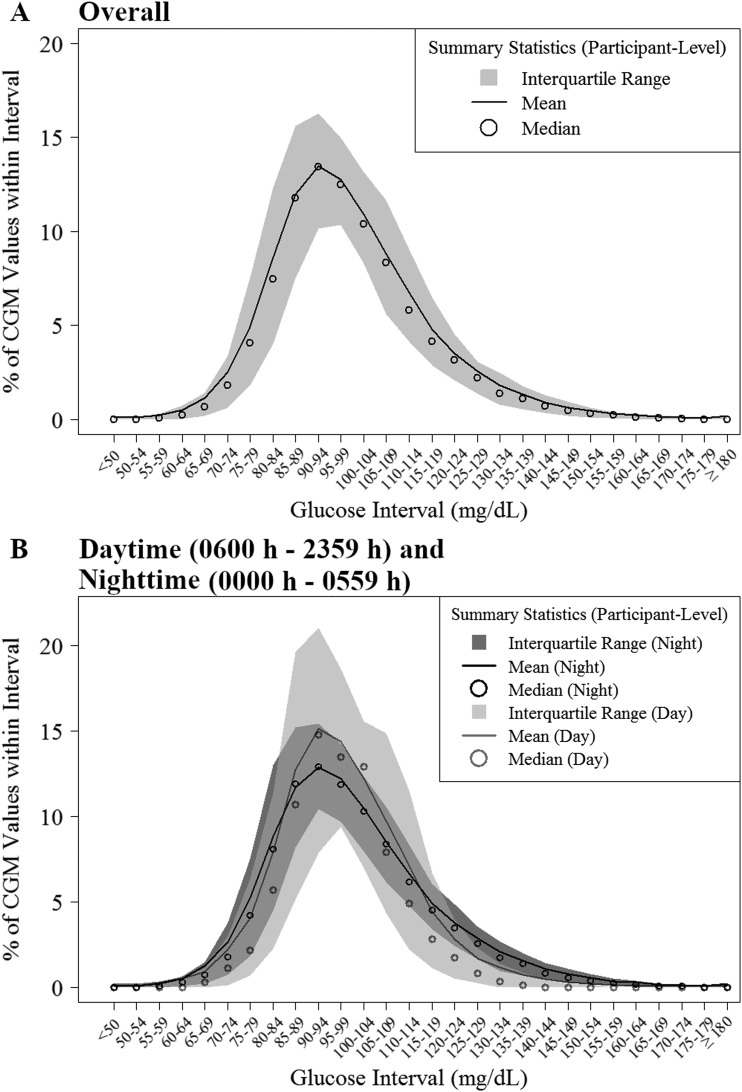
The cumulative frequency distributions of overall, daytime, and nighttime hypoglycemia and hyperglycemia are shown in Fig. 1 and 2. Overall, 35% of participants spent ≥2% of time with sensor glucose <70 mg/dL (almost 30 min/d), but only 1% of participants spent ≥2% of time <54 mg/dL. Overall, 51% of participants spent ≥2% of time with sensor glucose >140 mg/dL, but <1% of participants spent ≥2% of time >180 mg/dL (10). The distributions of CGM glucose values are displayed in Fig. 3. A standardized CGM glucose report [ambulatory glucose profile (11)] was created by aggregating the glucose profiles and CGM metrics from all 153 study participants (10).
Figure 1.
Cumulative distribution function of hypoglycemia (n = 153). (A) Overall. (B) Daytime (6:00 am to 11:59 pm). (C) Nighttime (12:00 am to 5:59 am).
Figure 2.
Cumulative distribution function of hyperglycemia (n = 153). (A) Overall. (B) Daytime (6:00 am to 11:59 pm). (C) Nighttime (12:00 am to 5:59 am). aNone of the participants had CGM readings >250 mg/dL during the night.
Figure 3.
Distribution of CGM glucose values (n = 153). (A) Overall. (B) Daytime (6:00 am to 11:59 pm) and nighttime (12:00 am to 5:59 am).
Mean HbA1c was 5.3 ± 0.2% for participants aged ≥60 years and ranged from a mean of 4.9% to a mean of 5.1% for all other age groups. Overall, the correlation was weak (r = 0.27; 95% confidence interval, 0.12 to 0.41) between mean glucose concentration and HbA1c levels, and there was a wide spread of HbA1c levels for a given mean glucose concentration (10).
In a sensitivity analysis after removing participants with first-degree family members with T1D (n = 101), mean CGM glucose, time spent in the 70 to 140 mg/dL range, and glycemic variability as measured by CV were not different from the main analysis (data not shown). Similarly, in a sensitivity analysis after removing implausible data, mean glucose, time spent in the 70 to 140 mg/dL range, and CV were not different from the main analysis; however, the percentage of participants who had a hypoglycemic event was lower after removing implausible data (10). When data were assessed by days of sensor wear, more hypoglycemia was seen during the first 48 hours of sensor wear than on subsequent days (data not shown).
Discussion
Our study reports CGM profiles in a large cohort of healthy, nondiabetic individuals with negative islet autoantibodies and normal HbA1c across different age groups. These individuals spent a median of 96% of the time with sensor glucose values between 70 and 140 mg/dL. Readings >180 and <54 mg/dL were relatively infrequent in all age groups. The current consensus conferences have somewhat arbitrarily set time in ranges for CGM sensor glucose levels for patients with T1D, with time in target identified as being between 70 to 180 mg/dL (12, 13). Our study provides data for how often this target range and other ranges are achieved in individuals without diabetes. These data can be used as a benchmark for new diabetes technology and treatments. Although it is likely that incremental benefits in glycemic control will be seen in the coming years with advanced closed-loop systems, creation of more physiologic insulin delivery, and adjunctive therapies for use in T1D, the ability of those with T1D to achieve the target glucose range between 70 and 140 seen in our healthy, nondiabetic control subjects may remain elusive. However, characterizing the glycemic benefits they afford will be important to place these therapies into a clinical context.
CGM profiles also provide the best window available to date to evaluate the frequency of biochemical hypoglycemia in clinical trials of new drugs and devices for the treatment of diabetes. Our findings support the recent consensus conference recommendation (12, 13) that sensor glucose values <54 mg/dL represent a more meaningful cut-off than 70 mg/dL to define clinically important hypoglycemia because CGM glucose levels below 54 mg/dL were uncommon, whereas most participants had some glucose levels of 55 to 69 mg/dL.
Further narrowing the time in the target range of 70 to 120 mg/dL, there was a lower frequency of sensor glucose values that fell within this range moving across the age cohorts, with participants 6 to 11 years old having a median of 90% of their readings in this range, as compared with only 81% in the cohort over age 60 years. The percentage of time between 70 to 120 mg/dL was quite different for daytime vs nighttime in the 60+ age group (78% vs 92%, respectively).
In this healthy population, even at the same HbA1c, mean glucose was variable. This is similar to what has been observed in the T1D population and highlights the limitation of HbA1c to reflect true glycemic status for some individuals (14). However, the correlation between HbA1c and glucose is lower than what others have found (15). There are two possible factors that may have contributed to this weaker-than-expected correlation: (1) HbA1c was obtained prior to collection of CGM glucose data and (2) there are at most 10 days of CGM data (16).
The overall results of our study are in agreement with previous studies (3–7, 17) despite the different generations of CGM systems used in most of the studies. Because the majority of our participants were white (93%), we were unable to evaluate whether results were consistent across races. Strengths of our study include the large sample size crossing the lifespan, multiple centers conducting the study, use of the latest generation technology, and up to 10 days of CGM wear time. Although pressure on the sensor during sleep may have resulted in inaccuracy of some CGM readings, we are unable to determine how many of the nocturnal hypoglycemic events were due to pressure on the sensor because of movement or position of the participant during sleep. After removing implausible data, glycemic metrics, including mean glucose, time in target range, and time spent hypoglycemic, remained similar, but the percentage of participants with a hypoglycemic event was much lower in the sensitivity analyses after removing implausible data. Furthermore, this study defined CGM-based glucose profile in healthy nondiabetic participants, and therefore these results may not be generalizable for capillary glucose or laboratory measured glucose profiles.
As the assessment of outcomes of treatment of T1D and type 2 diabetes in clinical practice and research trials moves beyond the narrow focus of HbA1c levels to include the results of sensor glucose profiles (14), there is an increased need for a repository of “normative” CGM data with the most up-to-date and widely used devices. Although our study fills the current gap in the data, it will be important to update the results in nondiabetic individuals periodically as the duration of wear and accuracy of CGM devices improve further.
Acknowledgments
Participating T1D Exchange Clinic Network sites with principal investigators (PI), coinvestigators (I), and coordinators (C) ordered by the number of participants recruited per site are as follows: Henry Ford Health System, Detroit, MI (n = 25), Davida Kruger (PI), Natalie Corker (C), Ana Tassopoulos (C), and Heather Remtema (C); University of Colorado/Denver, Barbara Davis Center Adult Clinic, Aurora, CO (n = 25), Viral Shah (PI), and Prakriti Joshee (C); International Diabetes Center, St. Louis Park, MN (n = 23), Richard Bergenstal (PI), Kathy McCann (C), and Sean Dunnigan (C); Indiana University, Indianapolis, IN (n = 20), Stephanie Woerner (PI), Linda DiMeglio (I), Devyn Purtlebaugh (C), and Megan Hildinger (C); University of Oklahoma Health Sciences Center, Oklahoma City, OK (n = 17), David Sparling (PI), Joni Beck (I), and Linda Weber (C); SUNY Upstate Medical University, Syracuse, NY (n = 16), Ruth Weinstock (PI), Suzan Bzdick (C), and Patricia Conboy (C); University of Southern California, Los Angeles, CA (n = 15), Anne Peters (PI) and Mark Harmel (C); Yale University, New Haven, CT (n = 15), Jennifer Sherr (PI) and Amy Steffen (C); University of Miami/Diabetes Research Institute, Miami, FL (n = 14), Francesco Vendrame (PI), Natalia Sanders-Branca (C), and Della Matheson (C); University of Iowa, Iowa City, IA (n = 13), Michael Tansey (PI), Eva Tsalikian (I), Julie Coffey (C), Joanne Cabbage (C), and Rachel Bisbee (C); University of Louisville Physicians – Pediatric Endocrinology, Louisville, KY (n = 9), Sara Watson (PI), Suzanne Kingery (I), Heather Rush (I), Manuel Rodriguez-Luna (C), Lauren Rayborn (C), and Gwen Pierce (C); Central Ohio Pediatric Endocrine and Diabetes Services, Columbus, OH (n = 9), Jennifer Dyer (PI), Diane Seiple (C), and Megan Jaycox (C). The authors thank Jeffrey Saunders, Publications Manager at the Jaeb Center for Health Research, for assistance with manuscript preparation and submission.
Financial Support: This work was supported by the Leona M. and Harry B. Helmsley Charitable Trust.
Disclosure Summary: V.N.S. has received research grants and sits on the Advisory Board for Dexcom and Sanofi US. R.S.W.’s nonprofit employer has received grant support from Medtronic, Minimed, Oramed Ltd, Kowa Research Institute, Mylan GmbH, Calibra Medical Inc., and Diasome Pharmaceuticals. R.S.W. has received consultancy payments from Insulogenic, LLC.
Glossary
Abbreviations:
- CGM
continuous glucose monitoring
- CV
coefficient of variation
- HbA1c
glycated Hb
- IQR
interquartile range
- MARD
mean absolute relative difference
- T1D
type 1 diabetes
References and Notes
- 1. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 2. DeSalvo DJ, Miller KM, Hermann JM, Maahs DM, Hofer SE, Clements MA, Lilienthal E, Sherr JL, Tauschmann M, Holl RW, Exchange TD, Registries DPV; T1D Exchange and DPV Registries. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, Nerup J, Borch-Johnsen K, Witte DR, Group AS; ADAG Study Group. Real-life glycaemic profiles in non-diabetic individuals with low fasting glucose and normal HbA1c: the A1C-Derived Average Glucose (ADAG) study. Diabetologia. 2010;53(8):1608–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, Haug C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007;1(5):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox LA, Beck RW, Xing D; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33(6):1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazze RS, Strock E, Wesley D, Borgman S, Morgan B, Bergenstal R, Cuddihy R. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10(3):149–159. [DOI] [PubMed] [Google Scholar]
- 7. Zhou J, Li H, Ran X, Yang W, Li Q, Peng Y, Li Y, Gao X, Luan X, Wang W, Jia W. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care. 2009;32(7):1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389. [DOI] [PubMed] [Google Scholar]
- 9. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah VN, DuBose SN, Li Z, Beck RW, Peters AL, Weinstock RS, Kruger D, Tansey M, Sparling D, Woerner S, Vendrame F, Bergenstal R, Tamborlane WV, Watson SE, Sherr J. Data from: Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. Dryad; 2018. Deposited 14 May 2019. 10.5061/dryad.h7d11cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergenstal RM, Ahmann AJ, Bailey T, Beck RW, Bissen J, Buckingham B, Deeb L, Dolin RH, Garg SK, Goland R, Hirsch IB, Klonoff DC, Kruger DF, Matfin G, Mazze RS, Olson BA, Parkin C, Peters A, Powers MA, Rodriguez H, Southerland P, Strock ES, Tamborlane W, Wesley DM. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther. 2013;15(3):198–211. [DOI] [PubMed] [Google Scholar]
- 12. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ III, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip M. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maahs DM, Buckingham BA, Castle JR, Cinar A, Damiano ER, Dassau E, DeVries JH, Doyle FJ III, Griffen SC, Haidar A, Heinemann L, Hovorka R, Jones TW, Kollman C, Kovatchev B, Levy BL, Nimri R, O’Neal DN, Philip M, Renard E, Russell SJ, Weinzimer SA, Zisser H, Lum JW. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care. 2016;39(7):1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values [published correction appears in Diabetes Care. 2009;32(1):207]. Diabetes Care. 2008;31(8):1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riddlesworth TD, Beck RW, Gal RL, Connor CG, Bergenstal RM, Lee S, Willi SM. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20(4):314–316. [DOI] [PubMed] [Google Scholar]
- 17. Sundberg F, Forsander G. Continuous glucose monitoring in healthy children aged 2-8 years. Diabetes Technol Ther. 2018;20(2):113–116. [DOI] [PubMed] [Google Scholar]