Abstract
Objective
Transcatheter closure is the first-choice strategy for the management of appropriate patients with patent ductus arteriosus (PDA). The management of large PDAs is challenging due to the limited available sizes of approved devices and the inherent risks of surgical ligation, especially in adults with calcified PDAs. This study aimed to assess the outcomes of the off-label use of large occluders at a tertiary center.
Methods
This retrospective review included patients who underwent transcatheter PDA closure with large occluders (≥16 mm) over 16 years. The baseline patient data, procedural details, angiograms, and immediate outcomes were recorded and patients were followed up at 3, 6, 12 months after the intervention and annually thereafter.
Results
Of the 685 patients who underwent transcatheter PDA closure, 36 patients (mean age 16.6 ± 12.5 years) needed occluders ≥ 16 mm in size. Cocoon duct occluder, Cera duct occluder, Amplatzer atrial septal occluder (ASO), and Cera muscular ventricular septal defect occluders were used for PDA closure. There was no device embolization, one patient in whom ASO was used had residual shunt with intravascular hemolysis requiring surgery, and one patient had mild left pulmonary artery narrowing after the intervention, which was managed conservatively. No patient had residual shunt and one patient had persistent pulmonary hypertension at an intermediate duration of follow-up.
Conclusion
Transcatheter PDA closure with the use of large devices, which are available in Asia and Europe, is an effective and safe method, especially in adolescents and adults. However, a close follow-up of these patients is mandatory.
Keywords: Patent ductus arteriosus, Device closure, Cocoon duct occluder, Cera duct occluder, Large PDA
1. Introduction
Patent ductus arteriosus (PDA), which accounts for approximately 5–10% of all congenital heart defects, is a pathological communication between the descending thoracic aorta distal to the left subclavian artery and the pulmonary artery (PA) due to abnormal persistent patency of the fetal ductus arteriosus.1, 2, 3 The hemodynamic consequences of PDA are determined by the size of the shunt, the difference between systemic and PA pressure and vascular resistance, and the length and narrowest diameter of the PDA.1,2 The natural history of PDA varies from an asymptomatic, incidentally detected defect to congestive heart failure, atrial arrhythmias, endocarditis, ductal aneurysm, pulmonary vascular disease, and Eisenmenger's syndrome.4 The successful closure of PDA reduces mortality and decreases the incidence of endocarditis. Hence, surgical or transcatheter closure is indicated for all patients with PDA except those with small, silent defects and patients with irreversible pulmonary artery hypertension (PAH).1 Transcatheter closure has become the mainstay of treatment for PDA and surgical ligation is reserved for complex, large defects not suitable for device closure or PDA in very young infants and neonates.4,5 Although percutaneous closure is the preferred treatment of PDA for most children as well as adults, many patients, especially adolescents and adults with very large defects, are subjected to surgery because of the lack of appropriate sized devices.6 Most of the available literature of PDA device closure have established the efficacy of the Amplatzer duct occluders (ADO and ADO II, AGA Medical Corporation, Golden Valley, Minnesota, USA).7, 8, 9, 10, 11 The largest available size of the ADO is 16/14 mm and can be used to close PDAs up to 11–12 mm in size.4,7 However, larger devices are available from other manufacturers [i.e. Cera duct occluder, Lifetech Scientific, Shenzhen, China and Cocoon duct occluder, Vascular Innovations, Nonthaburi, Thailand] and can be used for the closure of defects not amenable to ADO and ADO II.4,12 There is limited data regarding the transcatheter closure of PDA with devices ≥16 mm.4,13,14 We hereby report our experience of PDA device closure with the use of large devices, which are not FDA-approved.
2. Methods
2.1. Study design and patient selection
This was a retrospective, single-center review of patients who underwent transcatheter PDA device closure with large occluders (≥16 mm) over the last 16 years (2003–2018) at a tertiary referral center in North India. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki and was reviewed and cleared by the ethics committee of the institute. Pre-procedural informed consent was obtained from all patients or appropriate legally authorized representatives. The baseline patient data, procedure and device-related details, angiograms, and immediate outcomes of all patients were recorded.
2.2. Procedural strategy
A pre-procedure echocardiogram was performed using Philips IE33/Philips EPIQ 7 (Philips Healthcare™, Amsterdam, Netherlands) and all interventions were performed by an experienced operator (the corresponding author). Transcatheter PDA device closure was attempted in consecutive patients with echocardiography proven PDA other than those who developed Eisenmenger's syndrome, had a pulmonary vascular resistance of >8 Woods unit/m2, or irreversible PAH.
The procedure was performed under local anesthesia. A single dose of weight-based intravenous antibiotic (cefotaxime) was administered 30 min prior to the procedure. The femoral artery and femoral vein were accessed using the Seldinger's technique. An aortic angiogram was performed with a 5F or 6F pigtail catheter (Medtronic Co, USA) in the descending thoracic aorta and pressure injector in the extreme lateral and right anterior oblique with 45° cranial angulation to visualize the ductus, its narrowest and widest diameters, and to assess the ductal anatomy. Ductal anatomy was classified as per the classification proposed by Krichenko et al.15 Baseline hemodynamic data, including pressures and shunt calculations, were recorded as deemed appropriate by the operator. The decision regarding the reversibility of PAH was based on multiple factors including clinical examination, chest X-ray, 12-lead ECG, 2-D echocardiogram, and cardiac catheterization. Patients with obvious signs of left ventricular volume overload (enlarged left atrium and left ventricle on echocardiogram) and normal size of right atrium and ventricle, net left-to-right shunt > 1.5:1 by catheterization/echocardiogram, PVR/SVR < 0.5, and pulmonary artery systolic pressure < 2/3rd of the systemic pressure were considered suitable for device closure. In doubtful cases, reversibility testing was done using either vasoreactive agents (100% oxygen) or balloon/device occlusion. After pulmonary vasoreactive testing or balloon/device occlusion, a reduction in the peak PA pressure >20 mm Hg from baseline without any drop in the aortic systolic pressure was considered a sign of reversible PAH (Fig. 1).
Fig. 1.
Catheterization tracings of a representative patient with PDA and pulmonary hypertension before (A) and after (B) device occlusion trial shows a significant reduction in PA pressure (red arrows) without a significant change in the aortic pressure.
The ductus was crossed with a straight tip 0.035″ Terumo guide wire (Terumo Inc, Japan). A 5F multipurpose catheter (MPA) (Medtronic Co, USA) was advanced into the descending thoracic aorta and exchanged with a delivery sheath over an exchange length 0.035” J-tip super-stiff Amplatz wire (Boston Scientific, Natick, MA, USA). This was followed by device positioning and deployment in accordance with the established method.4,7A descending aortogram was performed 10 min after device deployment to confirm the position of the device and evaluate any persistent flow across the device. After 10 min, PA pressure was measured to look for reversibility of PAH. After the confirmation of appropriate device positioning and decrease in PA pressure, the device was released as per the established technique.7 Subsequently, a pullback gradient from ascending to descending aorta was obtained to evaluate any device-induced aortic obstruction. The arterial and venous sheaths were removed and hemostasis achieved with manual compression followed by a tight compression bandage.
2.3. Device details
The device used was based upon the size and anatomy of the PDA and the availability of device sizes. The size of the pulmonary end of the device was at least 2 mm larger than the narrowest diameter of the PDA in children and was twice the narrowest diameter in adolescents and adults with severe PAH. The devices used for occlusion were Cocoon duct occluder, Cera duct occluder, Cera muscular ventricular septal defect occluder (MVSDO) (Lifetech Scientific, Shenzhen, China), and Amplatzer atrial septal occluder (ASO, AGA Medical Corporation, Golden Valley, Minnesota, USA). Custom-made devices were used for five patients who needed devices larger than 24 mm, as previously described.6
2.4. Follow-up
Follow-up echocardiogram was performed on the next day after the catheterization to assess the device position and residual shunt and was repeated at 3, 6, and 12 months after the intervention. Infective endocarditis prophylaxis was advised for the initial 6 months after the procedure. These patients were followed up annually subsequently for a few years after the intervention.
2.5. Statistical analysis
All data were collected retrospectively and entered into a spreadsheet (Microsoft Excel 2016™, Microsoft Corporation, USA). Statistical analysis was done using the Statistical package for social sciences (SPSS Inc. version 23.0™, IBM Corporation, Chicago, USA). All continuous variables were summarized as mean ± SD or median [interquartile range (IQ)]. Categorical variables were described as proportions and frequencies (%).
3. Results
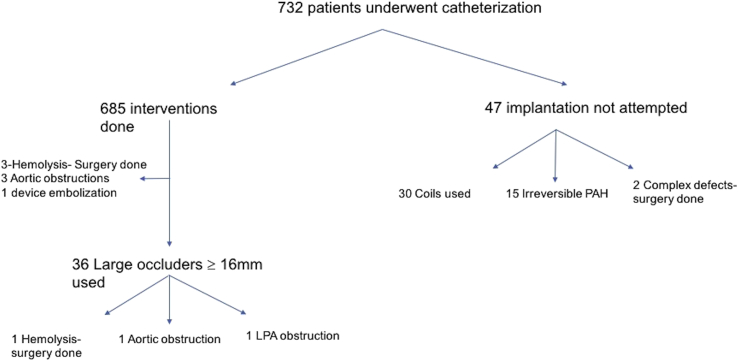
Of the 685 patients who underwent transcatheter device closure during the 16-year study period, 36 patients needed occluders with the size of the pulmonary end ≥16 mm, with the largest device size of 30/28 mm (custom-made Cera duct occluders) in 05 patients (Fig. 2). The mean age was 16.6 ± 12.5 years. The mean of mean PA pressure at baseline was 51.88 ± 24.28 mm Hg (Table 1).
Fig. 2.
STROBE diagram of patients in the study. PAH= pulmonary artery hypertension; LPA = left pulmonary artery.
Table 1.
Characteristics of patients who needed large occluders (≥16 mm).
| CHARACTERISTIC | N = 36 |
|---|---|
| Age (years), mean ± SD | 16.6 ± 12.5 |
| Female, n (%) | 19 (52.77%) |
| Weight (kg), mean ± SD | 39.62 ± 16.7 |
| Mean PA pressure at baseline (mm Hg), mean ± SD | 51.88 ± 24.28 |
| Mean PA pressure after balloon/device occlusion (mm Hg), mean ± SD | 27.2 ± 16.2 |
| PA systolic pressure/aortic systolic pressure ratio at baseline | 0.6 ± 0.2 |
| PA systolic pressure/aortic systolic pressure ratio after balloon/device occlusion | 0.31 ± 0.12 |
| Size of PDA (mm), median (range) | 14 (12–20) |
| PDA anatomy | |
| Type A | 23 |
| Type B | 2 |
| Type C | 11 |
| Type D | 0 |
| Type E | 0 |
| Devices used | |
| Cocoon duct occluder, n | 20 |
| Cera duct occluder, n | 13 |
| Cera MVSDO, n | 2 |
| Amplatzer septal occluder, n | 1 |
| Device sizes used | |
| 18/16 mm, n | 13 |
| 20/18 mm, n | 14 |
| 24/22 mm, n | 1 |
| 24 mm (ASO), n | 1 |
| 24 mm (Cera MVSDO), n | 2 |
| 30/28 mm, n | 5 |
| Procedural complications | |
| Death, n | 0 |
| Device embolization, n | 0 |
| Partial obstruction of PA, n | 1 |
| Aortic obstruction, na | 1 |
| Hemolysis due to residual shunt, n | 1 |
ASO = Amplatzer atrial septal occluder; PA= pulmonary artery; MVSDO = muscular ventricular septal defect occluder; PDA= patent ductus arteriosus.
Types of PDA were in accordance with Krichenko et al.15
Aortic obstruction was managed by removal of unreleased device and using a smaller device.
3.1. Procedural complications
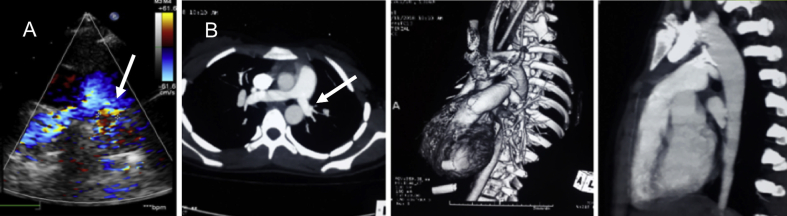
There was no death or device embolization in the study cohort. In one patient, there was 90% obstruction of the aorta before the release of a 30/28 mm Cera occluder, which was tackled by removing the unreleased device and deploying a smaller 24 mm Cera MVSDO, as previously described (Fig. 3).6 One patient developed hemolysis due to residual flow after the deployment of a 24 mm ASO and needed surgical intervention. One patient had partial obstruction of the left PA (gradient 30 mm Hg on echocardiography), which was managed conservatively in view of the absence of symptoms (Table 1). A repeat echocardiogram at 1-year follow-up showed reduction in gradient (15 mm Hg) (Fig. 4). At the end of the procedure, angiographic trivial or mild residual flow across the device was seen in 10 patients.
Fig. 3.
Aortogram of an 11-year-old patient in the 45° right anterior oblique projection shows a 14 mm PDA (A). After positioning a 30/28 mm Cera duct occluder, there was significant obstruction of the aorta (B) by the aortic rim of the device. After downsizing the device to a 24 mm Cera muscular ventricular septal defect occluder, there was no residual shunt or aortic obstruction (C).
Fig. 4.
Follow-up transthoracic echocardiogram shows mild turbulence in the left pulmonary artery (peak gradient 15 mm Hg) (A, arrow). A computed tomography angiogram showed the device-in-situ, normal sized right PA (12 mm) and diffusely narrow left PA (8 mm) (B, arrow).
3.2. Follow-up
Echocardiogram was performed for all patients on post-catheterization day 1 and at 3,6, and 12 months after the intervention. Although one patient with a 20/18 mm Cocoon duct occluder was found to have trivial flow across the device on echocardiogram at 1-year follow-up, an invasive aortogram showed appropriately positioned device with no residual flow. In all the other cases, an echocardiogram at 3-month follow-up showed no residual shunt. While all patients have been followed up for 2 years after the intervention, twenty patients have been followed up for 4 years and are currently asymptomatic without any significant late complications, except one patient described below.
Oral pulmonary vasodilators were stopped in most of the patients since there was a significant fall in PA pressure after the intervention (Table 1). Three patients who continued to have mean PA pressure >50 mm Hg after the intervention were discharged on oral Sildenafil. Of these three patients, pulmonary vasodilators were discontinued in two patients at 3-month follow-up as they had a significant reduction of PA pressure. One patient who had demonstrated >20% percent reduction in PA pressure after PDA occlusion showed increasing tricuspid regurgitation gradient at 3 and 6-month follow-up. At 1-year follow-up, he continued to have an estimated mean PA pressure >50 mm Hg and had class II exertional dyspnea, for which he was managed with Sildenafil and Bosentan. None of the remaining patients demonstrated pulmonary hypertension or left ventricular dysfunction at an intermediate duration of follow-up.
4. Discussion
Although surgical ligation was considered the standard of care for the management of PDA in the 20th century, it is associated with considerable morbidity and a longer duration of hospital stay.16 Surgical ligation or division of the ductus is associated with several complications such as infections, respiratory compromise, recurrent laryngeal nerve palsy, pleural effusion, pneumothorax, and chylothorax.17,18 The major aim of transcatheter techniques of PDA closure is to avoid the small but attendant risks of surgical ligation. Since the advent of coils and percutaneous closure devices, device technology has evolved considerably and a variety of occluders are currently available for the transcatheter closure of PDA. Although the transcatheter closure of various sizes of PDA with the use of several devices is now well established,4,8,9,11, 12, 13,19, 20, 21, 22 there is limited data for the use of devices ≥16 mm in size and patients with very large defects are frequently subjected to MVSDOs, which are much costlier than duct occluders, or to surgical ligation,6 which is associated with several complications due to calcification and friable nature of the ductus and the frequent requirement of cardiopulmonary bypass in adolescents and adults.2,6,23,24 The maximum available size of US-FDA approved devices ADO and ADO II is 16/14 mm and can only be used for PDAs up to 11–12 mm, while there is limited data regarding the use of Amplatzer vascular plug,25 and the PFM Nit-Occlud device has only been tested in patients with PDAs < 4 mm in size.26 Additional data are required to assess the safety and efficacy of non-FDA approved large devices for the management of very large PDAs.
The management of very large PDAs with associated PAH is a challenge and often entails the off-label use of large-sized occluders including Cera duct occluder, Cocoon duct occluder, Occlutech duct occluder, ASO, and MVSDO.4,12, 13, 14,19,21,27 There are limited studies that have assessed the role of these large occluders in transcatheter closure of PDAs. Garcia-Montes et al21 and Froehle et al20 established the role of ASO in the management of large PDA. In the present study, we used a 24 mm ASO in only one patient, who was found to have residual flow after the release and symptomatic intravascular hemolysis, necessitating surgical intervention. Since the ASO has a lower density and a smaller waist height, it may predispose to residual shunt and hemolysis, making it unsuitable for the closure of large PDAs.28 Hence, we did not use the ASO further. Although Thanopoulos et al27 established the role of Amplatzer ventricular septal occluder in large PDA with PAH, it is associated with significant complications.14,29 Additionally, most of these studies have used small-sized (8–14 mm) occluders. In the current study, we could successfully close the PDA with 24 mm Cera MVSDO in two patients. Bhalgat et al29 described the successful transcatheter closure of 10 patients with occluders larger than 14 mm with the use of Cocoon or Cera duct occluders in eight patients and Amplatzer MSVDO in two cases. Although there were some episodes of aortic or LPA obstruction, none of them were hemodynamically significant so as to warrant surgical intervention and none had any progression in peak velocities at follow-up. Additionally, there was only one episode of self-limiting hemolysis. Yang et al28 established the safety of China-made mushroom-shaped occluders (Starway Medical Technology Inc) in 47 patients with PDAs larger than 13 mm. There were no episodes of device dislocation, no LPA/aortic obstruction, and two patients developed self-limiting hemolysis. The current and the previously described cases6 establish safety and efficacy and are consistent with the existing literature on the use of large devices. However, a close lookout for procedural complications is mandatory and as previously reported,6 these devices should be used cautiously in children less than 12 years of age. To the best of our knowledge, our study and a previously published report from China28 are the only studies with a sufficient sample size to assess the role of different devices ≥16 mm in the transcatheter management of PDA.
Although successful transcatheter closure of PDA with severe PAH has been reported, extreme caution is advocated in selection of appropriate patients with reversible PAH for device closure. Clinical examination, chest X-ray, ECG, arterial saturation, echocardiogram, hemodynamic data from cardiac catheterization, and use of pulmonary vasodilator testing help in the clinical decision-making.30 The use of pulmonary vasodilator prior to closure may have a favorable role in post-procedure outcomes in borderline cases.31 However, a small percentage of patients with borderline hemodynamic data may worsen after PDA closure due to progressive and irreversible PAH, and pulmonary vascular disease (PVD),30,32 as seen in one of our patients. Hence, a close clinical and echocardiographic follow-up of these patients is important.
There are several limitations to the study. First, all the limitations of a retrospective observational study apply to our study as well. In view of the non-randomized sample, we are unable to compare the outcomes of device closure vs surgical ligation in the management of large PDAs. Additionally, intermediate duration follow-up data are available for half of the patients and long-term safety and clinical status remain unknown. The data related to Qp/Qs, PVR/SVR at baseline are not available for all patients due to retrospective design and the study dating back to 2003.
To conclude, use of large and custom-made devices appears an attractive method for PDA closure, especially in adolescents and adults, and avoids the complications of open surgery. However, a close follow-up of patients who receive these large devices is mandatory.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
All authors have none to declare.
References
- 1.Schneider D.J., Moore J.W. Patent ductus arteriosus. Circulation. 2006 Oct 24;114(17):1873–1882. doi: 10.1161/CIRCULATIONAHA.105.592063. [DOI] [PubMed] [Google Scholar]
- 2.Baruteau A.-E., Hascoët S., Baruteau J. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis. 2014 Feb 1;107(2):122–132. doi: 10.1016/j.acvd.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell S.C., Korones S.B., Berendes H.W. Congenital heart disease in 56,109 births: incidence and natural history. Circulation. 1971;43:323–332. doi: 10.1161/01.cir.43.3.323. [DOI] [PubMed] [Google Scholar]
- 4.Sinha S.K., Razi M., Pandey R.N. Prospective evaluation of the feasibility, safety, and efficacy of Cocoon Duct Occluder for transcatheter closure of large patent ductus arteriosus: a single-center study with short- and medium-term follow-up results. Anatol J Cardiol. 2017 Nov;18(5):321–327. doi: 10.14744/AnatolJCardiol.2017.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhakar P., Jose J., George O.K. Contemporary outcomes of percutaneous closure of patent ductus arteriosus in adolescents and adults. Indian Heart J. 2018;70(2):308–315. doi: 10.1016/j.ihj.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohit M.K., Gupta A. Transcatheter closure of large patent ductus arteriosus using custom made devices. Cathet Cardiovasc Interv. 2017 May 1;89(6):E194–E199. doi: 10.1002/ccd.25349. [DOI] [PubMed] [Google Scholar]
- 7.Boehm W., Emmel M., Sreeram N. The Amplatzer duct occluder for PDA closure: indications, technique of implantation and clinical outcome. Images Paediatr Cardiol. 2007;9(2):16–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Pass R.H., Hijazi Z., Hsu D.T., Lewis V., Hellenbrand W.E. Multicenter USA Amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol. 2004 Aug 4;44(3):513–519. doi: 10.1016/j.jacc.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 9.Faella H.J., Hijazi Z.M. Closure of the patent ductus arteriosus with the amplatzer PDA device: immediate results of the international clinical trial. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2000 Sep;51(1):50–54. doi: 10.1002/1522-726x(200009)51:1<50::aid-ccd11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Butera G., De Rosa G., Chessa M. Transcatheter closure of persistent ductus arteriosus with the Amplatzer duct occluder in very young symptomatic children. Heart Br Card Soc. 2004 Dec;90(12):1467–1470. doi: 10.1136/hrt.2003.025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruenstein D.H., Ebeid M., Radtke W., Moore P., Holzer R., Justino H. Transcatheter closure of patent ductus arteriosus using the AMPLATZERTM duct occluder II (ADO II) Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2017 May;89(6):1118–1128. doi: 10.1002/ccd.26968. [DOI] [PubMed] [Google Scholar]
- 12.Fiszer R., Szkutnik M., Chodor B., Bialkowski J. Preliminary experience in the use of CERA occluders for closure of different intracardiac and extracardiac shunts. J Invasive Cardiol. 2014 Aug;26(8):385–388. [PubMed] [Google Scholar]
- 13.Fernando R., Koranne K., Loyalka P., Kar B., Gregoric I. Patent ductus arteriosus closure using an Amplatzer(TM) ventricular septal defect closure device. Exp Clin Cardiol. 2013;18(1):e50–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayalakshmi I.B., Setty N., Narasimhan C., Singla V., Manjunath C.N. Percutaneous device closure of patent ductus arteriosus with pulmonary artery hypertension: long-term results. J Intervent Cardiol. 2014;27(6):563–569. doi: 10.1111/joic.12156. [DOI] [PubMed] [Google Scholar]
- 15.Krichenko A., Benson L.N., Burrows P. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63:877–880. doi: 10.1016/0002-9149(89)90064-7. [DOI] [PubMed] [Google Scholar]
- 16.Mavroudis C., Backer C.L., Gevitz M. Forty-six years of patient ductus arteriosus division at Children's Memorial Hospital of Chicago: standards for comparison. Ann Surg. 1994;1220:402–409. doi: 10.1097/00000658-199409000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankowski T., Aboul-Hassan S.S., Marczak J., Szymanska A., Augustyn C., Cichon R. Minimally invasive thoracoscopic closure versus thoracotomy in children with patent ductus arteriosus. J Surg Res. 2017;208:1–9. doi: 10.1016/j.jss.2016.08.097. [DOI] [PubMed] [Google Scholar]
- 18.Chen H., Weng G., Chen Z. Comparison of posterolateral thoracotomy and video-assisted thoracoscopic clipping for the treatment of patent ductus arteriosus in neonates and infants. Pediatr Cardiol. 2011 Apr;32(4):386–390. doi: 10.1007/s00246-010-9863-x. [DOI] [PubMed] [Google Scholar]
- 19.Abdelbasit M.A.E., Alwi M., Kandavello G., Che Mood M., Samion H., Hijazi Z.M. The new Occlutech® PDA occluder: initial human experience. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2015 Jul;86(1):94–99. doi: 10.1002/ccd.25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Froehle M., Haas N.A., Sandica E., Happel C., Laser K.T. Percutaneous closure of a gigantic patent ductus arteriosus (PDA) with pulmonary hypertension with an atrial septal defect occluder in a 35-year-old woman. Clin Res Cardiol Off J Ger Card Soc. 2014 Apr;103(4):319–323. doi: 10.1007/s00392-013-0650-6. [DOI] [PubMed] [Google Scholar]
- 21.García-Montes J.A., Camacho-Castro A., Sandoval-Jones J.P. Closure of large patent ductus arteriosus using the Amplatzer Septal Occluder. Cardiol Young. 2015 Mar;25(3):491–495. doi: 10.1017/S1047951114000183. [DOI] [PubMed] [Google Scholar]
- 22.Bruckheimer E., Godfrey M., Dagan T., Levinzon M., Amir G., Birk E. The Amplatzer Duct Occluder II Additional Sizes device for transcatheter PDA closure: initial experience. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2014 Jun 1;83(7):1097–1101. doi: 10.1002/ccd.25445. [DOI] [PubMed] [Google Scholar]
- 23.Weisz D.E., Giesinger R.E. Surgical management of a patent ductus arteriosus: is this still an option? Semin Fetal Neonatal Med. 2018;23(4):255–266. doi: 10.1016/j.siny.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Lam J.Y., Lopushinsky S.R., Ma I.W.Y., Dicke F., Brindle M.E. Treatment options for pediatric patent ductus arteriosus: systematic review and meta-analysis. Chest. 2015 Sep;148(3):784–793. doi: 10.1378/chest.14-2997. [DOI] [PubMed] [Google Scholar]
- 25.Delaney J.W., Fletcher S.E. Patent ductus arteriosus closure using the Amplatzer® vascular plug II for all anatomic variants. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2013 Apr;81(5):820–824. doi: 10.1002/ccd.24707. [DOI] [PubMed] [Google Scholar]
- 26.Moore J.W., Greene J., Palomares S. Results of the combined U.S. Multicenter Pivotal Study and the Continuing Access Study of the Nit-Occlud PDA device for percutaneous closure of patent ductus arteriosus. JACC Cardiovasc Interv. 2014 Dec;7(12):1430–1436. doi: 10.1016/j.jcin.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Thanopoulos B.D., Tsaousis G.S., Djukic M., Al Hakim F., Eleftherakis N.G., Simeunovic S.D. Transcatheter closure of high pulmonary artery pressure persistent ductus arteriosus with the Amplatzer muscular ventricular septal defect occluder. Heart. 2002 Mar;87(3):260–263. doi: 10.1136/heart.87.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang S.-W., Zhou Y.-J., Hu D.-Y. Feasibility and safety of transcatheter intervention for complex patent ductus arteriosus. Angiology. 2010 May;61(4):372–376. doi: 10.1177/0003319709351874. [DOI] [PubMed] [Google Scholar]
- 29.Bhalgat P.S., Pinto R., Dalvi B.V. Transcatheter closure of large patent ductus arteriosus with severe pulmonary arterial hyperten- sion: short and intermediate term results. Ann Pediatr Cardiol. 2012;5:135–140. doi: 10.4103/0974-2069.99614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tharakan J., Venkateshwaran S. Large patent ductus arteriosus: to close or not to close. Ann Pediatr Cardiol. 2012;5(2):141–144. [PMC free article] [PubMed] [Google Scholar]
- 31.Dimopoulos K., Peset A., Gatzoulis M.A. Evaluating operability in adults with congenital heart disease and the role of pretreatment with targeted pulmonary arterial hypertension therapy. Int J Cardiol. 2008 Sep 26;129(2):163–171. doi: 10.1016/j.ijcard.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph A.M., Nadas A.S. The pulmonary circulation and congenital heart disease. Considerations of the role of the pulmonary circulation in certain systemic-pulmonary communications. N Engl J Med. 1962 Nov 8;267:968–974 contd. doi: 10.1056/NEJM196211082671906. [DOI] [PubMed] [Google Scholar]