Abstract
There is an increasing recognition of patients presenting with cryptococcal meningitis despite having a negative CSF cryptococcal antigen (CrAg). In this report, we describe three cases of patients with advanced immunosuppression who presented to hospital with “false negative” CSF cryptococcal antigen, two of whom had a positive fungal culture. We describe the challenge of CSF-CrAg negative cryptococcal meningitis and explore ways to overcome this challenge using newer diagnostic techniques.
Keywords: Cryptococcal meningitis, Lateral flow assay, Prozone effect, HIV, Cryptococcosis
1. Introduction
Cryptococcal meningitis is responsible for an estimated 15% of AIDS-related mortality worldwide, three-quarters in sub-Saharan Africa [1]. Overtime, the diagnosis of cryptococcosis has improved with newer diagnostic assays like the cryptococcal antigen lateral flow assay which has a better sensitivity and specificity compared to its predecessor the cryptococcal antigen latex agglutination assay [2]. Nevertheless, false positive results have been reported when using the cryptococcal antigen lateral flow assay in patients with reported low cryptococcal antigen titers [3].
There is still a high early mortality rate of 33–41% reported among patients diagnosed with cryptococcal meningitis and may be related to delayed diagnosis, which results in advanced presentation and late commencement of effective antifungal therapy [[4], [5], [6]]. A false-negative cryptococcal antigen (CrAg) in cerebrospinal fluid (CSF) is one of the potential confounders in the diagnosis of cryptococcal meningitis in CSF samples during routine clinical care. Possible causes of a false negative CSF CrAg result in cryptococcal meningitis include poor assay sensitivity or very low CrAg concentrations that may fall below the limit of assay detection. This has been described with very early in the course of meningitis, low-burden of infection, or with variants of weakly-capsulated Cryptococcus sp. [7]. Paradoxically, false negative results can also occur with very high CrAg concentrations, a phenomenon best described as the “postzone” effect.
We present three cases of HIV-seropositive patients with positive serum CrAg and symptoms of meningitis who presented to Mulago National Referral Hospital in Kampala, Uganda with negative cryptococcal antigen in CSF. These cases illustrate disparate mechanisms for false negative CrAg results that can complicate the diagnosis of cryptococcal meningitis.
2. Case 1
A 38-year-old man with newly diagnosed HIV presented to hospital with a 2-week history of severe headache of gradual onset that was associated with blurred vision, hearing loss, altered mental state, and 3 episodes of seizures. He remained ART-naïve, and had no prior history of being treated for meningitis in the past. On physical examination, he was ill-appearing and confused, with a Glasgow Coma Scale (GCS) score of 14/15. He was noted to have a stiff neck and a positive Kerning's sign. Creamy white lesions in the oropharynx were thought consistent with oral thrush. Baseline CD4 T-cell count was 11 cells/μL. The rest of the examination was unremarkable. A finger prick CrAg by lateral flow assay (LFA; Immuno-Mycologics Inc., Norman, OK) was assessed at the bedside and showed a positive reaction, with a titer of >1:2560. A lumbar puncture was performed, and an opening pressure of 500 mmH2O was noted. Bedside CrAg testing of the cerebrospinal fluid (CSF) was performed according to the manufacturer's instructions, and surprisingly showed a negative reaction.
Despite the negative CSF CrAg, the probability of a cryptococcal meningitis was thought to be sufficiently high to warrant further assessment of CSF. After 1:5 dilution of CSF, a repeat CSF CrAg was positive. Evaluation of CSF CrAg using a new semi-quantitative CrAg LFA (CrAg SQ LFA; Immuno-Mycologics, Norman, OK) which we have been evaluating for research purposes was also 3+ positive. Subsequent CSF analysis was as follows: WBC count <5 cells/mm3 with no differential cells seen, Gram stain 2+ yeast cells, no red cells and total protein 27 mg/dl (Table 1). Baseline quantitative culture was positive, with a growth of 600,000 CFU/mL of Cryptococcus sp. after 10 days of incubation.
Table 1.
Summary of CSF results from cases.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| CrAg | |||
| Undiluted CSF | Negative | Negative | Negative |
| 1:10 dilution of CSF | Positive | Positive | Negative |
| CrAg SQ on undiluted CSF | 3+ positive | 4+ positive | Negative |
| Other CSF analysis | |||
| WBC count, cells/mm3 | <5 | 45 | <5 |
| Protein, mg/dL | 27 | 109 | 31 |
| Gram Stain | 2+ yeast cells | 2+ yeast cells | No organisms |
| QCC, CFU/mL | 600,000 | 520,000 | No growth |
Abbreviations: CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; SQ, semi-quantitative; WBC, white blood cell; QCC, quantitative cryptococcal culture; CFU, colony-forming units.
The patient received 7 doses of Amphotericin B (1 mg/kg/day) in addition to oral flucytosine (100 mg/kg/day) and high dose fluconazole (1200 mg/day) for induction therapy. He was discharged in stable condition, and continued to do well through 10-weeks of outpatient follow-up.
2.1. Case 2
A 31-year-old female with HIV, receiving combination antiretroviral therapy (ART) with tenofovir, lamivudine, and nevirapine for 8 years with poor adherence, was admitted as a referral from her primary HIV clinic with a 2-week history of a gradual headache associated with vomiting, blurred vision, seizures and a positive serum CrAg. On examination, a GCS of 15/15 was noted with signs of meningism. Her baseline CD4 T-cell count was 155 cells/μL and serum CrAg titer >1:2560. A lumbar puncture was performed, with an opening pressure of 400 mmH20. A CSF CrAg was noted to be negative.
As in the first case, we had a high index of suspicion for cryptococcal meningitis, and CrAg was positive following a 1:5 dilution of CSF. The semi-quantitative CrAg SQ LFA on undiluted CSF was 4+ positive. Subsequent CSF analysis yielded turbid appearance, WBC 45 cells, protein 109 mg/dl, 100% lymphocytes and 2+ yeast cells on Gram stain. Quantitative CSF culture yielded 520,000 CFU/ml of Cryptococcus sp. after 10 days of incubation.
The patient received 7 doses of Amphotericin B (1 mg/kg/day) in addition to flucytosine (100 mg/kg/day) and high dose fluconazole (1200 mg/day) for induction therapy, and was discharged in good condition. She continued to improve after 10-weeks of outpatient follow-up.
2.2. Case 3
A 36-year-old female with HIV, receiving combination ART with tenofovir, lamivudine, and ritonavir-boosted lopinavir, switched from first-line ART 8 months’ prior because of virologic failure, was admitted as a referral from her primary HIV clinic with a 1-week history of headache associated with photophobia and vomiting. A serum CrAg was obtained prior to referral and was positive, and a CD4 T-cell count was noted to be 75 cells/μL. On examination, a GCS of 15/15 was noted with no meningism. A serum CrAg was repeated, and titer of 1:160 was noted. A lumbar puncture was done with an opening pressure of 80 mmH2O.
A CSF CrAg was noted to be negative on both diluted and undiluted CSF. A semi-quantitative CrAg SQ LFA on undiluted CSF was also negative. Subsequent CSF analysis yielded a clear appearance, WBC<5 cells, protein 31 mg/dl and no organisms were seen on gram stain. An Xpert MTB/RIF performed on CSF was negative, as was mycobacterial lipoarabinomannan (LAM) antigen in urine that was obtained as part of TB screening program. CSF fungal culture was negative after 10 days.
The patient improved after 3 doses of Amphotericin B (1 mg/kg/day) in addition to high dose fluconazole (800 mg/day) and was discharged in stable condition.
3. Discussion
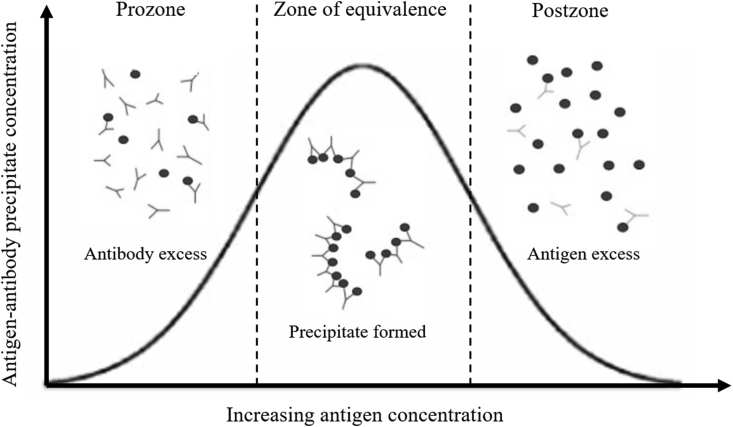
Although the CrAg LFA is highly sensitive test [8], false negative cryptococcal antigen results, as with any test, are possible. The cases above reflect two possible explanations for a false negative cryptococcal antigen test in CSF. We believe that cases 1 and 2 illustrate a postzone phenomenon, which can be a limitation of antigen-antibody capture assays. This phenomenon can occur when an excess of cryptococcal antigen in the setting of a high fungal burden leads to soluble immune complexes and lack of required agglutination reaction (Fig. 1) [9]. Case 3, on the other hand, demonstrates a newly recognized category of cryptococcosis, that has been termed “symptomatic antigenemia”, which is not well understood and may or may not represent true meningitis [10]. In these cases, a “false negative” CSF CrAg may be more a reflection of the disease state itself or an inherent limitation of the lateral flow assay, rather than a performance problem of the CrAg assay used.
Fig. 1.
Variation of antigen-antibody precipitate concentration with antigen concentration at any given antibody concentration.
At any given antibody concentration, the prozone phenomenon is seen when there is failure of development of an adequate antigen-antibody agglutination complex due to excess antibody relative to antigen. Failure of development of an adequate antigen-antibody agglutination complex in postzone phenomenon is due to antigen excess relative to the antibody.
Postzone effect, often mistakenly referred to as prozone effect (historically used to denote access antibody rather than access antigen), usually occurs in the context of extremely high concentrations of cryptococcal antigen, typically greater than 0.140 mg/ml, resulting in weak agglutination reactions and in rare circumstances yielding negative results [11]. The postzone effect in cryptococcal meningitis is thought to occur with a frequency of about 0.6% when using Immy CrAg LFA and has also been reported when using the CrAg latex agglutination assay but data on its frequency is limited [12,13]. However, as the first two cases illustrate, a high index of suspicion for meningitis in the setting of a positive serum CrAg, should prompt further examination of CSF. In our experience, following the semi-quantitative titration procedure described in the IMMY package insert resulted in positive test results at more diluted CSF concentrations, confirming the postzone phenomenon. In addition, we found that CrAg SQ LFA was also positive in the postzone cases. The semi-quantitative CrAg SQ LFA being developed by IMMY has a 98% sensitivity and specificity with an overall qualitative agreement of 99% on serum in reference to CrAg LFA [14]. Despite limited studies on the performance of CrAg SQ LFA in CSF, it appears to have utility in cases of cryptococcal meningitis.
This assay, unlike the standard qualitative CrAg LFA, has an inhibition test line containing immobilized CrAg antigen competing with specimen CrAg for the gold-conjugated CrAg antibody in specimen diluent, in addition to a line containing immobilized anti-CrAg monoclonal antibody. Not only does this allow for semi-quantification, it eliminates false negatives due the postzone phenomenon [15].
In contrast to the first two cases illustrating the postzone effect with advanced cryptococcal meningitis, Case 3 outlines a case of “symptomatic antigenemia” in a patient with meningitis symptoms and a negative CSF CrAg and fungal culture. Unlike postzone, this appears to be a relatively common phenomenon with an occurrence of about 7% in patients suspected of having cryptococcal meningitis. Importantly, in-hospital mortality among patients with symptomatic antigenemia appears to be similar to that of patients with culture confirmed cryptococcal meningitis [10].
While it remains a poorly understood phenomenon, symptomatic antigenemia is thought to be caused by early meningoencephalitis occurring in cryptococcal antigenemia patients as they advance toward developing meningitis. This hypothesis is further supported by a pilot study done by Ramachandran and colleagues who used metagenomics next generation sequencing (mNGS) on stored CSF samples of patients diagnosed with symptomatic antigenemia to demonstrated the presence of low levels of Cryptococcus neoformans DNA [7]. mNGS allows for sequencing the pathogen DNA molecules to generate millions to billions or short or long sequences per instrument run depending on the sequencing technology [16]. Since fungal cultures are also negative in these cases, a negative CSF CrAg can be considered a “false negative” insofar as the diagnostic reference standard used is an uncertain clinical diagnosis.
In conclusion, we have described three cases of “false negative” CSF CrAg in patients with cryptococcal meningitis, two of which were due to the postzone phenomenon and one which was due presumed culture-negative cryptococcal meningitis. These cases, which likely reflect inherent limitations in traditional antigen-capture assays and diagnostic uncertainty in cases of assumed early cryptococcal meningitis, rather than poor diagnostic performance of the CrAg LFA, are relatively common in clinical practice. Symptomatic meningitis in the context of a positive serum CrAg but negative CSF CrAg should prompt enhanced diagnostic testing to rule out the postzone effect such as repeating CrAg on diluted CSF or utilizing new diagnostic assays such as the CrAg SC LFA and mNGS.
Declaration of competing interestCOI
None.
Acknowledgments
The cases described were in the context of research studies supported by the United States United States Fogarty International Center (K01TW010268, R25TW009345), National Institute of Neurological Disorders and Stroke (R01NS086312), National Institute of Allergy and Infectious Diseases (T32AI055433), United Kingdom Medical Research Council/Wellcome Trust/Department for International Development (MRC MR/M007413/1) and Grand Challenges Canada (S4-0296-01).
References
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet. 2017;17(8):873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulware D.R., Rolfes M.A., Rajasingham R., von Hohenberg M., Qin Z., Taseera K., Schutz C., Kwizera R., Butler E.K., Meintjes G., Muzoora C., Bischof J.C., Meya D.B. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg. Infect. Dis. 2014;20(1):45–53. doi: 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubbels M., Granger D., Theel E.S. Low cryptococcus antigen titers as determined by lateral flow assay should be interpreted cautiously in patients without prior diagnosis of cryptococcal infection. J. Clin. Microbiol. 2017;55(8):2472–2479. doi: 10.1128/JCM.00751-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katchanov J., Von Kleist M., Arastéh K., Stocker H. QJM; 2014. “Time-to-amphotericin B” in Cryptococcal Meningitis in a European Low-Prevalence Setting: Analysis of Diagnostic Delays. [DOI] [PubMed] [Google Scholar]
- 5.Deming M., Mark A., Nyemba V., Heil E.L., Palmeiro R.M., Schmalzle S.A. Cognitive biases and knowledge deficits leading to delayed recognition of cryptococcal meningitis. IDCases. 2019;18:e00588. doi: 10.1016/j.idcr.2019.e00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French N., Gray K., Watera C., Nakiyingi J., Lugada E., Moore M., Lalloo D., Whitworth J.A.G., Gilks C.F. AIDS; 2002. Cryptococcal Infection in a Cohort of HIV-1-Infected Ugandan Adults. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran P.S., Cresswell F.V., Meya D.B., Langelier C., Crawford E.D., Derisi J.L., Boulware D.R., Wilson M.R. Detection of cryptococcus DNA by metagenomic next-generation sequencing in symptomatic cryptococcal antigenemia. Clin. Infect. Dis. 2019;68(11):1978–1979. doi: 10.1093/cid/ciy1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen J., Slechta E.S., Gates-Hollingsworth M.A., Neary B., Barker A.P., Bauman S., Kozel T.R., Hanson K.E. Large-scale evaluation of the immuno-mycologics lateral flow and enzyme-linked immunoassays for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin. Vaccine Immunol. 2013;20(1):52–55. doi: 10.1128/CVI.00536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teorell T. Quantitative aspects of antigen-antibody reactions. I. a theory and its corollaries. J. Hyg. (Lond) 1946 doi: 10.1017/S0022172400013449. [DOI] [PubMed] [Google Scholar]
- 10.Ssebambulidde K., Bangdiwala A.S., Kwizera R., Kandole T.K., Tugume L., Kiggundu R., Mpoza E., Nuwagira E., Williams D.A., Lofgren S.M., Abassi M., Musubire A.K., Cresswell F.V., Rhein J., Muzoora C., Hullsiek K.H., Boulware D.R., Meya D.B. Symptomatic cryptococcal antigenemia presenting as early cryptococcal meningitis with negative cerebral spinal fluid analysis. Clin. Infect. Dis. 2019;68(12):2094–2098. doi: 10.1093/cid/ciy817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IMMY . 2020. CrAg LFA Package Insert.https://immy.com/crag [Google Scholar]
- 12.Lourens A., Jarvis J.N., Meintjes G., Samuel C.M. Rapid diagnosis of cryptococcal meningitis by use of lateral flow assay on cerebrospinal fluid samples: influence of the high-dose “hook” effect. J. Clin. Microbiol. 2014;52(12):4172–4175. doi: 10.1128/JCM.01683-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamm A.M., Polt S.S. False-negative cryptococcal antigen test. JAMA, J. Am. Med. Assoc. 1980;244(12):1359. doi: 10.1001/jama.1980.03310120047024. [DOI] [PubMed] [Google Scholar]
- 14.Skipper C., Tadeo K., Martyn E., Nalintya E., Rajasingham R., Meya D.B., Kafufu B., Rhein J., Boulware D.R. Evaluation of serum cryptococcal antigen testing using two novel semiquantitative lateral flow assays in persons with cryptococcal antigenemia. J. Clin. Microbiol. 2020;58(4):e02046–19. doi: 10.1128/JCM.02046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IMMY . 2019. CrAg SQ Lateral Flow Assay Package Insert.www.immy.com [Google Scholar]
- 16.Simner P.J., Miller S., Carroll K.C. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin. Infect. Dis. 2018;66(5):778–788. doi: 10.1093/cid/cix881. [DOI] [PMC free article] [PubMed] [Google Scholar]