Abstract
Context
Data on hypothalamic-pituitary (HP) disorders in systematically evaluated childhood cancer survivors are limited.
Objective
To describe prevalence, risk factors, and associated adverse health outcomes of deficiencies in GH deficiency (GHD), TSH deficiency (TSHD), LH/FSH deficiency (LH/FSHD), and ACTH deficiency (ACTHD), and central precocious puberty (CPP).
Design
Retrospective with cross-sectional health outcomes analysis.
Setting
Established cohort; tertiary care center.
Patients
Participants (N = 3141; median age, 31.7 years) were followed for a median 24.1 years.
Main Outcome Measure
Multivariable logistic regression was used to calculate ORs and 95% CIs for associations among HP disorders, tumor- and treatment-related risk factors, and health outcomes.
Results
The estimated prevalence was 40.2% for GHD, 11.1% for TSHD, 10.6% for LH/FSHD, 3.2% for ACTHD, and 0.9% for CPP among participants treated with HP radiotherapy (n = 1089), and 6.2% for GHD, and <1% for other HP disorders without HP radiotherapy. Clinical factors independently associated with HP disorders included HP radiotherapy (at any dose for GHD, TSHD, LH/FSHD, >30 Gy for ACTHD), alkylating agents (GHD, LH/FSHD), intrathecal chemotherapy (GHD), hydrocephalus with shunt placement (GHD, LH/FSHD), seizures (TSHD, ACTHD), and stroke (GHD, TSHD, LH/FSHD, ACTHD). Adverse health outcomes independently associated with HP disorders included short stature (GHD, TSHD), severe bone mineral density deficit (GHD, LH/FSHD), obesity (LH/FSHD), frailty (GHD), impaired physical health-related quality of life (TSHD), sexual dysfunction (LH/FSHD), impaired memory, and processing speed (GHD, TSHD).
Conclusion
HP radiotherapy, central nervous system injury, and, to a lesser extent, chemotherapy are associated with HP disorders, which are associated with adverse health outcomes.
In a large cohort of systematically assessed childhood cancer survivors, associations between HP disorders, tumor and treatment variables, and adverse health outcomes were elicited.
Improvement in survival of children diagnosed with cancer and research demonstrating high rates of chronic disease in adult survivors of childhood cancer highlight the importance of characterizing clinical factors that contribute to these morbidities (1). Hypothalamic-pituitary (HP) disorders are frequently observed in childhood cancer survivors (2–4) and are associated with impaired growth, pubertal development and reproductive function, suboptimal body composition, decreased bone mineral density (BMD), inadequate response to acute illness, and poor quality of life (5, 6).
Radiotherapy and tumor location involving the HP region are established risk factors for HP disorders in childhood cancer survivors (7, 8). However, the association between HP disorders and chemotherapy or central nervous system (CNS) injury is not well documented in this population (9). In addition, data on the contribution of specific HP disorders to physical, psychosocial, and neurocognitive health are limited.
A previous report from the St. Jude Lifetime Cohort (SJLIFE) described the prevalence of HP disorders among childhood cancer survivors treated with radiotherapy to the CNS (10). SJLIFE expanded endocrine surveillance after 2015 from screening only at-risk participants (i.e., those with history of CNS irradiation), to screening all participants regardless of previous treatment. This change provided a unique opportunity to characterize potential risk factors for HP disorders in individuals without HP irradiation. Therefore, our aims for this study were to (1) describe the prevalence of HP disorders in long-term clinically assessed childhood cancer survivors, including those without HP irradiation; (2) evaluate associations between sociodemographic and treatment-related risk factors and HP disorders; and (3) describe associations between HP disorders and overall physical, psychosocial, and neurocognitive functioning.
Methods
SJLIFE study
Participants were enrolled in the previously described institutional review board–approved SJLIFE study (11, 12). Informed consent was obtained from all participants for all aspects of the study. SJLIFE is a retrospective cohort study initiated in October 2007, with prospective periodic follow-up and ongoing clinical data collection designed to facilitate clinical assessment of health outcomes among childhood cancer survivors.
Eligibility
Survivors diagnosed with cancer when they were 18 years old or younger, treated for childhood cancer at St. Jude Children’s Research Hospital, and who were at least 5 years from cancer diagnosis were considered eligible for the current analysis. Participants underwent a comprehensive clinical assessment between 2007 and 2016 on the St. Jude campus, including measures of physical, psychosocial, and neurocognitive health.
Tumor and treatment variables
CNS tumors were classified as involving the HP region if they included the thalamus, hypothalamus, optic chiasm, third ventricle, and sellar or para-sellar region. Dosimetry was available for 921 of 1086 participants who had undergone cranial irradiation. The radiotherapy dose absorbed by the HP region was based on prescribed doses for direct exposures. For indirect exposures, HP doses were estimated as pituitary doses derived from reconstructing radiotherapy fields on computational phantoms sized by ages at treatment (13). In the remaining 165 participants, the radiotherapy dose was quantified as the maximum tumor-prescribed dose to the brain. Cumulative treatment exposure for alkylating agents was quantified using the cyclophosphamide equivalent dose (CED) (14). Stroke was defined as any Common Terminology Criteria for Adverse Events (CTCAE) grade 2 or higher cerebrovascular accident, cerebrovascular disease, or intracranial hemorrhage. Survivors with previous placement of ventriculoperitoneal or ventriculoatrial shunts were classified with a history of hydrocephalus.
Diagnoses of HP disorders
In participants receiving hormone replacement therapy at the time of their visit, HP disorders were validated by medical record review; hormone replacement therapy was not interrupted. The diagnosis of a HP disorder was based on medical history assuming that previous diagnoses were valid and persistent. In participants not receiving hormone replacement therapy, HP disorders were determined from values in a morning blood sample at the SJLIFE visit, as described previously (10). Electrochemiluminescent immunometric assays (Roche Cobas 6000 analyzer; Roche Diagnostics, Indianapolis, IN) were used to measure concentrations of IGF-1, LH, FSH, total testosterone (males), estradiol (females), TSH, free thyroxine, and cortisol. GH deficiency (GHD) was considered present in individuals with a past history of GHD based on dynamic testing. In the absence of a known history of GHD, IGF-1 levels were used to determine these patients’ status. In these participants, GHD was considered present if age and sex-specific IGF-1 level was lower than −2 SD of normal values. Participants without available IGF-1 measurements or those with liver fibrosis and/or liver cirrhosis, conditions known to reduce synthesis of IGF-1, were excluded from GHD analysis. TSH deficiency (TSHD) was defined as free thyroxine level <0.9 ng/dL (laboratory normal value range, 1.0 to 2.0 ng/dL) and TSH level was <4 mIU/L (laboratory normal value range, 0.4 to 4 mIU/L). LH/FSH deficiency (LH/FSHD) in males was defined by total testosterone <200 ng/dL before 1 March 2012 and <250 ng/dL afterward (due to changes in assays used), in the presence of an LH concentration <7 IU/L and FSH concentration <9.2 IU/L. In females, LH/FSHD was defined if primary or secondary amenorrhea occurred before age 40 years and if estradiol and FSH levels were <17 pg/mL and <11.2 IU/L, respectively. ACTH deficiency (ACTHD) was defined among survivors exposed to HP radiotherapy if the morning serum cortisol level was <5 μg/dL. Central precocious puberty (CPP) was defined as pubertal onset before the age 8 years in girls and 9 years in boys. Pubertal onset was defined by Tanner stage 2 or greater breast development in girls and by Tanner stage 2 genital development and/or morning testosterone concentration >20 ng/dL in boys.
Physical assessment
Height was converted into an age- and sex-adjusted Z-score for participants younger than 20 years. For participants age 20 years or older, Z-score data for age 20 years were used. Short stature was defined as a height Z-score < −2. Depending on age at SJLIFE, obesity was defined as body mass index >30 kg/m2 in those ≥20 years old, or body mass index Z-score >2 in those younger than 20 years. BMD was assessed using dual x-ray absorptiometry. Average volumetric trabecular BMD for lumbar vertebrae L1 and L2 was calculated and reported as age- and sex-specific Z-scores. Low BMD was defined as a Z-score < −2 (15).
Physiologic frailty was defined as meeting three or more of the following criteria: decreased lean muscle mass, decreased vitality, poor physical activity, slowness, or weakness (Table 1) (16–20). The 6-minute walk test was used to evaluate exercise tolerance (21). Poor exercise tolerance was defined by <400 m of distance covered in 6 minutes. Hypertension, dyslipidemia, abnormal glucose metabolism, and cardiomyopathy were defined as CTCAE grade 2 or higher (12).
Table 1.
Criteria Used to Define Frailty in the SJLIFE Cohort
| Frailty Component | SJLIFE Criteria (Reference) | |||
|---|---|---|---|---|
| Decreased lean muscle mass | Lean muscle mass by dual x-ray absorptiometry below −1 age- and sex-specific SD when compared with data from a national sample (NHANES) (16) | |||
| Decreased vitality | Score ≤40 (1 SD, based on a standard normal distribution; this represents approximately the lowest 6.7% of the general population) on the Vitality Subscale of the Medical Outcomes Survey Short Form 36 (16). | |||
| Poor physical activity | Expended <383 Kcal/wk (males) or <270 Kcal/wk (females) during leisure time physical activity based on the NHANES Physical Activity Questionnaire (18, 19). | |||
| Walking speed (slowness) | Females <159 and males <173 centimeters tall were classified as slow if they took ≥7 seconds, and females ≥159 and males ≥173 centimeters tall were classified as slow if they took ≥6 seconds to walk 15 feet at their usual pace (20). | |||
| Hand grip strength (weakness) | Hand grip strength stratified by body mass index and sex (20). | |||
| Males | Females | |||
| BMI | Cut Point | BMI | Cut Point | |
| ≤ 24 kg/m2 | ≤29 kg | ≤ 23 kg/m2 | ≤17 kg | |
| 24.1–26 kg/m2 | ≤30 kg | 23.1–26 kg/m2 | ≤17.3 kg | |
| 26.1–28 kg/m2 | ≤30 kg | 26.1–29 kg/m2 | ≤18 kg | |
| > 28 kg/m2 | ≤32 kg | > 29 kg/m2 | ≤21 kg | |
Abbreviations: BMI, body mass index; NHANES, National Health and Nutrition Examination Survey.
Psychosocial and neurocognitive assessment
Health-related quality of life (HRQOL) was assessed using the physical and mental component summaries of the Medical Outcomes Study Short-Form 36 (22). Clinically meaningful impairment was assigned to those with scores ≤40, equivalent to ≥1 SD below the population mean of 50. Emotional distress was measured by the Brief Symptom Inventory, an 18-item questionnaire that provides a global measure of acute emotional distress as well as subscale scores for anxiety, depression, and somatization (23). Emotional distress was defined as scores ≥63 on each subscale, representing the highest 10th percentile of population norms. In males, sexual dysfunction was considered present when sexually active individuals scored ≤25 points on an abbreviated six-item version of the validated International Index of Erectile Function, embedded in the Men’s Health Questionnaire (24). In nonsexually active males, responses to two ancillary questions of the Men’s Health Questionnaire were used to assess sexual dysfunction (25). In sexually active females, sexual dysfunction was defined as a total score <10th percentile of population norms on the Sexual Functioning Questionnaire embedded in the Women’s Health Questionnaire (26). No ancillary questions were available in the Women’s Health Questionnaire to assess sexual dysfunction in sexually inactive females. Neurocognitive outcomes included memory, attention, processing speed, and executive function and were assessed with the instruments listed in Table 2 (27–30). For all four outcomes, a CTCAE grade 2 or higher, which reflected a domain score falling at least 2 SDs below population norms, was considered as impaired neurocognitive function.
Table 2.
Neurocognitive Measures and Assessment Instruments
| Neurocognitive Outcome | Assessment Instrument (Reference) |
|---|---|
| Memory | California Verbal Learning Test-ΙΙ: total learning, short-delay free recall and long-delay free recall (27) |
| Attention | Trail-Making Test Part A (28), Conners’ Continuous Performance Test-ΙΙ: Omissions and Variability (29), Digit Span Forward subtest of the WAIS-ΙΙΙ (30) |
| Processing speed | Coding and symbol search subtests of the WAIS-ΙΙΙ4, Continuous Performance Test-ΙΙ: Hit Rate (29) |
| Executive function | Trail-Making Test Part B (28), Controlled Oral Word Association Test (28), Digit Span Backward subtest of the WAIS-ΙΙΙ (30) |
Abbreviations: BMI, body mass index; WAIS-III, Wechsler Adult Intelligence Scale–ΙΙΙ.
Statistical analysis
χ 2, Fisher exact, t, and Wilcoxon rank-sum tests were used to compare demographic and treatment characteristics between participants and nonparticipants. Cumulative incidence was calculated using the Kaplan-Meier method and assigning the date of detection of an HP disorder as the date of occurrence and censoring participants without an event at last SJLIFE campus visit. Univariate log-binomial models were first used to examine associations between sociodemographic and treatment-related risk factors and HP disorders. For this analysis, participants with HP tumor involvement were excluded because their tumor location made them particularly prone to develop HP disorders. Variables with a P value ≤0.1 were included in multivariable log-binomial models for additional analysis. Second log-binomial regression was used to evaluate associations among HP disorders (GHD, TSHD, and LH/FSHD) and physical, psychosocial, and neurocognitive outcomes; however, association testing for ACTHD and CPP was not performed, because of the low prevalence of these disorders among survivors. For these analyses, participants with HP tumor involvement were included. Variables with P values ≤0.1 were included in multivariable models, together with predefined adjustments. SAS, version 9.4 (SAS Institute, Cary, NC), was used for all analyses.
Results
Study population
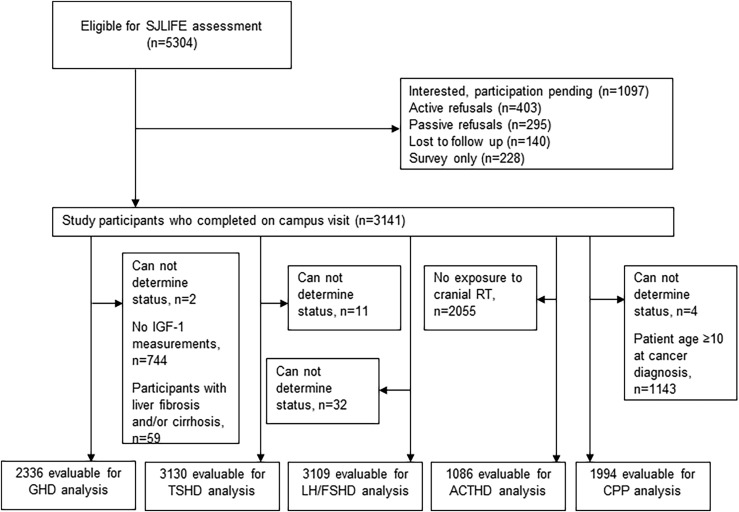
Among 5304 eligible survivors, 1066 (20.1%) were lost to follow-up or declined participation in the clinical assessment component of SJLIFE. Of the remaining 4238 survivors, 3141 (74.1%) completed an on-campus visit as of 30 June 2016 and had evaluable data (Fig. 1). Compared with nonparticipants (n = 2163), study participants were more likely to be female (47.9% vs 43.4%), white (81.7% vs 78.3%), leukemia survivors (37.2% vs 32.1%), and diagnosed with their primary malignancy at an older age (Table 3). In addition, participants were more likely to have received chemotherapy (85.2% vs 81.2%), alkylating agents (58.8% vs 54.8%), intrathecal chemotherapy (40.5% vs 37.1%), and radiotherapy including the HP region (34.6% vs 29.1%), compared with nonparticipants. Median age at assessment was 31.7 (range, 7.5 to 65.1) years, and median time since cancer diagnosis was 24.1 (range, 6.8 to 51.1) years. In 77 participants (2.5%), the CNS tumor involved the HP region (Table 4). Treatment exposures included HP radiotherapy in 1086 participants (34.6%) and alkylating agents in 1847 participants (58.8%).
Figure 1.
Flow diagram SJLIFE cohort. RT, radiation therapy.
Table 3.
Sociodemographic and Treatment Characteristics of Participants and Nonparticipants
| Variable | Participants (n = 3141) | Nonparticipants (n = 2163) | |||
|---|---|---|---|---|---|
| No. | % | No. | % | P Value | |
| Sex | |||||
| Male | 1638 | 52.1 | 1225 | 56.6 | 0.001 |
| Female | 1503 | 47.9 | 938 | 43.4 | |
| Race/ethnicity | |||||
| Non-Hispanic white | 2565 | 81.7 | 1694 | 78.3 | 0.004 |
| Non-Hispanic black | 465 | 14.8 | 363 | 16.8 | |
| Other | 111 | 3.5 | 106 | 4.9 | |
| Age at tumor diagnosis, median (range), y | 6.8 (0–18.0) | 6.0 (0–18.00) | <0.0001 | ||
| Age at SJLIFE evaluation, median (range), y | 31.7 (7.5–65.1) | — | — | ||
| Primary tumor diagnosis | |||||
| Leukemia | 1167 | 37.2 | 695 | 32.1 | <0.0001 |
| Lymphoma | 558 | 17.8 | 357 | 16.5 | |
| CNS tumor | 352 | 11.2 | 304 | 14.1 | |
| Craniopharyngioma | 26 | 7.4 | 17 | 5.6 | |
| Ependymoma | 38 | 10.8 | 26 | 8.6 | |
| Glial cell tumor | 174 | 49.4 | 151 | 49.7 | |
| Medulloblastoma | 87 | 24.7 | 62 | 20.4 | |
| Other CNS tumor | 27 | 7.7 | 48 | 15.8 | |
| Non-brain solid tumor of head and neck | 203 | 6.5 | 183 | 8.5 | |
| Other solid tumor | 795 | 25.3 | 549 | 25.4 | |
| Other | 66 | 2.1 | 75 | 3.5 | |
| HP involvement | |||||
| Yes | 77 | 2.4 | 57 | 2.6 | 0.68 |
| No | 3064 | 97.6 | 2106 | 97.4 | |
| Stroke | |||||
| Yes | 159 | 5.1 | — | — | Not applicable |
| No | 2982 | 94.9 | — | — | |
| Seizures | |||||
| Yes | 382 | 12.2 | — | — | Not applicable |
| No | 2759 | 87.8 | — | — | |
| Hydrocephalus with shunt placement | |||||
| Yes | 95 | 3.0 | 79 | 3.7 | 0.21 |
| No | 3046 | 97.0 | 2084 | 96.3 | |
| HP RT dose, Gy | |||||
| No cranial RT | 2055 | 65.4 | 1533 | 70.9 | <0.0001 |
| 1–19.9 | 399 | 12.7 | 183 | 8.5 | |
| 20–30 | 388 | 12.4 | 179 | 8.3 | |
| >30 | 298 | 9.5 | 267 | 12.3 | |
| Dose unknown | 1 | 0.03 | 1 | 0.1 | |
| Any chemotherapy | |||||
| Yes | 2676 | 85.2 | 1757 | 81.2 | 0.0001 |
| No | 465 | 14.8 | 406 | 18.8 | |
| Alkylating agent | |||||
| Yes | 1847 | 58.8 | 1186 | 54.8 | 0.004 |
| No | 1294 | 41.2 | 977 | 45.2 | |
| Intrathecal chemotherapy | |||||
| Yes | 1273 | 40.5 | 803 | 37.1 | 0.01 |
| No | 1868 | 59.5 | 1360 | 62.9 | |
Dashes indicate no data available in nonparticipants.
Abbreviation: RT, radiation therapy.
Table 4.
Sociodemographic and Treatment Characteristics of Participants With Tumor Involvement of the HP Region
| Variable | Participants (n = 77) | |
|---|---|---|
| No. | % | |
| Sex | ||
| Male | 44 | 57.1 |
| Female | 33 | 42.9 |
| Race/ethnicity | ||
| Non-Hispanic white | 63 | 81.8 |
| Non-Hispanic black | 12 | 15.6 |
| Other | 2 | 2.6 |
| Age at tumor diagnosis, median (range), y | 8.3 (0.4–17.8) | |
| Age at SJLIFE, median (range), y | 25.3 (19.2–40.5) | |
| Primary tumor diagnosis | ||
| Craniopharyngioma | 26 | 33.8 |
| Glial cell tumor | 46 | 59.7 |
| Other CNS tumor | 5 | 6.5 |
| Stroke | ||
| Yes | 12 | 15.6 |
| No | 65 | 84.4 |
| Seizure | ||
| Yes | 20 | 26.0 |
| No | 57 | 74.0 |
| Hydrocephalus with shunt placement | ||
| Yes | 25 | 32.5 |
| No | 52 | 67.5 |
| HP dose, Gy | ||
| No cranial RT | 37 | 48.1 |
| 1–19.9 | 0 | 0 |
| 20–30 | 0 | 0 |
| >30 | 40 | 51.9 |
| Alkylating agent | ||
| Yes | 4 | 5.2 |
| No | 73 | 94.8 |
| Intrathecal chemotherapy | ||
| Yes | 0 | 0 |
| No | 77 | 100 |
| HP disorder present at tumor diagnosis | ||
| Yes | 37 | 48.1 |
| One HP disorder | 15 | 40.5 |
| Multiple HP disorders | 22 | 59.5 |
| No HP disorder | 40 | 51.9 |
Abbreviation: RT, radiation therapy.
Risk factors of HP disorders
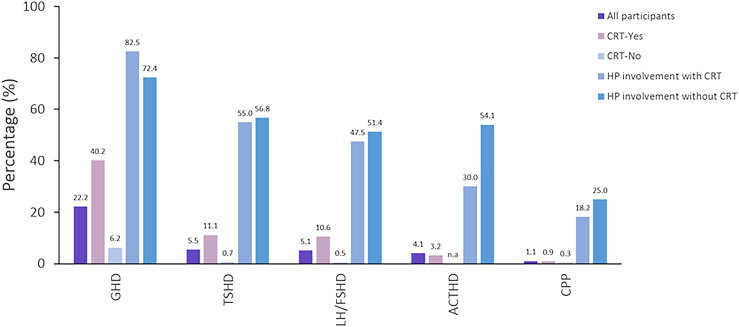
The estimated prevalence of all HP disorders is shown in Fig. 2. In the multivariable analysis, any radiotherapy dose to the HP region was associated with higher odds of GHD [1 to 19.9 Gy, (OR, 4.16; 95% CI, 2.94 to 5.89); 20 to 30 Gy, (OR, 5.71; 95% CI, 4.03 to 8.09); or >30 Gy (OR, 21.43; 95% CI, 14.41 to 31.86)]; TSHD [1 to 19.9 Gy (OR, 12.86; 95% CI, 6.61 to 25.03); 20 to 30 Gy (OR, 11.4; 95% CI, 5.45 to 23.98); or >30 Gy (OR, 33.53; 95% CI, 17.59 to 63.90)]; and LH/FSHD [1 to 19.9 Gy (OR, 7.59; 95% CI, 3.42 to 16.81); 20 to 30 Gy (OR, 13.05; 95% CI, 6.12 to 27.84); or >30 Gy (OR, 27.45; 95% CI, 12.98 to 58.04)], compared with no HP radiotherapy (Table 5). ACTHD was associated with HP radiotherapy dose >30 Gy vs no HP radiotherapy (OR, 4.41; 95% CI, 1.38 to 14.11). Stroke was associated with higher odds for all four HP disorders. In addition, the multivariable analysis demonstrated significant associations between GHD and male sex, younger age at cancer diagnosis, hydrocephalus with shunt placement, intrathecal chemotherapy, and alkylating agents at CED 8001 to 12,000 mg/m2 (OR, 1.55; 95% CI, 1.09 to 2.19) or >12,000 mg/m2 (OR, 1.79; 95% CI, 1.28 to 2.49). The risk factor analysis was repeated for GHD after stratification based on HP exposure to radiotherapy. The association between GHD and intrathecal chemotherapy remained significant only in individuals without HP exposure to radiotherapy (OR, 3.70; 95% CI, 2.29 to 5.98). The association between alkylating agents and GHD became nonsignificant in both of the subgroups; TSHD was associated with white ethnicity, younger age at SJLIFE, and seizures; LH/FSHD was associated with male sex, white ethnicity, younger age at cancer diagnosis, hydrocephalus with shunt placement, and alkylating agents at CED 1 to 4000 mg/m2 (OR, 2.26; 95% CI, 1.06 to 4.82) or >12,000 mg/m2 (OR, 2.21; 95% CI, 1.29 to 3.78); and ACTHD with younger age at SJLIFE and seizures. The limited number of participants with a history of CPP allowed only univariable analysis for this HP disorder (Table 6). Interestingly, nine individuals (42.8%) with a history of CPP were diagnosed with LH/FSHD later in life, most likely as a late effect of HP radiotherapy (n = 8) or because of tumor-related factors (n = 1).
Figure 2.
Prevalence of HP disorders. CRT, cranial radiation therapy.
Table 5.
Multivariable Logistic Regression Models for Risk Factors of HP Disorders
| Variable | GHD (n = 2267) | TSHD (n = 3053) | LH/FSHD (n = 3032) | ACTHD (n = 1046) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (Row %) | OR (95% CI) | P Value | No. (Row %) | OR (95% CI) | P Value | No. (Row %) | OR (95% CI) | P Value | No. (Row %) | OR (95% CI) | P Value | |
| Sex | ||||||||||||
| Male | 271 (22.9) | 1.00 | — | — | 95 (6.1) | 1.00 | 24 (4.2) | 1.00 | ||||
| Female | 194 (17.9) | 0.77 (0.61–0.98) | 0.03 | — | — | — | 25 (1.7) | 0.29 (0.18–0.47) | <0.0001 | 9 (1.9) | 0.46 (0.20–1.04) | 0.06 |
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | 399 (21.6) | 1.00 | 116 (4.7) | 1.00 | 112 (4.5) | 1.00 | — | — | ||||
| Other | 66 (15.6) | 0.83 (0.59–1.16) | 0.27 | 13 (2.3) | 0.43 (0.23–0.81) | 0.01 | 8 (1.4) | 0.38 (0.17–0.83) | 0.02 | — | — | — |
| Age at tumor diagnosis, mean (SD), y | 6.12 (4.19) | 0.92 (0.89–0.94) | <0.0001 | 6.5 (4.9) | 0.97 (0.93–1.02) | 0.20 | 6.7 (5.0) | 0.95 (0.90–0.99) | 0.03 | — | — | — |
| Age at SJLIFE evaluation, mean (SD), y | 32.6 (8.2) | — | — | 30.8 (7.4) | 0.96 (0.94–0.99) | 0.004 | 35.8 (9.6) | 1.02 (0.99–1.05) | 0.08 | 29.22 (5.8) | 0.94 (0.89–0.99) | 0.02 |
| Stroke | ||||||||||||
| No | 405 (18.9) | 1.00 | 101 (3.5) | 1.00 | 88 (3.1) | 1.00 | 22 (2.3) | 1.00 | ||||
| Yes | 60 (47.2) | 1.73 (1.12–2.67) | 0.01 | 28 (19.1) | 2.52 (1.47–4.33) | 0.001 | 32 (21.9) | 3.45 (2.02–5.90) | <0.0001 | 11 (10.5) | 3.12 (1.32–7.42) | 0.01 |
| Seizure | ||||||||||||
| No | 368 (18.6) | 1.00 | 89 (3.3) | 1.00 | 82 (3.1) | 1.00 | 19 (2.2) | 1.00 | ||||
| Yes | 97 (34.3) | 1.30 (0.93–1.81) | 0.12 | 40 (11.1) | 1.77 (1.12–2.80) | 0.02 | 38 (10.6) | 1.52 (0.93–2.46) | 0.09 | 14 (7.3) | 2.49 (1.11–5.59) | 0.03 |
| Hydrocephalus with shunt placement | ||||||||||||
| No | 431 (19.6) | 1.00 | 111 (3.7) | 1.00 | 103 (3.5) | 1.00 | 26 (2.6) | 1.00 | ||||
| Yes | 34 (54.8) | 2.74 (1.41–5.32) | 0.003 | 18 (25.7) | 1.82 (0.92–3.60) | 0.09 | 17 (24.3) | 2.93 (1.36–6.33) | 0.01 | 7 (14.0) | 1.32 (0.47–3.70) | 0.50 |
| HP dose, Gy | ||||||||||||
| No cranial RT | 82 (6.2) | 1.00 | 13 (0.7) | 1.00 | 10 (0.5) | 1.00 | — | |||||
| 1–19.9 | 103 (28.3) | 4.16 (2.94–5.89) | <0.0001 | 31 (7.8) | 12.86 (6.61–25.03) | <0.0001 | 19 (4.8) | 1.00 | 5 (1.3) | 1.00 | ||
| 20–30 | 147 (41.5) | 5.71 (4.03–8.09) | <0.0001 | 26 (6.7) | 11.43 (5.45–23.98) | <0.0001 | 44 (11.4) | 7.59 (3.42–16.81) | <0.0001 | 6 (1.6) | 1.57 (0.44–5.63) | 0.49 |
| >30 | 132 (56.7) | 21.43 (14.41–31.86) | <0.0001 | 59 (23.1) | 33.53 (17.59–63.90) | <0.0001 | 46 (17.9) | 13.05 (6.12–27.84) | <0.0001 | 22 (8.5) | 4.41 (1.38–14.11) | 0.01 |
| Cumulative equivalent dose, mg/m2 | ||||||||||||
| None | 148 (16.3) | 1.00 | — | — | — | 31 (2.56) | 1.00 | |||||
| 1–4000 | 37 (16.9) | 0.97 (0.06–1.53) | 0.91 | — | — | — | 12 (4.18) | 2.26 (1.06–4.82) | 0.03 | |||
| 4001–8000 | 54 (15.7) | 1.01 (0.67–1.51) | 0.97 | — | — | — | 15 (2.88) | 1.54 (0.73–3.23) | 0.25 | |||
| 8001–12,000 | 98 (24.6) | 1.55 (1.09–2.19) | 0.02 | — | — | — | 17 (3.48) | 1.59 (0.81–3.11) | 0.18 | |||
| >12,000 | 127 (32.7) | 1.79 (1.28–2.49) | 0.0006 | — | — | — | 45 (8.70) | 2.21 (1.29–3.78) | 0.004 | |||
| Intrathecal chemotherapy | ||||||||||||
| No | 180 (14.9) | 1.00 | — | — | — | 23 (5.6) | 1.00 | |||||
| Yes | 285 (26.9) | 2.38 (1.73–3.28) | <0.0001 | — | — | — | — | — | 10 (1.6) | 1.00 (0.33–3.03) | 0.99 | |
Variable not included in the model.
Abbreviation: RT, radiation therapy.
Table 6.
Univariable Logistic Regression for Risk Factors of CPP
| Variable | CPP (n = 1994) | ||
|---|---|---|---|
| Yes | No | P Value | |
| Sex | |||
| Male | 10 (47.6) | 1024 (51.9) | 0.70 |
| Female | 11 (52.4) | 949 (48.1) | |
| Race/ethnicity | |||
| Non-Hispanic white | 14 (66.7) | 1630 (82.6) | 0.02 |
| Non-Hispanic black | 4 (19.1) | 281 (14.2) | |
| Other | 3 (14.3) | 62 (3.1) | |
| Age at tumor diagnosis, mean (SD), y | 4.17 (2.1) | 4.4 (2.7) | 0.87 |
| Age at SJLIFE, mean (SD), y | 26.1 (4.0) | 31.0 (8.8) | 0.01 |
| HP involvement | |||
| Yes | 10 (47.6) | 36 (1.8) | <0.0001 |
| No | 11 (52.4) | 1937 (98.2) | |
| Stroke | |||
| Yes | 4 (19.1) | 105 (5.3) | 0.02 |
| No | 17 (80.9) | 1868 (94.7) | |
| Seizure | |||
| Yes | 7 (33.3) | 254 (12.9) | 0.01 |
| No | 14 (66.7) | 1719 (87.1) | |
| Hydrocephalus with shunt placement | |||
| Yes | 4 (19.1) | 51 (2.6) | 0.002 |
| No | 17 (80.9) | 1922 (97.4) | |
| HP dose, Gy | |||
| No cranial RT | 10 (47.6) | 1221 (61.9) | 0.004 |
| 1–19.9 | 2 (9.5) | 267 (13.5) | |
| 20–30 | 2 (9.5) | 309 (15.7) | |
| >30 | 7 (33.3) | 175 (8.9) | |
| Dose unknown | — | 1 (0.05) | |
| Alkylating agent | |||
| Yes | 8 (38.1) | 1088 (55.1) | 0.12 |
| No | 13 (61.9) | 885 (44.9) | |
| Intrathecal chemotherapy | |||
| Yes | 3 (14.3) | 921 (46.7) | 0.003 |
| No | 18 (85.7) | 1052 (53.3) | |
Data given as no., (%) unless otherwise indicated.
Abbreviation: RT, radiation therapy.
Cumulative incidence of HP disorders
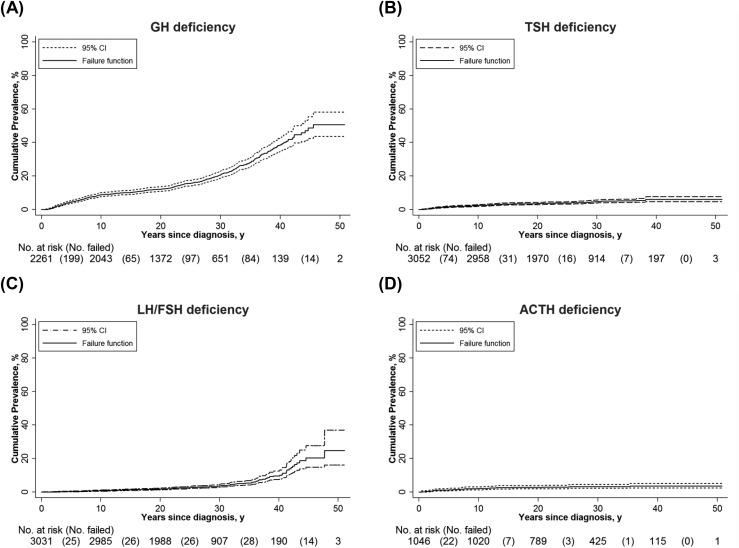
The estimated cumulative incidence at 40 years from cancer diagnosis was 38.7% (95% CI, 34.8 to 42.9) for GHD, 6.1% (95% CI, 4.8 to 7.7) for TSHD, 9.7% (95% CI, 7.5 to 12.5) for LH/FSHD, and 3.6% (95% CI, 2.5 to 5.3) for ACTHD (Fig. 3).
Figure 3.
Cumulative incidence of (A) GHD, (B) TSHD, (C) LH/FSHD, and (D) ACTHD from cancer diagnosis.
Physical health outcomes and HP disorders
In total, 24 of 519 participants (4.6%) with GHD, 163 of 172 participants (94.8%) with TSHD, 70 of 158 participants (44.3%) with LH/FSHD, and 44 of 45 participants (97.8%) with ACTHD were receiving hormone replacement therapy. Independent associations were observed between GHD and short stature, low BMD, frailty, and poor exercise tolerance (Table 7); between TSHD and short stature and poor exercise tolerance; and between untreated LH/FSHD and obesity and low BMD.
Table 7.
Multivariable Logistic Regression Models for HP Disorders and Physical Health Outcomes
| Physical Health Outcome | Clinical Outcome (Yes) | GHD | TSHD | Untreated LH/FSHD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GHD | No GHD | Multivariable Analysis | TSHD | No TSHD | Multivariable Analysis | LH/FSHD | No LH/FSHD | Multivariable Analysis | ||||||||
| No. (%) | No. (%) | No. (%) | OR | 95% CI | P Value | No. (%) | No. (%) | OR | 95% CI | P Value | No. (%) | No. (%) | OR | 95% CI | P Value | |
| Short stature (n = 2231)a | 209 (9.4) | 105 (23.1) | 104 (5.9) | 3.50 | 2.54 to 4.83 | <0.0001 | 33 (28.2) | 176 (8.3) | 1.90 | 1.16 to 3.10 | 0.01 | 16 (20.5) | 193 (9.0) | 0.95 | 0.50 to 1.79 | 0.87 |
| Obesity (n = 2210)b | 825 (37.3) | 199 (44.5) | 626 (35.5) | 1.20 | 0.94 to 1.54 | 0.15 | 48 (41.4) | 777 (37.1) | 0.83 | 0.54 to 1.27 | 0.39 | 49 (63.6) | 776 (36.4) | 2.62 | 1.56 to 4.41 | 0.0003 |
| Low BMD (n = 2035)c | 521 (25.6) | 164 (40.5) | 357 (21.9) | 2.16 | 1.68 to 2.78 | <0.0001 | 49 (47.6) | 472 (24.4) | 1.54 | 0.98 to 2.42 | 0.06 | 32 (55.2) | 489 (24.7) | 2.40 | 1.35 to 4.26 | 0.003 |
| Hypertension (n = 2203)d | 352 (16.0) | — | — | — | — | — | 16 (13.9) | 336 (16.1) | 1.09 | 0.60 to 1.97 | 0.78 | 19 (24.7) | 333 (15.7) | 1.13 | 0.61 to 2.08 | 0.70 |
| Dyslipidemia (n = 2150)e | 112 (5.2) | — | — | — | — | — | 7 (6.3) | 105 (5.2) | 1.31 | 0.56 to 3.07 | 0.53 | 9 (11.8) | 103 (5.0) | 1.49 | 0.67 to 3.30 | 0.32 |
| Abnormal glucose metabolism (n = 2265)f | 140 (6.2) | 40 (7.9) | 100 (5.7) | 1.42 | 0.92 to 2.18 | 0.11 | 13 (8.0) | 127 (6.0) | 1.31 | 0.68 to 2.54 | 0.43 | — | — | — | — | — |
| Frailty (n = 2064)g | 118 (5.7) | 41 (9.7) | 77 (4.7) | 1.87 | 1.21 to 2.91 | 0.01 | 14 (13.5) | 104 (5.3) | 1.87 | 0.96 to 3.65 | 0.07 | 9 (13.0) | 109 (5.5) | 1.57 | 0.71 to 3.50 | 0.26 |
| Poor exercise tolerance (n = 2087)h | 170 (8.2) | 49 (11.9) | 121 (7.2) | 1.51 | 1.02 to 2.23 | 0.04 | 18 (18.2) | 152 (7.7) | 2.15 | 1.17 to 3.95 | 0.01 | 11 (16.2) | 159 (7.9) | 1.44 | 0.69 to 3.03 | 0.33 |
| Cardiomyopathy (n = 2277)i | 139 (6.1) | 21 (4.2) | 118 (6.7) | 0.80 | 0.48 to 1.34 | 0.40 | 4 (2.5) | 135 (6.4) | 0.87 | 0.30 to 2.54 | 0.80 | — | — | — | — | — |
Short stature was adjusted for craniospinal radiotherapy and stem cell transplantation.
Increased body mass index was adjusted for sex, ethnicity/race, age at SJLIFE, cranial radiation therapy and age at cancer diagnosis.
Low BMD was adjusted for obesity (i.e., body mass index >30 kg/m2), prednisone equivalent dose, and total body irradiation.
Hypertension was adjusted for sex, age at SJLIFE, ethnicity/race, obesity, and age at cancer diagnosis.
Dyslipidemia was adjusted for sex, age at SJLIFE, ethnicity/race, obesity, and age at cancer diagnosis.
Abnormal glucose metabolism was adjusted for sex, age at SJLIFE, ethnicity/race, obesity, and age at cancer diagnosis.
Poor exercise tolerance was adjusted for sex, age at SJLIFE, and obesity.
Frailty was adjusted for age at cancer diagnosis and smoking status.
Cardiomyopathy was adjusted for sex, age at SJLIFE, ethnicity/race, obesity, previous anthracycline use, and chest irradiation.
Psychosocial and neurocognitive health outcomes and HP disorders
Substantial associations were observed between TSHD and worse HRQOL in the physical domain (Table 8). Participants with untreated LH/FSHD had higher odds for sexual dysfunction, although this association was only relevant for females. Participants with GHD and TSHD had higher odds of impaired memory and processing speed, compared with participants without these HP disorders.
Table 8.
Multivariable Logistic Regression Models for HP and Psychosocial and Neurocognitive Health Outcomes
| Clinical Outcome (Yes) | GHD | TSHD | Untreated LH/FSHD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GHD | No GHD | Multivariable Analysis | TSHD | No TSHD | Multivariable Analysis | LH/FSHD | No LH/FSHD | Multivariable Analysis | ||||||||
| No. (%) | No. (%) | No. (%) | OR | 95% CI | P Value | No. (%) | No. (%) | OR | 95% CI | P Value | No. (%) | No. (%) | OR | 95% CI | P Value | |
| Psychosocial health outcome | ||||||||||||||||
| PCS (n = 2036)a | 359 (17.6) | 84 (20.1) | 275 (17.0) | 1.26 | 0.91 to 1.75 | 0.16 | 31 (29.5) | 328 (17.0) | 2.38 | 1.45 to 3.92 | 0.0007 | 18 (24.7) | 341 (17.4) | 1.03 | 0.57 to 1.89 | 0.91 |
| GSI (n = 2051)b | 291 (14.2) | — | — | — | — | — | 14 (9.9) | 277 (14.5) | 0.89 | 0.49 to 1.61 | 0.70 | — | — | — | — | — |
| Anxiety (n = 2052)b | 242 (11.8) | — | — | — | — | — | 9 (6.3) | 233 (12.2) | 0.68 | 0.33 to 1.38 | 0.29 | — | — | — | — | — |
| Depression (n = 1992)b | 300 (15.1) | — | — | — | — | — | — | — | — | — | — | 17 (23.9) | 283 (14.7) | 1.75 | 0.97 to 3.14 | 0.06 |
| Sexual dysfunction (n = 1337)c | 438 (32.8) | 104 (40.0) | 334 (31.0) | 1.29 | 0.96 to 1.74 | 0.10 | 36 (46.8) | 402 (31.9) | 1.44 | 0.87 to 2.38 | 0.15 | 25 (54.4) | 413 (32.0) | 2.05 | 1.09 to 3.83 | 0.03 |
| Male sexual dysfunction (n = 645) | 177 (27.4) | 48 (33.3) | 129 (25.8) | 1.31 | 0.86 to 1.99 | 0.20 | — | — | — | — | — | 14 (42.4) | 163 (26.6) | 2.06 | 0.98 to 4.35 | 0.06 |
| Female sexual dysfunction (n = 692) | 261 (37.7) | 56 (48.3) | 205 (35.6) | 1.40 | 0.90 to 2.16 | 0.13 | 23 (50.0) | 238 (36.8) | 1.13 | 0.58 to 2.21 | 0.72 | 250 (36.8) | 11 (84.6) | 6.91 | 1.43 to 33.42 | 0.02 |
| Neurocognitive health outcomed | ||||||||||||||||
| Memory (n = 1370) | 290 (21.2) | 85 (37.8) | 205 (17.9) | 1.74 | 1.21 to 2.52 | 0.003 | 34 (54.8) | 256 (19.6) | 3.46 | 1.91 to 6.27 | <0.0001 | 15 (31.3) | 275 (20.8) | 0.56 | 0.27 to 1.15 | 0.11 |
| Attention (n = 1403) | 255 (18.2) | 62 (24.1) | 193 (16.8) | 1.12 | 0.75 to 1.67 | 0.57 | 27 (30.7) | 228 (17.3) | 1.63 | 0.95 to 2.79 | 0.08 | — | — | — | — | — |
| Processing speed (n = 1173) | 84 (7.2) | 38 (17.0) | 46 (4.9) | 1.80 | 1.01 to 3.21 | 0.05 | 19 (25.7) | 65 (5.9) | 2.12 | 1.07 to 4.21 | 0.03 | — | — | — | — | — |
| Executive function (n = 1194) | 275 (23.0) | 79 (34.1) | 196 (20.4) | 1.14 | 0.77 to 1.67 | 0.52 | 33 (41.3) | 242 (21.7) | 1.55 | 0.90 to 2.66 | 0.11 | |||||
Abbreviations: GSI, Global Severity Index; MCS, Mental Component Summary; PCS, Physical Component Summary.
PCS was adjusted for sex, age at SJLIFE, and cranial radiation therapy.
All parameters of emotional distress (i.e., GSI, anxiety, and depression) were adjusted for age at SJLIFE, sex, cranial radiation therapy, excessive alcohol use, illicit drug use, and smoking status.
Psychosexual dysfunction was adjusted for age at SJLIFE and ethnicity/race.
All four neurocognitive health outcomes were adjusted for cranial radiation therapy. Initially, the interaction term cranial radiation therapy dose and HP disorders was included in the model; however, the P value was >0.1, suggesting that this interaction term should not be included in the final model.
Discussion
Using a large, clinically and systematically assessed cohort of long-term adult survivors of childhood cancer, this study characterized prevalence, risk factors, and comorbidities associated with HP disorders in childhood cancer survivors. Our study findings identify treatment and clinical factors such as alkylating agents and CNS injury that may contribute to HP dysfunction in adult survivors of childhood cancer. HP disorders were also independently associated with impaired physical, sexual, and neurocognitive function. Importantly, because HP disorders were often untreated in our population, hormonal replacement therapy represents an intervention that may improve well-being.
Radiotherapy involving the HP region is a well-established risk factor for HP disorders (7, 8). TSHD and LH/FSHD are generally considered to occur at radiotherapy doses >30 Gy, on the basis of previous studies that did not include comparisons with survivors who did not undergo irradiation (9, 10, 31). Our findings suggest that TSHD and LH/FSHD may occur after HP radiotherapy doses <30 Gy with longer follow-up and that symptomatic individuals should be assessed for these deficits regardless of radiotherapy dose they received.
The contribution of chemotherapy to risk of HP injury remains controversial (2). HP dysfunction after chemotherapy, in the absence of HP exposure to radiotherapy, has only been reported in small case series (32–34). However, chemotherapy has been observed to add to risk conferred by irradiation, although the exact mechanism is unclear (5, 35). Our study demonstrated an important association between alkylating agents and GHD and LH/FSHD. The risk was higher at higher doses of alkylating agents for GHD but not for LH/FSHD. The significance of the association between alkylating agents and GHD was lost after stratification by exposure to HP radiotherapy, which may suggest a facilitating rather than an independent role, as previously reported (5, 35), but the small number of participants available for this subanalysis requires interpreting these data with caution. In contrast, the association between GHD and intrathecal chemotherapy remained substantial only in individuals not exposed to HP radiotherapy. Intrathecal chemotherapy has well-established potential for CNS toxicity but has not previously been linked to the development of HP dysfunction, to our knowledge (32, 36). Whether associations with alkylating agents and intrathecal chemotherapy are due to direct HP toxicity or potentiation of radiotherapy-related damage requires further study.
HP disorders after traumatic brain injury in children have been described with a prevalence ranging from 5% to 57% (37). Pathophysiological mechanisms for HP dysfunction in these children may result from ischemia of the normally highly vascularized pituitary gland associated with low cerebral perfusion (38). Other possible explanations include direct compression or mechanical trauma to the pituitary gland (39, 40). In our study, we observed substantial associations between HP disorders and conditions associated with CNS insult, such as stroke, seizures, and hydrocephalus with shunt placement. Unfortunately, whether the onset of HP disorders preceded the CNS insult could not be assessed with certainty. Therefore, it is unknown if these associations are causal or if they just reflect mutual etiological risk factors (i.e., radiotherapy, CNS tumor or surgery).
Consistent with prior studies, we found that the prevalence of HP disorders was highest among participants with tumor involvement in the HP region (8, 41, 42). As expected, CPP was primarily observed in participants with HP tumor involvement, and at similar percentages between 26% and 29%, as reported in literature on patients with HP tumor location (6, 42). Although CPP has been reported at a rate of 6.6% after radiotherapy in patients without HP tumor involvement, the occurrence of CPP in our cohort was very low (<1%), possibly due to the relatively young age of individuals surviving tumors associated with this condition (6).
This study systematically assesses the prevalence of HP disorders in survivors of childhood cancer with tumors that do not involve the HP region and those not previously exposed to radiotherapy involving the HP region. Among these, a small proportion demonstrated HP dysfunction, with GHD representing the most prevalent condition (6%). In these cases, other potential mechanisms may result in HP damage, such as leukemic infiltration, hydrocephalus with shunt placement, or other CNS insult (43, 44). Because the diagnosis of GHD was based on low IGF-1 levels (a nonstandard diagnostic test), there is a possibility for false-positive GHD cases among these participants. Although validation is required, our results emphasize the importance of monitoring growth and (pubertal) development of all survivors and having a low threshold for evaluation and endocrine referral of survivors with clinical signs of HP disorders, especially for GHD.
In our study, HP disorders significantly affected clinically assessed physical function and self-reported HRQOL in the physical domain, but not emotional well-being. Short stature, low BMD, frailty, and poor exercise tolerance were physical factors associated with GHD. Similar associations have been reported in survivors of childhood cancer, especially in studies comparing GH-treated and -untreated survivors (10, 45, 46). In a previous SJLIFE report, untreated LH/FSHD was also associated with low BMD, likely reflecting the known effect of decreased sex steroid levels on bone health (10). The association between untreated LH/FSHD and obesity may represent a cause or consequence, because testosterone levels tend to be low in obese men (47). Nutritional status may influence HP function and hormonal measures; the statistical analysis cannot establish causality in the context of the potentially bidirectional associations (9). Unexpectedly, we did not observe associations between adverse metabolic outcomes and HP disorders, despite the large proportion of patients who did not receive hormonal replacement therapy (48, 49). Interestingly, untreated LH/FSHD was associated with impaired sexual function. Women and men with LH/FSHD tended to report sexual dysfunction more frequently, although the association was only relevant for women. This is surprising because low testosterone levels in a previous SJLIFE report were strongly associated with impaired sexual functioning (25). It is possible the previous finding resulted from low testosterone levels primarily related to Leydig cell failure rather than central HP damage.
Endocrine disorders contribute to neurocognitive impairment in survivors of childhood cancer, but, to our knowledge, associations with specific endocrine disorders have not been previously identified (50). Our study revealed independent associations between impaired memory and processing speed and GHD and TSHD. Impaired neurocognitive functioning, especially memory, has been observed in small studies including patients with GH deficiency from the general population (51). In addition, hypothyroidism impairs neurocognitive development during childhood or neurocognitive functioning in adulthood, although this evidence originates from studies of primary and congenital forms of hypothyroidism (52). Although the important associations between HP disorders and impaired physical, psychosocial, and neurocognitive outcomes are concerning, these results may be the consequence of shared risk factors, such as CNS irradiation, rather than demonstrating causal relationships (53). These provocative results should be validated in other cohorts.
The current study has several limitations. Campus visits were completed by only 60% of eligible participants and there were differences in characteristics between study participants and nonparticipants. In addition, HP disorders were defined with screening modalities that often require additional testing to confirm the diagnosis. This may have resulted in false-positive and false-negative diagnoses. In particular, the use of a morning cortisol level for the diagnosis of ACTHD may have resulted in underestimating the prevalence of this deficit. Also, the proportions of risk- and nonrisk-based screened participants differ among different HP-axes, which limit the ability to compare overall prevalence and cumulative incidence between different HP axes. Limitations also include assumption of the validity and persistence of previously made diagnoses of HP dysfunction in individuals receiving hormone replacement for these deficits and GHD among those evaluated by dynamic testing before participation in SJLIFE. The use of IGF-1 to diagnose GHD is an additional limitation, given its low sensitivity in making a diagnosis of GHD in individuals treated with radiotherapy (9). Also, the distinction between central and primary endocrine deficits, which may overlap, was not always possible and may have resulted in misclassifying certain individuals. In addition, temporal onset of HP disorders in relation to CNS injury could not be determined. This has limited our ability to establish causal relationships between CNS injury and HP disorders. The need to retrospectively estimate radiotherapy doses, the use of total testosterone levels to assess males for LH/FSHD, and the potential interactions among ongoing treatments such as estrogen, anticonvulsants, and hormonal assays represent additional limitations.
In conclusion, our study demonstrates that survivors of childhood cancer are at increased risk for HP disorders predominantly related to primary cancer location and radiotherapy involving the HP region, but also to intrathecal chemotherapy and alkylating agents, and conditions associated with CNS injury, including seizure, strokes, and hydrocephalus. Survivors with HP disorders experience more adverse physical, sexual, and neurocognitive outcomes compared with survivors without HP disorders. Challenges in current follow-up care include providing adequate screening and hormone replacement therapy of HP disorders for all survivors at risk, as well as attention to the physical and psychosexual effects of these conditions.
Acknowledgments
Financial Support: L.L.R. was supported by the American Lebanese Syrian Associated Charities, the National Cancer Institute (Grants U01 CA195547 and P30 CA021765); L.v.I. was supported by the Royal Netherlands Academy of Arts and Sciences (Ter Meulen Grant TMB17316); and H.M.v.S. was supported by Stichting Kinderen Kankervrij (Grant 282).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
Glossary
Abbreviations:
- ACTHD
ACTH deficiency
- BMD
bone mineral density
- CED
cyclophosphamide equivalent dose
- CNS
central nervous system
- CPP
central precocious puberty
- CTCAE
Common Terminology Criteria for Adverse Events
- GHD
GH deficiency
- HP
hypothalamic-pituitary
- HRQOL
health-related quality of life
- LH/FSHD
LH/FSH deficiency
- SJLIFE
St. Jude Lifetime Cohort
- TSHD
TSH deficiency
References and Notes
- 1. Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, Chemaitilly W, Ehrhardt MJ, Bass J, Bishop MW, Shelton K, Lu L, Huang S, Li Z, Caron E, Lanctot J, Howell C, Folse T, Joshi V, Green DM, Mulrooney DA, Armstrong GT, Krull KR, Brinkman TM, Khan RB, Srivastava DK, Hudson MM, Yasui Y, Robison LL. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crowne E, Gleeson H, Benghiat H, Sanghera P, Toogood A. Effect of cancer treatment on hypothalamic-pituitary function. Lancet Diabetes Endocrinol. 2015;3(7):568–576. [DOI] [PubMed] [Google Scholar]
- 3. Chemaitilly W, Cohen LE. Diagnosis of endocrine disease: endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol. 2017;176(4):R183–R203. [DOI] [PubMed] [Google Scholar]
- 4. Chemaitilly W, Cohen LE, Mostoufi-Moab S, Patterson BC, Simmons JH, Meacham LR, van Santen HM, Sklar CA. Endocrine late effects in childhood cancer survivors. J Clin Oncol. 2018;36(21):2153–2159. [DOI] [PubMed] [Google Scholar]
- 5. Gleeson HK, Stoeter R, Ogilvy-Stuart AL, Gattamaneni HR, Brennan BM, Shalet SM. Improvements in final height over 25 years in growth hormone (GH)-deficient childhood survivors of brain tumors receiving GH replacement. J Clin Endocrinol Metab. 2003;88(8):3682–3689. [DOI] [PubMed] [Google Scholar]
- 6. Chemaitilly W, Merchant TE, Li Z, Barnes N, Armstrong GT, Ness KK, Pui CH, Kun LE, Robison LL, Hudson MM, Sklar CA, Gajjar A. Central precocious puberty following the diagnosis and treatment of paediatric cancer and central nervous system tumours: presentation and long-term outcomes. Clin Endocrinol (Oxf). 2016;84(3):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clement SC, Schouten-van Meeteren AY, Boot AM, Claahsen-van der Grinten HL, Granzen B, Sen Han K, Janssens GO, Michiels EM, van Trotsenburg AS, Vandertop WP, van Vuurden DG, Kremer LC, Caron HN, van Santen HM. Prevalence and risk factors of early endocrine disorders in childhood brain tumor survivors: a nationwide, multicenter study. J Clin Oncol. 2016;34(36):4362–4370. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, Hudson MM, Donaldson SS, King AA, Stovall M, Krull KR, Robison LL, Packer RJ. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sklar CA, Antal Z, Chemaitilly W, Cohen LE, Follin C, Meacham LR, Murad MH. Hypothalamic-pituitary and growth disorders in survivors of childhood cancer: an Endocrine Society Clinical practice guideline. J Clin Endocrinol Metab. 2018;103(8):2761–2784. [DOI] [PubMed] [Google Scholar]
- 10. Chemaitilly W, Li Z, Huang S, Ness KK, Clark KL, Green DM, Barnes N, Armstrong GT, Krasin MJ, Srivastava DK, Pui CH, Merchant TE, Kun LE, Gajjar A, Hudson MM, Robison LL, Sklar CA. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2015;33(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, Spunt SL, Metzger ML, Krull KR, Klosky JL, Srivastava DK, Robison LL. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, Green DM, Mulrooney DA, Armstrong GT, Brinkman TM, Klosky JL, Krull KR, Sabin ND, Wilson CL, Huang IC, Bass JK, Hale K, Kaste S, Khan RB, Srivastava DK, Yasui Y, Joshi VM, Srinivasan S, Stokes D, Hoehn ME, Wilson M, Ness KK, Robison LL. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5):666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, Kleinerman R. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1 Pt 2):141–157. [DOI] [PubMed] [Google Scholar]
- 14. Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, Neglia JP, Sklar CA, Kaste SC, Hudson MM, Diller LR, Stovall M, Donaldson SS, Robison LL. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cann CE, Genant HK, Kolb FO, Ettinger B. Quantitative computed tomography for prediction of vertebral fracture risk. Bone. 1985;6(1):1–7. [DOI] [PubMed] [Google Scholar]
- 16. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One .2009;4(9):e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Physical Activity and Physical Fitness - PAQ 2007. Available at: www.cdc.gov/nchs/data/nhanes/nhanes_07_08/paq07_08_eng.pdf. Accessed 30 September 2019. [Google Scholar]
- 19. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc . 2011;43(8):1575–1581. [DOI] [PubMed] [Google Scholar]
- 20. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 21. Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- 22. Waren JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF‐36 Health Survey. Lincoln, RI: QualityMetric Inc; 2000. [Google Scholar]
- 23. Derogatis L. Brief Symptom Inventory (BSI). Minneapolis, MN: NCS Pearson; 2000. [Google Scholar]
- 24. Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function.Urology.1999;54(2):346–351. [DOI] [PubMed] [Google Scholar]
- 25. van Iersel L, Li Z, Chemaitilly W, Schover LR, Ness KK, Hudson MM, Klosky JL. Erectile dysfunction in male survivors of childhood cancer. JAMA Oncol. 2018;4(11):1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Syrjala KL, Schroeder TC, Abrams JR, Atkins TZ, Brown WS, Sanders JE, Schubert MA, Heiman JR. Sexual function measurement and outcomes in cancer survivors and matched controls. J Sex Res. 2000;37(3):213–225. [Google Scholar]
- 27. Delis D, Kramer J, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed.San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 28. Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third ed.New York, NY: Oxford University Press; 2006. [Google Scholar]
- 29. Conners C. Conners’ Continuous Performance Test II. North Tonawanda, NY: MultiHealth Systems; 2001. [Google Scholar]
- 30. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 31. Constine LS, Woolf PD, Cann D, Mick G, McCormick K, Raubertas RF, Rubin P. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. [DOI] [PubMed] [Google Scholar]
- 32. Rose SR, Schreiber RE, Kearney NS, Lustig RH, Danish RK, Burghen GA, Hudson MM. Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab. 2004;17(1):55–66. [DOI] [PubMed] [Google Scholar]
- 33. Voorhess ML, Brecher ML, Glicksman AS, Jones B, Harris M, Krischer J, Boyett J, Forman E, Freeman AI. Hypothalamic-pituitary function of children with acute lymphocytic leukemia after three forms of central nervous system prophylaxis. A retrospective study. Cancer. 1986;57(7):1287–1291. [DOI] [PubMed] [Google Scholar]
- 34. Birkebaek NH, Fisker S, Clausen N, Tuovinen V, Sindet-Pedersen S, Christiansen JS. Growth and endocrinological disorders up to 21 years after treatment for acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 1998;30(6):351–356. [DOI] [PubMed] [Google Scholar]
- 35. Spoudeas HA, Hindmarsh PC, Matthews DR, Brook CG. Evolution of growth hormone neurosecretory disturbance after cranial irradiation for childhood brain tumours: a prospective study. J Endocrinol. 1996;150(2):329–342. [DOI] [PubMed] [Google Scholar]
- 36. Hasle H, Helgestad J, Christensen JK, Jacobsen BB, Kamper J. Prolonged intrathecal chemotherapy replacing cranial irradiation in high-risk acute lymphatic leukemia: long-term follow up with cerebral computed tomography scans and endocrinological studies. Eur J Pediatr. 1995;154(1):24–29. [DOI] [PubMed] [Google Scholar]
- 37. Casano-Sancho P. Pituitary dysfunction after traumatic brain injury: are there definitive data in children? Arch Dis Child. 2017;102(6):572–577. [DOI] [PubMed] [Google Scholar]
- 38. Ghigo E, Masel B, Aimaretti G, Léon-Carrión J, Casanueva FF, Dominguez-Morales MR, Elovic E, Perrone K, Stalla G, Thompson C, Urban R. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19(9):711–724. [DOI] [PubMed] [Google Scholar]
- 39. Kelly DF, Gonzalo IT, Cohan P, Berman N, Swerdloff R, Wang C. Hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a preliminary report. J Neurosurg. 2000;93(5):743–752. [DOI] [PubMed] [Google Scholar]
- 40. Dusick JR, Wang C, Cohan P, Swerdloff R, Kelly DF. Pathophysiology of hypopituitarism in the setting of brain injury. Pituitary. 2012;15(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kendall-Taylor P, Jönsson PJ, Abs R, Erfurth EM, Koltowska-Häggström M, Price DA, Verhelst J. The clinical, metabolic and endocrine features and the quality of life in adults with childhood-onset craniopharyngioma compared with adult-onset craniopharyngioma. Eur J Endocrinol. 2005;152(4):557–567. [DOI] [PubMed] [Google Scholar]
- 42. Gan HW, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine morbidity after pediatric optic gliomas: a longitudinal analysis of 166 children over 30 years. J Clin Endocrinol Metab. 2015;100(10):3787–3799. [DOI] [PubMed] [Google Scholar]
- 43. Quigg TC, Haddad NG, Buchsbaum JC, Shih CS. Hypothalamic obesity syndrome: rare presentation of CNS+ B-cell lymphoblastic lymphoma. Pediatr Blood Cancer. 2012;59(5):930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Merchant TE, Rose SR, Bosley C, Wu S, Xiong X, Lustig RH. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol. 2011;29(36):4776–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cohen LE, Gordon JH, Popovsky EY, Sainath NN, Feldman HA, Kieran MW, Gordon CM. Bone density in post-pubertal adolescent survivors of childhood brain tumors. Pediatr Blood Cancer. 2012;58(6):959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tamhane S, Sfeir JG, Kittah NEN, Jasim S, Chemaitilly W, Cohen LE, Murad MH. GH therapy in childhood cancer survivors: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(8):2794–2801. [DOI] [PubMed] [Google Scholar]
- 47. Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16(7):581–606. [DOI] [PubMed] [Google Scholar]
- 48. Murray RD, Darzy KH, Gleeson HK, Shalet SM. GH-deficient survivors of childhood cancer: GH replacement during adult life. J Clin Endocrinol Metab. 2002;87(1):129–135. [DOI] [PubMed] [Google Scholar]
- 49. Follin C, Thilen U, Osterberg K, Bjork J, Erfurth EM. Cardiovascular risk, cardiac function, physical activity, and quality of life with and without long-term growth hormone therapy in adult survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2010;95(8):3726–3735. [DOI] [PubMed] [Google Scholar]
- 50. Cheung YT, Brinkman TM, Li C, Mzayek Y, Srivastava D, Ness KK, Patel SK, Howell RM, Oeffinger KC, Robison LL, Armstrong GT, Krull KR. Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2018;110(4):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nyberg F, Hallberg M. Growth hormone and cognitive function. Nat Rev Endocrinol. 2013;9(6):357–365. [DOI] [PubMed] [Google Scholar]
- 52. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158(13):1413–1418. [DOI] [PubMed] [Google Scholar]
- 53. Krull KR, Li C, Phillips NS, Cheung YT, Brinkman TM, Wilson CL, Armstrong GT, Khan RB, Merchant TE, Sabin ND, Srivastava D, Pui CH, Robison LL, Hudson MM, Sklar CA, Chemaitilly W. Growth hormone deficiency and neurocognitive function in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2019;125(10):1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]