Highlights
-
•
Trabectedin confers clinical benefit in patients with recurrent/metastatic uterine leiomyosarcoma.
-
•
Our data are similar to those previously reported in clinical studies.
-
•
Trabectedin is especially effective when administered in earlier lines.
-
•
Trabectedin has a manageable safety profile.
Keywords: Uterine leiomyosarcoma, Metastatic, Trabectedin, Efficacy, GEICO
Abstract
Objective
We assessed trabectedin in patients with advanced uterine leiomyosarcoma (uLMS) in real-life clinical practice given according to the marketing authorization.
Methods
Thirty-six women from 11 tertiary hospitals across Spain who received trabectedin after anthracycline-containing regimen/s were retrospectively analyzed. The primary endpoint was progression-free survival (PFS).
Results
Median PFS and overall survival (OS) since starting trabectedin treatment were 5.4 (95%CI: 3.5–7.3) and 18.5 months (95%CI: 11.5–25.6), respectively. Median OS was significantly higher (P = 0.028) in patients receiving trabectedin in ≤ 2nd line (25.3 months) than in ≥ 3rd (15.1 months) and with ECOG performance status ≤ 1 at trabectedin start (19.8 months) than ECOG 2–3 (6.0 months, P = 0.013). When calculating OS since diagnosis, patients had longer OS with localized disease at diagnosis (87.4 months) vs. locally advanced (30.0 months) or metastatic (44.0 months, P = 0.041); and patients who received adjuvant therapy (87.4 months) compared with those who did not (30.0 months, P = 0.003), especially when receiving radiochemotherapy (106.7 months, P = 0.027). One patient (2.8%) had a complete response (CR) and nine patients (25.0%) achieved a partial response (PR) for an objective response rate of 27.8% with median response duration of 11 months (range: 4–93). Eighteen patients (50.0%) had disease stabilization for a disease control rate (DCR) of 77.8%. More patients receiving trabectedin in 1st-line of advanced disease achieved CR (16.7%) and PR (50.0%) than those in ≥ 2nd line/s (0.0% and 20.0%), whereas the DCR was similar across treatment lines. Reversible neutropenia was the most common grade 3/4 laboratory abnormality (19.4%).
Conclusions
Trabectedin confers clinical benefit in patients with recurrent/metastatic uLMS, given after failure to an anthracycline-based regimen being comparable to those reported in clinical trials and with a manageable safety profile.
1. Introduction
Uterine leiomyosarcoma (uLMS) is an uncommon condition that accounts for approximately 1% of all uterine malignancies, and whose annual incidence is 0.6 per 100,000 women (D'Angelo and Prat, 2010). The uLMS is a very aggressive tumor, with a 5-year survival ranging between 16% and 57% depending on the disease stage at diagnosis (Kapp et al., 2008). In fact, the median overall survival in advanced or recurrent disease is less than 12 months (Zivanovic et al., 2009). Surgical resection is probably the only potential option for cure so far (Korets and Curtin, 2012). Despite adequate surgical resection, 40–70% of patients subsequently develop a recurrence (Arend et al., 2018). Drugs for the treatment of uLMS include doxorubicin (objective response rate [ORR]: 13–25%), ifosfamide (ORR: 17%), and gemcitabine (ORR: 21%) (Look et al., 2004, Amant et al., 2015). Studies evaluating the combination of gemcitabine and docetaxel have reported response rates ranging between 27% and 53%, depending on the number of previous chemotherapy lines (Hensley et al., 2002, Hensley et al., 2008, Hensley et al., 2008). Other cytotoxic agents, such as temozolomide and paclitaxel, have demonstrated more modest results (Gallup et al., 2003, Anderson and Aghajanian, 2005).
Trabectedin (Yondelis®; PharmaMar, S.A., Madrid, Spain) is a natural drug derived from the marine tunicate Ecteinascidia turbinata and currently produced synthetically (Monk et al., 2012). Trabectedin is indicated for the treatment of adult patients with advanced soft tissue sarcoma (STS) after failure of previous treatment with anthracycline and ifosfamide or are not a candidate to receive them (European Medicines Agency, 2019). The efficacy and manageable safety profile of trabectedin for the treatment of advanced uLMS have been demonstrated in diverse clinical trials (Monk et al., 2012, Hensley et al., 2017, Gadducci et al., 2018, Pautier et al., 2015), retrospective studies (Judson et al., 2010, Sanfilippo et al., 2011), and case series (Tewari et al., 2006, Amant et al., 2009, Corrado et al., 2011, Payne et al., 2014, Bongiovanni et al., 2015, Tavella et al., 2017, Nteli et al., 2018, Henry et al., 2019). Gadducci et al. (2018) have recently reported the results from a phase II study on the activity of the single-agent trabectedin 1.3 mg/m2 in 126 pretreated patients with metastatic or locally relapsed uLMS. The study met the condition for trabectedin activity as progression-free survival (PFS) rate after 6 months from the study entry, i.e., the primary end-point of this study, was 35.2% (95% confidence interval, CI 95%CI, 26.2–45) of patients with no difference in PFS according with the number of previous chemotherapy lines. Noteworthy, Hensley et al. (2017) conducted a post hoc subgroup analysis of patients with uLMS pretreated with anthracycline-based therapy in the pivotal phase III SAR-3007study (Demetri et al., 2016), which evaluated the efficacy and safety of trabectedin vs. dacarbazine in patients with unresectable, locally advanced or metastatic liposarcoma or leiomyosarcoma. Overall, 232 of 577 patients had uLMS and were treated with trabectedin 1.5 mg/m2 by 24-hour intravenous infusion (n = 144) or dacarbazine 1 g/m2 by 20–120-minute IV infusion (n = 88) once every three weeks. PFS for trabectedin was 4.0 months compared with 1.5 months for dacarbazine (hazard ratio [HR] = 0.57; 95% CI: 0.41–0.81; p = 0.012). Toxicity profile of trabectedin was similar to that reported in the overall leiomyosarcoma and liposarcoma population and were consistent with the known safety profile of trabectedin. Additionally, a retrospective analysis of 66 previously-treated unselected patients with metastatic uLMS treated with trabectedin 1.0 mg/m2 to 1.5 mg/m2 observed a partial response (PR) in eleven (16%) patients, whereas 23 (35%) patients achieved stable disease (SD) (Sanfilippo et al., 2011). Recorded responses as well as median PFS and OS of 3.3 months and 14.4 months, respectively, were consistent with those seen in more selective populations enrolled in clinical trials.
The aim of this retrospective analysis was to review the data of patients with advanced uLMS treated with anthracycline-containing regimens in tertiary hospitals across Spain to assess the efficacy of trabectedin in real-life routine clinical practice according to SmPC.
2. Methods
2.1. Study design
This retrospective, observational, multicenter study involved patients who received trabectedin after an anthracycline-containing regimen/s for uLMS. Eligible patients were patients ≥ 18 years old with unresectable advanced or metastatic uLMS who had experienced either relapse or disease progression at the time of starting trabectedin treatment. All patients were treated between September 17th, 2007 and April 30th, 2016 with at least three cycles of trabectedin, administered according to the marketing authorization at a recommended dose of 1.5 mg/m2 through a central venous line as a 24-h continuous infusion every 3 weeks. In accordance with the Summary of Product Characteristics (SmPC) (European Medicines Agency, 2019), other eligibility criteria included adequate baseline renal, hepatic and bone marrow function according to laboratory standard parameters before the first treatment cycle and complete recovery from any toxicity derived from prior treatment/s. The only exclusion criterion was not having available information from the medical record. As per SmPC patients treated in 1st-line of advanced disease with trabectedin were not candidates to receive more anthracyclines after having been treated with these drugs in the adjuvant setting. In the present manuscript, first line of treatment in advanced disease represents the first systemic therapy in this setting, regardless adjuvant chemotherapy. All women signed the written consent to participate in the study, except for those who died before the analysis or who were not possible to contact. Procedures were in accordance with the Declaration of Helsinki and approved by Ethics Committees according to national legislation.
2.2. Endpoints and variables
The primary endpoint included the efficacy of trabectedin in relapsed/metastatic disease, as defined by PFS. The PFS was defined as the elapsed time between the date of starting trabectedin treatment (first dose) and disease progression or death, by any cause. Secondary endpoints included the OS, the determination of the best tumor response to trabectedin (with the special attention to the number of treatment line in advanced disease), and the safety profile of trabectedin. The OS was calculated as the elapsed time between the date of starting trabectedin treatment (first dose) and death, by any cause. Tumor response was assessed following the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (Eisenhauer et al., 2009). The ORR was calculated as the sum of complete response (CR) and PR, whereas DCR as the sum of CR, PR, and SD. The adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
2.3. Statistical analysis
Continuous variables were expressed as median and ranges; whereas categorical ones as absolute and relative frequencies (%). Comparison in tumor response to trabectedin regarding the line in advanced disease was performed by an X-square test. Time-to-event endpoints (PFS and OS) and their fixed-time estimations were estimated according to the Kaplan–Meier method and comparisons of survival curves considering characteristics of patients and treatments were done using the log-rank test. Multivariate survival analysis was performed by using Cox regression. The statistical significance was established when P = 0.05. All statistical procedures were carried out by using SPSS version 20 and all data were obtained from medical records.
3. Results
3.1. Characteristics of patients and treatments
Thirty-six patients with metastatic uLMS from 11 Spanish tertiary hospitals were included in the study (Table 1). At diagnosis, patients had a median age of 54 years (range: 29–68), mostly with an Eastern Cooperative Oncology Group (ECOG) performance status 0–1 (92.9%), tumor size ≥ 50 mm (82.1%), and high-grade disease (72.0%). Twenty-one patients (58.4%) received adjuvant therapy, mainly radiotherapy (27.8%), chemotherapy (13.9%), or both (16.7%). A total of 34 patients (out of 36) underwent a hysterectomy for the primary tumor. In the remaining 2 patients, hysterectomy was contraindicated by the metastatic disease. Thirty patients (83.3%) treated with trabectedin had received prior chemotherapy for relapsed disease (median number of lines 2; range 0–3). Six patients had not received any chemotherapy line before trabectedin since they were not candidates to receive more anthracyclines after having been treated with these drugs in the adjuvant setting. The median number of trabectedin cycles was 6 (range: 3–25). Reasons for trabectedin discontinuation included: tumor progression (n = 30), patient’s decision (n = 1), physician’s decision (n = 1), CR (n = 1), and unacceptable toxicity (hematological toxicity in two patients, and hepatic one in one patient).
Table 1.
Demographic and clinical information of patients, and treatments.
| Patients (n = 36) | |
|---|---|
| AT DIAGNOSIS | |
| Age, median years (range) | 54 (29–68) |
| Disease, n (%) | |
| Localized | 19 (52.8) |
| Locally advanced | 4 (11.1) |
| Metastatic | 13 (36.1) |
| Tumor size, n (%) * | |
| <50 mm | 5 (17.9) |
| ≥50 mm | 23 (82.1) |
| Histologic grade, n (%) * | |
| Low | 7 (28.0) |
| High | 18 (72.0) |
| Mitotic index, n (%) * | |
| 0–15/10 HPF | 11 (50.0) |
| >15/10 HPF | 11 (50.0) |
| PREVIOUS TREATMENTS | |
| Adjuvant therapy, n (%) | 21 (58.4) |
| Radiotherapy | 10 (27.8) |
| Chemotherapy | 5 (13.9) |
| Both | 6 (16.7) |
| Chemotherapy prior to trabectedin (any line for relapsed disease), n (%) | 30 (83.3) |
| Number of lines, median (range) | 2 (0–3) |
| Anthracycline therapy immediately before trabectedin, n (%) | 5 (16.7) |
| TRABECTEDIN TREATMENT | |
| Age at onset of trabectedin treatment, median years (range) | 57 (36–70) |
| ECOG performance status at starting treatment, n (%) * | |
| 0–1 | 28 (87.5) |
| 2–3 | 4 (12.5) |
| Number of trabectedin cycles, median (range) | 6 (3–25) |
| Reasons for trabectedin discontinuation, n (%) | |
| Tumor progression | 30 (83.3) |
| Intolerance | 3 (8.3) |
| Complete tumor response | 1 (2.8) |
| Patient’s decision | 1 (2.8) |
| Physician’s decision | 1 (2.8) |
ECOG, Eastern Cooperative Oncology Group; HPF, high-power fields
Data not available for some patients.
3.2. Efficacy
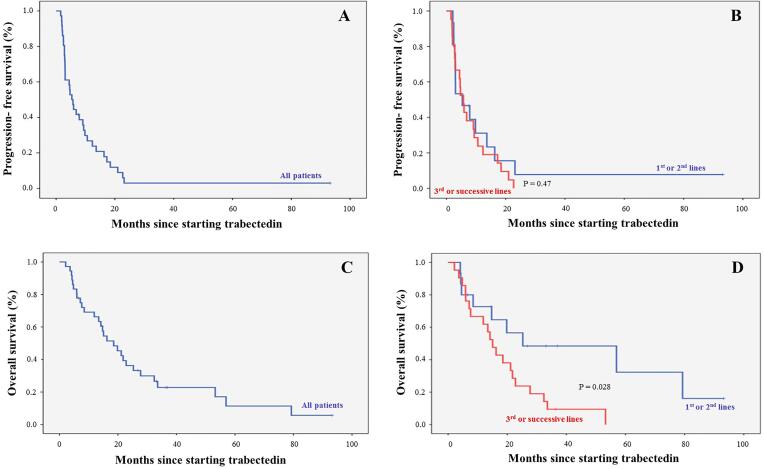
After a median follow-up of 33 months (range: 7–93), median PFS and OS since starting trabectedin treatment were 5.4 months (95%CI: 3.5–7.3; Fig. 1A) and 18.5 months (95%CI: 11.5–25.6; Fig. 1C), respectively. No differences in PFS were found between trabectedin administered in 1st–2nd lines of advanced disease, and 3rd and successive lines (Fig. 1B). In contrast, median OS for patients receiving trabectedin in 1st or 2nd line in advanced disease (25.3 months, 95%CI: 0.0–66.7) was significantly higher than in ≥ 3rd line/s (15.1 months, 95%CI: 10.9–19.2; P = 0.028; Fig. 1D). Furthermore, the median OS was significantly longer in patients with ECOG performance status 0–1 at trabectedin start (19.8 months, 95%CI: 12.9–26.7) than ECOG 2–3 (6.0 months, 95%CI: 2.4–9.6; P = 0.013). When calculating OS since diagnosis patients with localized disease at diagnosis had longer OS (87.4 months, 95%CI: 28.2–146.5) compared with locally advanced (30.0 months, 95%CI: 0.0–68.8) and metastatic disease (44.0 months, 95%CI: 19.4–68.6; P = 0.041). Additionally, patients who received adjuvant therapy had a longer OS (87.4 months, 95%CI: 25.8–148.9) compared with those who did not (30.0 months, 95%CI: 3.9–56.2; P = 0.003), especially when receiving radiotherapy and chemotherapy (106.7 months, 95%CI: 0.0–235.6; P = 0.027) (Table 2). One patient (2.8%) had a CR and nine patients (25.0%) achieved a PR for an ORR of 27.8% with a median response duration of 11 months (4–93; Table 3). Eighteen patients (50.0%) had SD for a DCR of 77.8%. Tumor responses to trabectedin were significantly different (P = 0.041) regarding the line in advanced disease. More patients receiving trabectedin in 1st line of advanced disease achieved CR (16.7%) and PR (50.0%) than those in ≥ 2nd line/s (0.0% and 20.0%, respectively). Similarly, fewer patients in 1st of advanced disease line had SD (16.7%) and PD (16.7%) than in ≥ 2nd line/s (56.7% and 23.3%). The DCR was similar across treatment lines (83.4% in 1st line of advanced disease vs. 76.7% in ≥ 2nd line/s).
Fig. 1.
Progression-free survival and overall survival since starting trabectedin treatment, in all patients and considering the line of treatment in advanced disease.
Table 2.
Survival analysis considering characteristics of patients or treatments.
|
Since trabectedin treatment |
Since diagnosis |
|||||
|---|---|---|---|---|---|---|
| Progression-free survival | P | Overall survival | P | Overall survival | P | |
| Median line of trabectedin treatment of advanced disease | ||||||
| 1st-2nd (n = 15) | 5.4 (0.0–11.1) | 0.470 | 25.3 (0.0–66.7) | 0.028 | 87.4 (22.6–152.1) | 0.310 |
| 3rd or successive (n = 21) | 5.7 (3.8–7.7) | 15.1 (10.9–19.2) | 44.7 (42.4–47.0) | |||
| ECOG at trabectedin start * | ||||||
| 0–1 (n = 28) | 5.4 (3.7–7.0) | 0.260 | 19.8 (12.9–26.7) | 0.013 | 46.0 (32.7–59.9) | 0.930 |
| 2–3 (n = 4) | 3.1 (1.7–4.5) | 6.0 (2.4–9.6) | 30.0 (28.1–31.9) | |||
| Disease at diagnosis | ||||||
| Localized (n = 19) | 3.1 (0.0–6.9) | 0.410 | 21.1 (11.1–31.2) | 0.130 | 87.4 (28.2–146.5) | 0.041 |
| Locally advanced (n = 4) | 3.0 (1.4–4.6) | 4.1 (0.4–7.9) | 30.0 (0.0–68.8) | |||
| Metastatic (n = 13) | 6.8 (3.9–9.8) | 18.5 (8.0–29.1) | 44.0 (19.4–68.6) | |||
| Adjuvant therapy | ||||||
| Yes (n = 21) | 4.6 (0.6–8.6) | 0.830 | 19.8 (11.9–27.8) | 0.170 | 87.4 (25.8–148.9) | 0.003 |
| No (n = 15) | 5.9 (3.3–8.6) | 15.1 (0.0–30.9) | 30.0 (3.9–56.2) | |||
| Type of adjuvant therapy | ||||||
| Radiotherapy (n = 10) | 5.7 (0.0–15.0) | 0.088 | 21.8 (7.8–35.8) | 0.370 | 87.4 (21.9–152.8) | 0.027 |
| Chemotherapy (n = 5) | 13.6 (0.0–33.0) | 18.5 (10.4–26.7) | 63.7 (25.7–101.8) | |||
| Both (n = 6) | 2.9 (2.2–3.6) | 8.5 (3.6–13.4) | 106.7 (0.0–235.6) | |||
Values are expressed as median (range). ECOG, Eastern Cooperative Oncology Group.
Data not available for 4 patients.
Table 3.
Best tumor response to trabectedin.
|
Tumor response, n (%) |
|||||||
|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | ORR | DCR | P | |
| Overall (n = 36) | 1 (2.8%) | 9 (25.0%) | 18 (50.0%) | 8 (22.2%) | 10 (27.8%) | 28 (78.8%) | |
| Trabectedin line of advanced disease | |||||||
| 1st line (n = 6) | 1 (16.7%) | 3 (50.0%) | 1 (16.7%) | 1 (16.7%) | 4 (66.7%) | 5 (83.4%) | 0.041 |
| 2nd or successive lines (n = 30) | 0 (0.0%) | 6 (20.0%) | 17 (56.7%) | 7 (23.3%) | 6 (20.0%) | 23 (76.7%) | |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate.
3.3. Safety
Treatment-emergent AEs observed were consistent with established safety and toxicity profiles of trabectedin. Most frequent hematological AES were reversible neutropenia (11 patients, 30.5%), mainly grade 3/4 (7 patients, 19.4%), and anemia (9 patients, 25.0%), especially grade 1/2 (7 patients, 19.4%), whereas most frequent non-hematological AEs included fatigue (25.0%), nausea (13.9%), and vomiting (11.1%) (Table 4).
Table 4.
Adverse events associated with trabectedin treatment.
| Adverse event, n (%) | Hematological toxicity |
||
|---|---|---|---|
| Overall | Grade 1/2 | Grade 3/4 | |
| Neutropenia | 11 (30.5) | 4 (11.1) | 7 (19.4) |
| Anemia | 9 (25.0) | 7 (19.4) | 2 (5.6) |
| Febrile neutropenia | 2 (5.6) | 0 (0.0) | 2 (5.6) |
| Thrombocytopenia | 1 (2.7) | 0 (0.0) | 1 (2.7) |
| Non-hematological toxicity | |||
| Fatigue | 9 (25.0) | 8 (22.2) | 1 (2.7) |
| Nausea | 5 (13.9) | 5 (13.9) | 0 (0.0) |
| Vomiting | 4 (11.1) | 4 (11.1) | 0 (0.0) |
| Diarrhea | 2 (5.6) | 2 (5.6) | 0 (0.0) |
| Decreased appetite | 2 (5.6) | 2 (5.6) | 0 (0.0) |
| Elevation in ALT | 2 (5.6) | 2 (5.6) | 0 (0.0) |
| Elevation in AST | 1 (2.7) | 1 (2.7) | 1 (2.7) |
| Constipation | 1 (2.7) | 1 (2.7) | 0 (0.0) |
| Myalgia | 1 (2.7) | 1 (2.7) | 0 (0.0) |
| Hypotension | 1 (2.7) | 1 (2.7) | 0 (0.0) |
| Hypocalcemia | 1 (2.7) | 1 (2.7) | 0 (0.0) |
| Mucositis | 1 (2.7) | 1 (2.7) | 0 (0.0) |
| Infection | 1 (2.7) | 0 (0.0) | 1 (2.7) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
4. Discussion
Results from clinical studies have demonstrated that trabectedin is an appropriate second-line option for advance uLMS as it provides long-term tumor stabilization and good tolerability (Hensley et al., 2017, Gadducci et al., 2018, Hindi and Martin-Broto, 2017). Although randomized controlled clinical trials are the gold standard of medical evidence, their applicability to daily clinical practice and generalizability to diverse patient populations needs to be verified in non-interventional studies to gain real-life validity. Despite the fact that the eligibility criteria of this study were less restrictive than those of prospective clinical trials, the patients were treated according to the terms of the marketing authorization. Thus, the results from our study are representative of patient demographics, clinical practice, and outcomes in the real-life practice across Spain.
In the present study patients’ baseline demographic and disease characteristics were comparable with those included in the randomized phase II/III studies (Hensley et al., 2017, Gadducci et al., 2018) (Table 5). In our study, trabectedin administration resulted in an ORR of 27.8%, and a median PFS (primary end-point) and OS of 5.4 and 18.5 months, respectively. Recognizing that comparisons cannot be established, and only with the aim of putting the findings in wider context, the benefit in PFS and OS in this analysis was particularly similar to those observed in the phase II study, which reported a median PFS of 4.1 and a median OS of 20.6 months, respectively (Gadducci et al., 2018). In addition, similar as observed in the phase II study we observed no difference in PFS according to the number of previous chemotherapy lines (Gadducci et al., 2018). In our retrospective, real-life analysis the benefit of trabectedin in PFS was also supported by other secondary endpoints as trabectedin therapy resulted in higher than expected ORR (27.8%) and DCR (77.8%), which compares favorably with previous clinical study reports (Table 5). It is necessary to indicate that in our study the evaluation of trabectedin was carried out after 3 cycles of treatment, in agreement with previous studies suggesting a delated response by trabectedin (Sanfilippo et al., 2011, Le Cesne et al., 2005, Grosso et al., 2007, Le Cesne et al., 2015, Endo et al., 2020).On the other hand, trabectedin-related AEs experienced by our patients were in concordance with extensive prior experience and reports reflecting the well-characterized transient and non-cumulative toxicities of bone marrow suppression and hepatotoxicity (Le Cesne et al., 2015, Samuels et al., 2013). In our study, the patients received a median of 6 trabectedin cycles, suggestive of an acceptable safety profile that allowed prolonged treatment up to 25 cycles. No new or unexpected adverse reactions or qualitative differences in the adverse reactions were observed.
Table 5.
Relevance of our results as compared with prospective clinical data obtained in patients with uLMS.
| Studies | Number of patients (n) |
Median age years (range) | ECOG PS score 1/2 (%) |
CR (%) |
PR (%) |
ORR (%) |
DCR (%) |
Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|---|
|
Phase II study Gadducci et al. 2018 |
115* | 57 (34–76) |
100.0 | 7.0 | 16.5 | 23.4 | 60.8 | 4.1 | 20.6 |
|
Phase III study Hensley et al. 2017 |
232 | 54 (27–81) |
100.0 | 0.0 | 11.0 | 11.0 | 31.0 | 4.0 | 13.4 |
| Real-world outcomes | |||||||||
| Rubio et al. | 36 | 54 (29–68) |
92.9 | 2.8 | 25.0 | 27.8 | 77.8 | 5.4 | 18.5 |
CR, complete response; DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; ORR, overall response rate; OS, overall survival; PFS; progression-free survival; PR, partial response; PS, performance status.
Population per protocol.
Regarding major limitation of this analysis, the retrospective nature and the low number of participating women were the main weaknesses of the study. Therefore, our results should be interpreted with caution as they may be subject to bias.
In conclusion, trabectedin confers clinical benefit in patients with recurrent/metastatic uLMS, after failure to an anthracycline-containing regimen, especially when administered in earlier lines, with the manageable safety profile of trabectedin. Real-life results from the present study are largely comparable to previously shown in clinical studies.
Funding
This study was supported by funding from PharmaMar (Madrid, Spain).
CRediT authorship contribution statement
María Jesús Rubio: Methodology, Investigation, Formal analysis, Writing - original draft, Writing - review & editing, Supervision. María José Lecumberri: Investigation, Writing - review & editing. Silvia Varela: Investigation, Writing - review & editing. Jesús Alarcón: Investigation, Writing - review & editing. María Eugenia Ortega: Investigation, Writing - review & editing. Lydia Gaba: Investigation, Writing - review & editing. Jaime Espinós: Investigation, Writing - review & editing. Julia Calzas: Investigation, Writing - review & editing. Pilar Barretina: Investigation, Writing - review & editing. Isabel Ruiz: Investigation, Writing - review & editing. Gloria Marquina: Investigation, Writing - review & editing. Ana Santaballa: Methodology, Investigation, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
All of the authors declare that have disclosured conflict of interests within the Conflict of Interest Forms.
References
- D'Angelo E., Prat J. Uterine sarcomas: a review. Gynecol. Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Kapp D.S., Shin J.Y., Chan J.K. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112:820–830. doi: 10.1002/cncr.23245. [DOI] [PubMed] [Google Scholar]
- Zivanovic O., Leitao M.M., Iasonos A., Jacks L.M., Zhou Q., Abu-Rustum N.R. Stage-specific outcomes of patients with uterine leiomyosarcoma: a comparison of the International Federation of Gynecology and Obstetrics and American Joint Committee on Cancer Staging Systems. J. Clin. Oncol. 2009;27:2066–2072. doi: 10.1200/JCO.2008.19.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korets S.B., Curtin J.P. Surgical options for recurrent uterine sarcomas. Am. Soc. Clin. Oncol. Educ. Book. 2012:362–366. doi: 10.14694/EdBook_AM.2012.32.70. [DOI] [PubMed] [Google Scholar]
- Arend R.C., Toboni M.D., Montgomery A.M., Burger R.A., Olawaiye A.B., Monk B.J. Systemic treatment of metastatic/recurrent uterine leiomyosarcoma: A changing paradigm. Oncologist. 2018;23:1533–1545. doi: 10.1634/theoncologist.2018-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look K.Y., Sandler A., Blessing J.A., Lucci J.A., 3rd, Rose P.G. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) Study. Gynecol. Oncol. 2004;92:644–647. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Amant F., Lorusso D., Mustea A., Duffaud F., Pautier P. Management strategies in advanced uterine leiomyosarcoma: Focus on trabectedin. Sarcoma. 2015;2015 doi: 10.1155/2015/704124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley M.L., Maki R., Venkatraman E., Geller G., Lovegren M., Aghajanian C. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J. Clin. Oncol. 2002;20:2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- Hensley M.L., Blessing J.A., Degeest K., Abulafia O., Rose P.G., Homesley H.D. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol. Oncol. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley M.L., Blessing J.A., Mannel R., Rose P.G. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol. Oncol. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup D.G., Blessing J.A., Andersen W., Morgan M.A. Evaluation of paclitaxel in previously treated leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol. Oncol. 2003;89:48–51. doi: 10.1016/s0090-8258(02)00136-1. [DOI] [PubMed] [Google Scholar]
- Anderson S., Aghajanian C. Temozolomide in uterine leiomyosarcomas. Gynecol. Oncol. 2005;98:99–103. doi: 10.1016/j.ygyno.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Monk B.J., Dalton H., Benjamin I., Tanović A. Trabectedin as a new chemotherapy option in the treatment of relapsed platinum sensitive ovarian cancer. Curr. Pharm. Des. 2012;18:3754–3769. doi: 10.2174/138161212802002814. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Yondelis (trabectedin). European public assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/yondelis. Accessed date: 24 March 2019.
- B.J. Monk, J.A. Blessing, D.G. Street, C.Y. Muller, J.J. Burke, M.L., A phase II evaluation of trabectedin in the treatment of advanced, persistent, or recurrent uterine leiomyosarcoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 124 (2012) 48–52. [DOI] [PMC free article] [PubMed]
- Hensley M.L., Patel S.R., von Merhren M., Ganjoo K., Jones R.L., Staddon A. Efficacy and safety of trabectedin or dacarbazine for the treatment of patients with uterine leiomyosarcoma after prior chemotherapy: A subgroup analysis of the randomized phase 3 SAR-3007 study. Gynecol. Oncol. 2017;146:531–537. doi: 10.1016/j.ygyno.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadducci A., Grosso F., Scambia G., Raspagliesi F., Colombo N., Grignani G. A phase II randomised (calibrated design) study on the activity of the single-agent trabectedin in metastatic or locally relapsed uterine leiomyosarcoma. Br. J. Cancer. 2018;119:565–571. doi: 10.1038/s41416-018-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautier P., Floquet A., Chevreau C., Penel N., Guillemet C., Delcambre C. Trabectedin in combination with doxorubicin for first-line treatment of advanced uterine or soft-tissue leiomyosarcoma (LMS-02): a non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2015;16:457–464. doi: 10.1016/S1470-2045(15)70070-7. [DOI] [PubMed] [Google Scholar]
- Judson I.R., Blay J., Chawla S.P., Radford J.A., Le Cesne A., Verweij J. Trabectedin (Tr) in the treatment of advanced uterine leiomyosarcomas (U-LMS): Results of a pooled analysis of five single-agent phase II studies using the recommended dose. Clin. Oncol. 2010;28:10028. [Google Scholar]
- Sanfilippo R., Grosso F., Jones R.L., Banerjee S., Pilotti S., D'Incalci M. Trabectedin in advanced uterine leiomyosarcomas: a retrospective case series analysis from two reference centers. Gynecol. Oncol. 2011;123:553–556. doi: 10.1016/j.ygyno.2011.08.016. [DOI] [PubMed] [Google Scholar]
- D. Tewari, B. Saffari, C. Cowan, A.C. Wallick, M.Z. Koontz, B.J., Activity of trabectedin (ET-743, Yondelis) in metastatic uterine leiomyosarcoma. Gynecol. Oncol. 102 (2006) 421–424. [DOI] [PubMed]
- Amant F., Coosemans A., Renard V., Everaert E., Vergote I. Clinical outcome of ET-743 (Trabectedin; Yondelis) in high-grade uterine sarcomas: report on five patients and a review of the literature. Int. J. Gynecol. Cancer. 2009;19:245–248. doi: 10.1111/IGC.0b013e31819c0f59. [DOI] [PubMed] [Google Scholar]
- Corrado G., Salutari V., Fuoco G., Lucidi A., Ferrandina G. Prolonged clinical response to trabectedin in a heavily pretreated patient with advanced uterine leiomyosarcoma: a case report and literature review. Gynecol. Oncol. 2011;121:416–417. doi: 10.1016/j.ygyno.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Payne M.J., Macpherson R.E., Bradley K.M., Hassan A.B. Trabectedin in advanced high-grade uterine leiomyosarcoma: A case report illustrating the value of (18)FDG-PET-CT in assessing treatment response. Case Rep. Oncol. 2014;7:132–138. doi: 10.1159/000355224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni A., Riva N., Ricci M., Mercatali L., Liverani C., La Manna F. Long-lasting activity of trabectedin in refractory uterine leiomyosarcoma: a case report. BMC Cancer. 2015;15:998. doi: 10.1186/s12885-015-2038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavella K., Villanucci A., Vannini L., Lavacchi D., Montelatici S., Amunni G. Stable disease in a patient with metastatic leiomyosarcoma treated with trabectedin. Anticancer Drugs. 2017;28:465–468. doi: 10.1097/CAD.0000000000000485. [DOI] [PubMed] [Google Scholar]
- Nteli V.A., Knauf W., Janton-Klein A., El-Safadi S. Long-lasting response to trabectedin in a patient with metastatic uterine leiomyosarcoma: A case report. Case Rep. Oncol. 2018;11:81–89. doi: 10.1159/000486638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T., Fabre E., Baccar L.S., Lamuraglia M. Longer survival in patients with metastatic uterine leiomyosarcoma treated with trabectedin: A case report. Mol. Clin. Oncol. 2019;10:387–390. doi: 10.3892/mco.2019.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G.D., von Mehren M., Jones R.L., Hensley M.L., Schuetze S.M., Staddon A. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J. Clin. Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hindi N., Martin-Broto J. Current and future systemic treatment options for advanced soft-tissue sarcoma beyond anthracyclines and ifosfamide. Cancer Transl. Med. 2017;3:20–28. [Google Scholar]
- Le Cesne A., Blay J.Y., Judson I., Van Oosterom A., Verweij J., Radford J. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- Grosso F., Jones R.L., Demetri G.D., Judson I.R., Blay J.Y., Le Cesne A. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- Le Cesne A., Ray-Coquard I., Duffaud F., Chevreau C., Penel N., Bui Nguyen B. Trabectedin in patients with advanced soft tissue sarcoma: a retrospective national analysis of the French Sarcoma Group. Eur. J. Cancer. 2015;51:742–750. doi: 10.1016/j.ejca.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Endo M., Takahashi S., Araki N., Sugiura H., Ueda T., Yonemoto T. Time lapse analysis of tumor response in patients with soft tissue sarcoma treated with trabectedin: A pooled analysis of two phase II clinical trials. Cancer Med. 2020;Mar 27 doi: 10.1002/cam4.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels B.L., Chawla S., Patel S., von Mehren M., Hamm J., Kaiser P.E. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann. Oncol. 2013;24:1703–1709. doi: 10.1093/annonc/mds659. [DOI] [PubMed] [Google Scholar]