Abstract
Brachybacterium is a genus of Gram-positive bacteria that rarely causes infections in humans. Here we report the case of an 8-month-old infant who presented with an acute febrile illness. During the diagnostic process, a blood culture was positive with Gram-positive cocci that were identified as Brachybacterium nesterenkovii by MALDI-TOF. As a result of the unclear clinical significance of this isolate and the continuous febrile state, a second blood culture was taken and returned B. nesterenkovii once more. To our knowledge this is the first time that B. nesterenkovii has been isolated from human blood cultures during the course of a systemic infection.
Keywords: Blood culture, bloodstream infection, Brachybacterium nesterenkovii, human, matrix-assisted laser desorption ionization time-of-flight mass spectrometry
Introduction
With the description of Brachybacterium faecium, the genus Brachybacterium was first proposed by Collins et al. in 1988 [1]. Since then, a total of 22 Brachybacterium species have been identified in various ecological niches, in animal and human specimens [[2], [3], [4]]. Infections in humans, in particular systemic infections, are rare.
Brachybacterium nesterenkovii was first described by Gvozdyak et al. in 1992 and was only the second identified species belonging the genus Brachybacterium [5]. It is non-motile, non-spore-forming, not acid fast, Gram-positive, either cocci or bacilli depending on growth cycle stage, and forms yellowish colonies [5,6]. Brachybacterium nesterenkovii has been isolated from milk products, bioaerosols and root canal infections [5,7,8]. However, to our knowledge, no systemic infection with this particular species has been reported so far. Here we report the first documented case of a bloodstream infection caused by B. nesterenkovii.
Case
An 8-month old male infant was presented to a migrant clinic on the Thailand-Myanmar border with a 2-day history of fever, watery stool, vomiting and reduced urine output. The infant was born at term at an estimated gestational age of 38+2 (weeks+days), to a mother with an unremarkable pregnancy. Before the onset of symptoms, the infant's development was normal, and the vaccination schedule was up to date.
On presentation, the infant was alert with a Blantyre coma scale score of 5. Examination of heart, lungs and the abdomen were unremarkable but there were signs of dehydration and the weight was measured at 7.5 kg (8th centile according to the WHO weight-for-age growth scale). The initial heart rate was 165 beats/minute, the respiratory rate 40 breaths/minute, peripheral oxygen saturation 97% on room air and the body temperature was 38.4°C.
A peripheral malaria smear and a microscopic stool examination for intestinal helminth and protozoal infection were negative. The white blood cell (WBC) count was 11.1 × 109/L (5.82 × 109 to 13.77 × 109/L, population adjusted reference interval (RI) for this age group [9]), with a 74.4% neutrophil predominance in the differential count (RI 9%–50%) and C-reactive protein was 30.6 mg/L (reference <8 mg/L). At 106 g/L (RI 103–150) and 35.1% (RI 30.4%–46.0%), respectively, the haemoglobin and haematocrit were normal. Moreover, an aerobic blood culture was obtained (BacT/ALERT® PF, bioMérieux, Marcy l'Etoile, France).
The infant was admitted and symptomatic treatment in the form of parenteral fluid replacement (20 mL/kg normal saline bolus, followed by a 2 mL/kg Ringer lactate maintenance dose), oral rehydration (240 mL STAT dose) and paracetamol (15 mg/kg four times a day) was provided. Additionally, as per local treatment protocols, zinc (15 mg once daily) and vitamin B1 (50 mg once daily) were supplemented.
On the second day of admission, the infant was still febrile and in addition to the gastrointestinal symptoms, crackles over both lungs had developed and chest indrawing was noted. There were still signs of dehydration and the infant was increasingly irritable. As there was concern of sepsis and as the infant did not tolerate oral medication, empiric antibiotic treatment with parenteral ceftriaxone (50 mg/kg) was commenced.
On day three the BacT/ALERT® microbial detection system indicated a positive result and Gram-positive cocci were seen. The bacterial colonies were non-haemolytic on the sheep blood agar plate, catalase positive, coagulase negative (Staphaurex™ Latex Agglutination Test; Thermo Fisher Scientific, Waltham, MA, USA) and DNase negative. Further identification of the isolate by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) (VITEK MS, bioMérieux, Marcy l'Etoile, France) confirmed B. nesterenkovii with a 99.9% certainty.
As it was not clear whether this isolate represented a true bacteraemia or a contaminant, and as the infant was still febrile and showed clinical symptoms that were compatible with a lower respiratory tract infection, it was decided to repeat the blood culture. In the meantime, the antibiotic regimen was continued and while the result of the second blood culture was still pending, the clinical condition improved and the fever subsided.
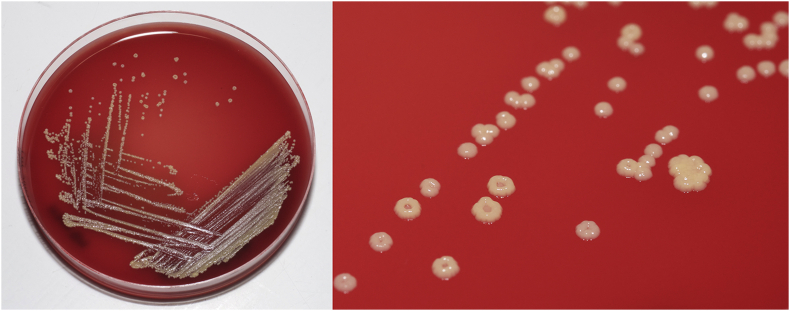
Four days after the second blood culture was taken, the BacT/ALERT® microbial detection system flagged positive and once again non-haemolytic, catalase-positive, coagulase-negative, DNase-negative, Gram-positive cocci with a pale yellow/cream tinge were isolated (Fig. 1). As before, the isolate was identified as B. nesterenkovii by MALDI-TOF with a confidence of 99.9%.
Fig. 1.
Brachybacterium nesterenkovii colonies after 48 hours incubation on blood agar.
When this second result became known 4 days after specimen collection, the infant was already free of symptoms and 7 days of antibiotic treatment were completed. White blood cell counts (11.3 × 109/L) and C-reactive protein (<8 mg/L) were within normal limits in a repeated complete blood count. Anaemia was noted (haemoglobin 95 g/L), so anaemia treatment with ferrous sulphate, folic acid and multivitamin supplementation was prescribed, and the infant was discharged home on day nine after admission. Two weeks after initial presentation, when the infant was followed up in the outpatient department, all symptoms had subsided, and he was fully recovered.
Discussion
In a comprehensive search of published literature, no case of a systemic infection with B. nesterenkovii was identified, making this report the first one, to our knowledge.
Over recent years, MALDI-TOF has become an established tool for accurate identification of a broad spectrum of bacteria in clinical microbiology [10]. With the identification of a broader spectrum of pathogens, clinicians are increasingly confronted with rarely identified bacterial species, and the associated question of whether isolates represent a true infection or a contaminant; misinterpretation of findings can lead to dire consequences [11]. Initially it was not entirely clear whether the isolated bacterium was a true infection or due to contamination. The facts that this particular bacterium was isolated twice from the same patient and no other agent that could explain the patient's symptoms was identified are strong indicators for a true infection [11].
Reports of bacteraemia caused by Brachybacteria in humans are extremely scarce. Unclassified Brachybacteria strains were isolated by lysis filtration from blood samples of children who underwent dental procedures [12]. These isolates were taken from healthy children without signs of systemic infections. Interestingly, Brachybacteria were more often isolated at baseline, before the dental procedures (three before versus two after dental procedures) [12].
To our knowledge there is only one report of a symptomatic systemic infection with a Brachybacterium reported by Tamai et al., who isolated a strain that was closely related to Brachybacterium squillarum in a blood culture [13]. In this region Brachybacteria species have been detected in nasopharyngeal swabs using 16S rRNA sequencing [14], but no systemic infections have been encountered.
The empiric antibiotic choice was based on the patient's presentation and as there was uncertainty of the significance of this isolate, the clinical course determined the subsequent management. As a result of the absence of antimicrobial susceptibility testing protocols, no antibiogram was available. Although the infant was still febrile when the second blood culture was acquired, the overall condition was stable and shortly thereafter the clinical symptoms subsided. Consequently, a full course of ceftriaxone was given and led to clinical cure. Similarly, in the case reported by Tamai et al., cefazolin, a first-generation cephalosporin, was clinically effective for a different Brachybacterium sp.
Brachybacterium nesterenkovii was first isolated from milk products [5] and also recovered from dental root canal infections [8]. Unfortunately, no apparent source of the bloodstream infection was identified in this case.
In conclusion, this is the first documented case of a systemic and symptomatic bloodstream infection in a human caused by B. nesterenkovii.
Acknowledgments
We would like to thank the mother of the infant described in this manuscript for giving consent to share this case and we wish to thank the SMRU clinic and laboratory staff for their hard work and dedication.
Conflict of interest
The authors declare no competing or conflicts of interests.
Funding
The Shoklo Malaria Research Unit is part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme supported by the Wellcome Trust of Great Britain (Major Overseas Programme).
References
- 1.Collins M.D., Brown J., Jones D. Brachybacterium faecium gen. nov., sp. nov., a coryneform bacterium from poultry deep litter. Int J Syst Bacteriol. 1988;38:45–48. [Google Scholar]
- 2.Tuo L., Yan X.-R., Li F.-N., Bao Y.-X., Shi H.-C., Li H.-Y. Brachybacterium endophyticum sp. nov., a novel endophytic actinobacterium isolated from bark of Scutellaria baicalensis Georgi. Int J Syst Evol Microbiol. 2018;68:3563–3568. doi: 10.1099/ijsem.0.003032. [DOI] [PubMed] [Google Scholar]
- 3.Kuete E., Mbogning Fonkou M.D., Mekhalif F., Anani H., Baudoin J.-P., Raoult D. Brachybacterium timonense sp. nov., a new bacterium isolated from human sputum. New Microb New Infect. 2019;31:100568. doi: 10.1016/j.nmni.2019.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekhalif F., Tidjani Alou M., Zgheib R., Lo C.I., Fournier P.-E., Raoult D. Brachybacterium massiliense sp. nov., a new bacterium isolated from stool from a healthy Senegalese child. New Microb New Infect. 2019;31:100588. doi: 10.1016/j.nmni.2019.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gvozdyak O.R., Nogina T.M., Schumann P. Taxonomic study of the genus Brachybacterium: Brachybacterium nesterenkovii sp. nov. Int J Syst Bacteriol. 1992;42:74–78. doi: 10.1099/00207713-42-1-74. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi M., Fang C.X., Yokota A. Taxonomic study of the genus Brachybacterium: proposal of Brachybacterium conglomeratum sp. nov., nom. rev., Brachybacterium paraconglomeratum sp. nov., and Brachybacterium rhamnosum sp. nov. Int J Syst Evol Microbiol. 1995;45:160–168. [Google Scholar]
- 7.Druckenmüller K., Gärtner A., Jäckel U., Klug K., Schiffels J., Günther K. Development of a methodological approach for the characterization of bioaerosols in exhaust air from pig fattening farms with MALDI-TOF mass spectrometry. Int J Hyg Environ Health. 2017;220:974–983. doi: 10.1016/j.ijheh.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Siqueira J.F., Rôças I.N., Paiva S.S.M., Magalhães K.M., Guimarães-Pinto T. Cultivable bacteria in infected root canals as identified by 16S rRNA gene sequencing. Oral Microbiol Immunol. 2007;22:266–271. doi: 10.1111/j.1399-302X.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- 9.Viprakasit V., Suwanthol L., Sangpraypan T., Glomglao W., Utto W., Veerakul G. Hematological parameters and red blood cell indices in healthy Thai children: a revision for 2005. J Med Assoc Thai. 2005;88(Suppl. 8):S188–S196. [PubMed] [Google Scholar]
- 10.Murray P.R. What is new in clinical microbiology—microbial identification by MALDI-TOF mass spectrometry. J Mol Diagn. 2012;14:419–423. doi: 10.1016/j.jmoldx.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall K.K., Lyman J.A. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19:788–802. doi: 10.1128/CMR.00062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonbol H., Spratt D., Roberts G.J., Lucas V.S. Prevalence, intensity and identity of bacteraemia following conservative dental procedures in children. Oral Microbiol Immunol. 2009;24:177–182. doi: 10.1111/j.1399-302X.2008.00492.x. [DOI] [PubMed] [Google Scholar]
- 13.Tamai K., Akashi Y., Yoshimoto Y., Yaguchi Y., Takeuchi Y., Shiigai M. First case of a bloodstream infection caused by the genus Brachybacterium. J Infect Chemother. 2018;24:998–1003. doi: 10.1016/j.jiac.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Salter S.J., Turner C., Watthanaworawit W., de Goffau M.C., Wagner J., Parkhill J. A longitudinal study of the infant nasopharyngeal microbiota: the effects of age, illness and antibiotic use in a cohort of South East Asian children. PLOS Neglect Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005975. [DOI] [PMC free article] [PubMed] [Google Scholar]