Abstract
Cancer cachexia is a multifactorial syndrome characterized by a progressive loss of skeletal muscle mass, along with adipose tissue wasting, systemic inflammation and other metabolic abnormalities leading to functional impairment. Cancer cachexia has long been recognized as a direct cause of complications in cancer patients, reducing quality of life and worsening disease outcomes. Some related conditions, like sarcopenia (age‐related muscle wasting), anorexia (appetite loss) and asthenia (reduced muscular strength and fatigue), share some key features with cancer cachexia, such as weakness and systemic inflammation. Understanding the interplay and the differences between these conditions is critical to advance basic and translational research in this field, improving the accuracy of diagnosis and contributing to finally achieve effective therapies for affected patients.
Keywords: Cachexia, Sarcopenia, Anorexia, Asthenia, Muscle wasting, Cancer
1. Introduction
Coming from the Greek words ‘kakos’ and ‘hexis’, cachexia means ‘bad condition’ and has been clinically described as long as 2000 years ago by Hippocrates.1, 2, 3, 4, 5, 6, 7 Cachexia is a multifactorial syndrome associated with numerous chronic or end stage diseases, such as cancer, acquired immunodeficiency syndrome (AIDS), congestive heart failure, chronic obstructive pulmonary disease, rheumatoid arthritis and tuberculosis among others.2, 5, 7, 8, 9, 10, 11
Cachexia is a complex systemic disease, involving several metabolic pathways in different tissues and organs, and is characterized by systemic inflammation, progressive weight loss and depletion of adipose tissue and skeletal muscle that cannot be fully reversed by conventional nutritional support.2, 3, 7, 12, 13, 14, 15, 16
Metabolically, there is resistance to anabolic signals, an overall catabolic state and a negative energy balance.15 Anorexia, asthenia, sarcopenia and anaemia are also involved in the clinical features of cachexia, contributing to further reduce quality of life.2, 3, 17 Although weight loss is a key feature of cachexia, it is important to emphasize that its wasting process is remarkably different from starvation‐associated wasting.15 Unlike starvation, where lean mass is preserved and adipose tissue is primarily affected, in cachectic patients, the most important event is the wasting of skeletal muscle, with or without fat loss.15, 18 In fact, cachexia shares some similarities and clear differences with other syndromes like age‐related loss of muscle mass (sarcopenia), anorexia, malabsorption, hyperthyroidism and starvation.12, 19
As previously mentioned, cachexia is associated with multiple chronic or end stage conditions and develops through similar pathways, regardless of the primary disease.2, 3, 7, 8, 9, 10, 11, 20, 21 Recently, the wasting process was proposed to follow a specific metabolic pattern, most often associated with advanced stages of the underlying condition, that is characterized by a persistent increase of catabolic turnover and a non‐compensatory anabolic activity.21
Cancer‐associated cachexia has been the most studied and is the best characterized. Cachexia occurs in up to 80% of cancer patients and is recognized as a direct cause of reduced quality of life, contributing to at least 20% of cancer‐associated deaths and limiting therapeutic options for cancer patients.5, 15, 22, 23, 24 It is also associated with high costs concerning the healthcare.25, 26
The specific aetiology and causes of cachexia are complex and only partially understood.12, 27, 28 Consequently, it is very difficult to assess cachexia objectively, particularly in its initial phase.15, 29, 30
It is essential to understand the pathophysiological basis of cancer cachexia and to be able to distinguish it from other related syndromes: only so will we be able to establish an early and accurate diagnosis and adopt timely therapeutic measures.3, 7, 12, 29, 31, 32, 33
In this review, we address these issues, bringing together recent data concerning the molecular signalling pathways involved in cachexia, particularly those that may offer therapeutic opportunities. Similarities and differences with other related syndromes — sarcopenia, anorexia and asthenia — are also discussed.
2. Cancer cachexia
Several research teams have proposed definitions of cachexia and tried to establish criteria for an accurate and timely diagnosis.2, 12, 27, 34, 35, 36 Different frameworks have been proposed, including a generic approach for cachexia associated with any underlying disease and frameworks to specifically assess cancer cachexia.2, 12, 37, 38, 39 In 2011, Fearon et al. proposed the most accepted framework for diagnosing cancer cachexia.2 This approach is based on three key features: a weight loss >5% over past 6 months (in the absence of simple starvation), a body mass index <20 and any degree of weight loss >2%, or an appendicular skeletal muscle index consistent with sarcopenia (male patients <7.26kg/m2, female patients <5,45kg/m2) and any degree of weight loss >2%.2 Because cancer cachexia can co‐occur with obesity, fluid retention and large tumours, all of which can mask weight loss, this framework also recommends a direct measure of muscularity.2, 40
The same framework also defined three stages in cancer cachexia, namely pre‐cachexia, cachexia and refractory cachexia.2, 30 An early diagnosis is much more efficient at preserving the patient's quality of life than a late one.30, 41
The presence of cancer cachexia does not depend on the tumour size.42 In fact, the incidence of cancer cachexia varies with the tumour type: in gastric or pancreatic cancer patients, the incidence is over 80%; nearly 50% of patients with lung, prostate, or colon cancer are affected and approximately 40% of patients with breast tumours, advanced head and neck and some leukaemias develop the syndrome.3, 43, 44, 45 Furthermore, within a given type of cancer, the degree and extension of cachexia depend on tumour stage.13, 27, 46
The pancreatic cancer is the tumour type in which cancer cachexia is more frequent.47 In fact, the pancreatic insufficiency may be previous to the tumour emergence.47 The loss of insulin production can constitute an additional problem that aggravates cachexia.47 The imbalance between insulin production and secretion leads to a loss of the anabolic function of insulin and its metabolic control in all cachectic patients.15, 47 Indeed, several cancer patients present insulin resistance that may be promoted by the tumour.15 This resistance became gradually worst during the cancer cachexia development and promotes muscle wasting.15
Importantly, chemotherapy and radiotherapy may contribute to this cachectic syndrome.13 Chemotherapy exacerbates cancer cachexia, because some chemotherapeutic agents induce pro‐cachectic molecules and can up‐regulate muscle wasting.19, 48, 49, 50 Cancer treatment based on platinum was described to be associated with weight loss, fatigue and inflammation in cancer patients, because it induces pro‐cachectic cytokines and myostatin, leading to muscle wasting.48 For instance, cisplatin is able to regulate muscle wasting through the activation of the nuclear factor‐kappa B (NF‐κB) signalling pathway, which has been convincingly associated with cachexia.48, 49 For the first time, Pin et al. demonstrated that cancer‐induced and chemotherapy‐induced cachexia are characterized by a few shared metabolic abnormalities.19
2.1. Inflammation
Inflammation is a major driver of cachexia, affecting the function of several tissues including skeletal muscle, fat, brain and liver.14, 15 In 1985, Cerami's group proved that circulating mediators could cause cachexia, identifying tumour necrosis factor alpha (TNF‐α), initially termed ‘cachectin’.51, 52 In cancer cachexia, pro‐inflammatory cytokines produced by immune cells and tumour cells most prominently TNF‐α; interleukin‐1, ‐6 and ‐8 (IL‐1, IL‐6 and IL‐8); and interferon gamma (IFNγ) help driving the wasting phenotype associated with this syndrome and are classified as procachetic factors by Argilés and Lopez‐Soriano.3, 15, 53, 54, 55
TNF‐α has a direct catabolic effect on skeletal muscle, by activating the NF‐κB pathway and inducing ubiquitin‐mediated proteasome degradation (UPR) of muscle protein.56, 57, 58, 59 TNF‐α is largely responsible for increased gluconeogenesis, proteolysis and loss of adipose tissue in cachectic patients and is associated with up‐regulation of uncoupling proteins (UCPs) 2 and 3 in cachectic skeletal muscle.28, 56, 60, 61, 62 Additionally, TNF‐α promotes the accumulation of neutrophils and macrophages in skeletal muscle.63 The increase of neutrophils and neutrophil infiltration in the tumour are associated with poor outcomes and more severe manifestations of cachexia.64 Furthermore, neutrophil‐derived proteases and angiotensin II may trigger a cascade of events required for the progression toward cachexia.65 Curiously, it seems that membrane‐bound cathepsin G expressed on neutrophils may generate angiotensin II from angiotensin I and angiotensinogen.66 Furthermore, elevated levels of angiotensin II in plasma may cause muscle protein degradation and inhibition of protein synthesis and thus promote cancer cachexia.65, 67, 68
The association of IFNγ with cachexia is still not clear, but studies have shown that IFNγ can synergize with TNF‐α to promote muscle wasting.15, 58, 69, 70 IFNγ is also an inhibitor of myosin mRNA in skeletal muscle cells, and it is able to activate ubiquitin gene expression.61, 69, 71
Serum concentrations of IL‐1 increase in cachectic patients, but its role in tissue wasting remains a matter of debate.58, 72 On the one hand, IL‐1 is thought to induce anorexia in cachectic patients by increasing tryptophan plasma concentrations, leading to increased serotonin levels and causing early satiety and suppression of appetite.15, 28, 71, 73 Opposite results from other studies show that high circulating IL‐1 failed to affect food intake or weight loss, suggesting that IL‐1 may have a local tissue‐specific effect or must be present in high pharmacologic doses to produce cachectic response.15, 74
The pro‐inflammatory cytokine IL‐6 is found in high levels in cachectic patients and is correlated with weight loss.28, 61, 75 IL‐6 was associated with cachexia in rodent models58, 61, 76 and is thought to induce the activation of inflammatory and catabolic pathways, resulting in the suppression of protein synthesis in muscle cells by Janus kinase (JAK) signalling.58, 61, 75, 76, 77 Inflammation by IL‐6 also induces lipolysis and the browning of the white adipocytes, apparently through up‐regulation of UCP1.3, 78, 79
Elevated serum IL‐8 level was associated with weight loss and was significantly correlated with cachexia in pancreatic cancer patients.54, 55, 80 In gastric cancer patients, an IL‐8 genetic polymorphism was associated with the onset and development cachexia.55, 80
Decreased expression of anti‐inflammatory cytokines such as IL‐4, IL‐10 and IL‐12 accompanies the up‐regulation of pro‐inflammatory cytokines, further disrupting the balance between pro‐ and anti‐inflammatory stimuli.15, 58
2.2. Skeletal and cardiac muscle wasting
The loss of skeletal muscle tissue is a key feature of cancer cachexia and its best studied aspect (Figure 1).3, 4, 23 The muscles are a source of amino acids that may be released for energy production during catabolic processes.81 Muscle homeostasis is maintained by a balance between the synthesis and degradation of muscle protein.15 However, when excessive protein degradation and/or decreased protein synthesis occurs in skeletal muscle, the imbalance can cause muscle wasting and cachexia.4, 15, 81 Skeletal muscle wasting involves several molecular alterations (Figures 1 and 2), all of which are associated with inflammation, protein metabolism, apoptosis and decreased tissue regeneration.3
Figure 1.

Molecular signalling involved in muscle wasting during cancer cachexia. Inflammatory mediators, such as pro‐inflammatory cytokines (interleukin‐1 and tumour necrosis factor‐α) and myostatin, and proteolysis‐inducing factor (PIF), derived from the tumour and/or immune cells, activate intracellular signals. Cytokines and PIF, through nuclear factor‐kappa B (NF‐κB), activate forkhead box O (FOXO) leading to increased transcription of ubiquitin ligase genes—Atrogin 1 and muscle RING finger‐containing protein 1 (MURF1)—that promote muscle protein degradation. The activation of p38 and Janus kinase/mitogen‐activated protein kinase (JAK/MAPK) cascades by PIF, cytokines and myostatin, leads to apoptosis mediated by caspases. Myostatin can also activate protein degradation through FOXOs. Additionally, myostatin may decrease protein synthesis, inhibiting protein kinase B (AKT) through SMAD. Insulin‐like growth factor‐1 (IGF‐1) is decreased during muscle wasting, suppressing the IGF‐1 pathway (dashed lines) and therefore inhibiting protein synthesis. Peroxisome proliferator‐activated receptor‐γ co‐activator 1α (PGC1α) increases uncoupling protein (UCP) expression, leading to mitochondrial dysfunction. The consumption of high levels of amino acids, such as glutamine, by the tumour increases protein breakdown in skeletal muscle, contributing to cancer cachexia. PIFR, PIF receptor; ACTRIIB, activin receptor type IIB; IGF1R, insulin‐like growth factor‐1 receptor; PI3K, phosphatidylinositol 3‐kinase; mTOR, mammalian target of rapamycin; UPR, ubiquitin‐mediated proteasome degradation; REE, resting energy expenditure
Figure 2.

Impaired regeneration capacity during cancer cachexia. Satellite cells are dysregulated during cancer cachexia: although they are able to be activated and proliferate, they cannot complete their differentiation process, because of persistent expression of Paired box 7 (PAX7), via nuclear factor‐kappa B (NF‐κB) activation. PAX7 negatively regulates MyoD and myogenin, which mediate differentiation
There are three main pathways associated with protein degradation described in skeletal muscle: the UPR pathway, the autophagy pathway and calcium‐activated protease calpains.15, 82
The UPR system is specifically up‐regulated by skeletal muscle cells during cancer cachexia through the expression of ubiquitin‐ligases: the muscle RING finger‐containing protein 1 (MURF1) and Atrogin‐1.15, 69, 82, 83 The expression of these ligases involved in muscular proteolysis and muscle wasting is increased by the activation of forkhead box O (FOXO) family transcription factors.3, 15 FOXO activation occurs via NF‐κB signalling, which is activated by cytokines such as TNF‐α and IL‐1 and by proteolysis‐inducing factor (PIF).3, 15, 84
Importantly, the p38 and JAK/mitogen‐activated protein kinase (MAPK) cascades may also be activated by the same cytokines and PIF, activating caspases and, consequently, apoptosis.3 Myostatin, which acts through activin receptor type IIB (ACTRIIB)‐mediated signalling, can also activate protein degradation by the FOXO and can trigger apoptosis via MAPK cascade.3
In the last 15 years, autophagy has also been recognized as a main promoter of proteolysis in skeletal muscle and as an important player in cancer cachexia.3, 15, 82, 85 Calcium‐activated protease calpains have also been associated with initiation of protein breakdown during cachexia.15, 82, 86
In parallel with the activation of catabolic pathways, there is also inhibition of anabolic pathways contributing to muscle wasting during cancer cachexia.15 Insulin‐like growth factor‐1 (IGF‐1) generally activates insulin‐like growth factor‐1 receptor (IGF1R)/phosphatidylinositol 3‐kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signalling, promoting protein synthesis and repressing protein degradation (via FOXO phosphorylation).3 Interestingly, both cancer patients and mouse models of cancer cachexia present low circulating levels of IGF‐1.3, 81, 87 In fact, during muscle wasting, IGF‐1 is decreased, effectively suppressing protein synthesis.3, 4 Additionally, the insulin resistance present in this condition promotes the muscle wasting too.15 Myostatin can also decrease protein synthesis through the activation of the SMAD complex and consequent repression of AKT.3, 88 In addition, myostatin decreases myoblast proliferation because of its inhibition of the myogenic programme.3, 88
Normally, the skeletal muscle regenerates by the activation and differentiation of specific stem cells, known as satellite cells.15, 89 However, in murine cancer models and in patients with cancer cachexia, these cells are dysregulated.4, 15, 89 Although satellite cells can still be activated and proliferate, they cannot differentiate into skeletal muscle cells because of a persistent expression of the self‐renewing factor Paired box 7 (Pax7) mediated by NF‐κB (Figure 2).15, 89 Pax7 negatively acts on MyoD and myogenin expression, essential mediators of differentiation, impairing the regeneration process.89
PIF can also increase protein degradation through the UPR pathway and inhibit protein synthesis by phosphorylating eukaryotic translation initiation factor 2α (eIF2α).3, 50, 84, 90
Moreover, the down‐regulation of protein synthesis and stimulation of protein degradation may result from a decreased availability of amino acids.82 Cancer cells require amino acids, namely glutamine, resulting in low circulating glutamine levels and breakdown of skeletal muscle protein to release amino acids from muscle cells.3, 87, 91 Thus, glutamine supplementation has been suggested to attenuate the muscle wasting in cancer patients.92
Cachectic muscles also show impaired mitochondrial metabolism.3, 15, 50 During cachexia, skeletal muscle mitochondria presents an ineffective ATP synthesis, because the proton gradient that drives this synthesis is disrupted by activation of UCPs (UCP2 and UCP3),3, 15, 93 causing a general increase of resting energy expenditure described in cachectic patients.3, 15, 93 The increased expression of UCPs may be the result of cytokine‐induced stabilization and activation of peroxisome proliferator‐activated receptor‐γ co‐activator 1α (PGC‐1α).3, 50, 94
As with skeletal muscle, the cardiac muscle is an important target in cancer cachexia and the loss of body weight is often accompanied by wasting of cardiac muscle.13, 15, 95, 96 Identical observations were made in laboratory rodents bearing cachexia‐inducing tumours, where cardiac atrophy is frequently observed.97 In the mouse colon‐26 cancer cachexia model, cardiac alterations include marked fibrosis, disrupted myocardial ultrastructure and altered composition of contractile proteins (e.g. troponin I and myosin heavy chain).98
As with skeletal muscle, pro‐inflammatory cytokines play a major role in mediating cardiac dysfunction and NF‐κB inhibition protected tumour bearing mice against cachexia.13, 15, 99 Proteolysis of cardiac muscle is mediated by the UPR, and cachectic tumour‐bearing mice show high levels of protein ubiquitylation and up‐regulated MURF1 and atrogin‐1 expression.13, 98 Furthermore, in mice with cancer cachexia, cardiac atrophy has been associated with growth inhibition through the activation of ACTRIIB signalling mediated by a subset of TGFβ family ligands including myostatin, activin and growth/differentiation factor 11 (GDF11).100
2.3. Adipose tissue: lipolysis and browning
The importance of pathologic changes in adipose tissue has been increasingly recognized in recent years, and some of those changes are involved in cachexia (Figure 3).13, 15, 50, 79 Histologically, there are two types of adipose tissue: white adipose tissue (WAT), which is mostly involved in energy storage in the form of triglycerides, and brown adipose tissue (BAT) involved in thermoregulation.79, 101, 102, 103, 104 Until recently, BAT was thought to be functionally present only on neonates, but in fact is also present in adults.79
Figure 3.
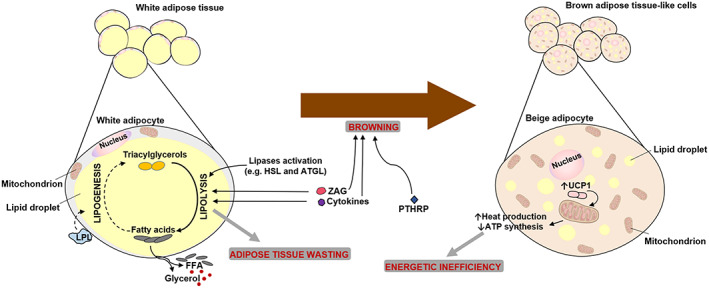
Adipose tissue lipolysis and browning during cancer cachexia. In cancer cachexia, adipose tissue wasting is observed. High levels of circulating free fatty acids (FFA) and glycerol are observed, because of a massive lipolysis in white adipose tissue (WAT), promoted by lipases activation, zinc‐α2‐glycoprotein (ZAG) and cytokines. Additionally, these high levels of FFA may also result from lipoprotein lipase (LPL) decreased activity that reduces lipogenesis (inhibition represented by the dashed line). Moreover, WAT can acquire features of brown adipose cells, a process called ‘WAT browning’. In these beige adipocytes, uncoupling protein 1 (UCP1) is expressed, promoting uncoupling mitochondrial respiration. This results in heat production and less ATP synthesis, leading to an energetic inefficiency. This browning can be promoted by cytokines, ZAG and tumoural‐derived compounds such as parathyroid‐hormone‐related protein (PTHRP). HSL, hormone‐sensitive lipase; ATGL, adipose triglycerides lipase
Cachectic patients often show high levels of circulating free fatty acids and glycerol resulting from a massive lipolysis in WAT through the activation of lipases like hormone‐sensitive lipase and adipose triglycerides lipase.3, 13, 15, 105, 106, 107 IL‐6, TNF‐α and zinc‐α2‐glycoprotein (ZAG, also called lipid‐mobilizing factor) are able to promote lipolysis in cancer cachexia.78, 79, 84, 106, 108 Additionally, inhibition of adipogenesis resulting from down‐regulation of adipogenic factors and reduced lipogenesis because of decreased activity of lipoprotein lipase (LPL) may also contribute to WAT wasting during cancer cachexia.3, 79, 106, 109, 110
During cancer cachexia, WAT cells may acquire some of the features that characterize BAT cells (‘beige adipocytes’),3, 50 a process known as ‘WAT browning’ that takes place during the initial steps of cancer cachexia, before muscle wasting.13, 70, 101, 105, 111 BAT is an active tissue associated with hypermetabolism because of the presence of UCP1.13 When the switch from WAT to BAT‐like cells (beige adipocytes) occurs, UCP1 expression is increased and promotes uncoupling mitochondrial respiration towards thermogenesis rather than ATP synthesis, leading to heat production and energetic inefficiency.13, 50, 70, 102 Thus, WAT browning contributes to increased systemic energy expenditure and, therefore, to cancer cachexia.13, 70 WAT browning can be triggered by pro‐inflammatory mediators, such IL‐6, ZAG, or by tumour‐derived compounds like parathyroid‐hormone‐related protein (PTHRP).50, 70, 79, 112, 113
2.4. Liver
The liver undergoes multiple metabolic and histological changes during cancer cachexia that help driving tissue wasting and also reflect hepatic overload and damage.13, 114, 115, 116
In the context of cancer, the liver often contributes to amplify systemic inflammation by producing acute phase proteins, which may help driving the breakdown of muscle proteins into amino acids.13, 15, 114, 116 It occurs an increase in the hepatic production of glucose and in the Cori Cycle activity.15, 26, 117 Protein breakdown produces a flow of nitrogen in form of amino acids, mainly alanine into the liver.3, 13 Hepatic gluconeogenesis is then able to use amino acids, glycerol (from adipose tissue), and lactate to produce glucose.3, 15 However, this pathway is extremely demanding, contributing to energetic inefficiency and a negative balance, promoting hepatic damage and weight loss.3, 5, 15, 118 In fact, hepatomegally has been described in cachectic patients in association with increased IL‐6 circulating levels and hepatic metabolic overload.15, 114, 115, 119 Inflammation and steatosis are typical features of hepatic damage associated with and cachexia.15, 78, 120, 121
2.5. Brain
In cancer patients, the brain areas that control energy homeostasis may suffer from functional alterations that may contribute to the onset and progression of some features of cancer cachexia, including anorexia, and increased catabolism in muscles and adipose tissue.50, 122, 123 Neuroinflammation seems to accompany tumour growth and to contribute to cancer cachexia, because hypothalamic inflammation can act both as receiver and as amplifier of systemic inflammation.50, 122, 123 Inflammation increases serotonin availability in the hypothalamus.13, 122, 124, 125 The alteration of serotonin levels was associated with changes in food intake of cachectic tumour‐bearing mice.124 Using murine‐derived neuropeptide‐Y (NPY)‐secreting hypothalamic cell lines, it was described that the serotonin can reduce the secretion levels of hypothalamic NPY, leading to a decrease of food intake.122, 124
Hypothalamic inflammation with increased IL‐1β levels also triggers the hypothalamic‐pituitary‐adrenal axis, resulting in increased release of glucocorticoids that promote the breakdown of adipose tissue and skeletal muscle, directly causing cachexia.50, 122, 123
2.6. The gastrointestinal tract and the gut microbiota
In cancer patients, altered intestinal permeability may occur because of the cytotoxic agents used in cancer therapies.126 These intestinal permeability alterations together with translocation of bacteria or bacterial components (e.g. lipopolysaccharide) may also contribute to systemic inflammation and, ultimately, to cachexia.13, 15, 127 Gut barrier dysfunction is a syndrome characterized by dysruption and leakage of the gut epithelial barrier.13, 128 Experimental data from a mouse model of colon cancer cachexia show that dysruption of the gut barrier accompanied tumour growth, leading to endotoxemia and systemic inflammation.128 Gut barrier dysfunction is also associated with malabsorption of nutrients, further contributing to the energy imbalance observed in cancer cachectic patients.13
Changes in the gut microbiota may also play a role in cancer cachexia, as different bacterial strains have different abilities to produce nutrients and pro‐inflammatory molecules that may induce muscle and adipose tissue wasting.13, 129 Experimental data from mouse models support this hypothesis and help associating alterations in the gut microbiome with cancer cachexia.130, 131
Ghrelin, a peptide that is mainly produced in the stomach and increases appetite, stimulating hunger and food intake, is highly increased in patients with cancer cachexia.3, 13, 132, 133, 134, 135 Ghrelin can also directly affect skeletal muscle cells, inhibiting protein degradation induced by cytokines.3, 136, 137, 138, 139 In this context, increased ghrelin levels are likely to be a compensatory mechanism to buffer cachexia and to represent a mechanism to counter‐balance anorexia.3, 13, 15
2.7. Therapeutic options
The more effective way to treat cancer cachexia is to cure the underlying condition, i.e., cancer.140, 141 However, curing many types of cancer remains an unmet challenge.140, 141 Therefore, therapeutics options for cancer cachexia often aim to treat and ameliorate cachexia itself.3, 12, 141 Unfortunately, the therapeutic options for this syndrome are limited and not always effective.3, 12 Cancer cachexia cannot be totally reversed or prevented by using conventional nutritional support or even total parenteral nutrition.7, 12, 13
Several therapeutic interventions with multiple agents have been described and tested.7, 32, 140, 142 Supplementation with omega‐3 fatty acids that reduce IL‐1 and TNF‐α production and improve the efficacy of nutritional support has been tested.7, 140, 141 Glucocorticoids have also been used in order to mitigate some symptoms in patients, by inhibiting the synthesis/release of pro‐inflammatory cytokines and enhancing NPY levels, and show a rapid onset of effect on appetite.7, 140, 141 Non‐steroidal anti‐inflammatory drugs and drugs leading to cytokine inhibition have also been hypothesized to have a promising positive effect in cancer cachexia, by diminishing the inflammatory status and improving muscle wasting.7, 50, 140, 141 Amino acid supplementation could also be an approach to consider (like glutamine supplementation that may attenuate muscle wasting in cancer patients).15, 92, 143 The use of megestrol acetate also shows some good results in cancer cachectic patients.30, 41 Other agents causing an appetite stimulation (like cannabinoids or erythropoietin), antidopaminergics (like metoclopramide), or muscle synthesis stimulation (as branched‐chain amino acids) have been described as possible therapeutic approaches too.7, 140, 144
Additionally, physical activity seems to be an important element for the treatment of cancer cachexia.32, 50, 145, 146 Exercise training programs (such as strength and/or aerobic training) demonstrated to have a very clear anti‐inflammatory effect, decreasing pro‐inflammatory cytokines and increasing anti‐inflammatory cytokines.146 Exercise may act as a promoter of metabolic and inflammatory pathways disruption during cancer cachexia, improving functional status, body composition and longevity.32, 146 Nonetheless, additional studies are needed to determine the effectiveness and safety of exercise in cancer cachexia patients.32
Because cancer cachexia has a multidimensional background, multimodal therapeutic approaches are a preferred strategy, as a single approach is unlikely to be effective.3, 141, 142, 147 These multimodal approaches consist of combinations of interventions, including not only pharmacological drugs and/or nutritional supplementation, but also a program of moderate physical exercise.13, 32, 141, 142
3. Sarcopenia
Advancing adult age is associated with severe changes in body composition, which affect skeletal muscle mass most severely and may lead to decreased muscle strength and functionality.148, 149 Irwin Rosenberg proposed the term ‘sarcopenia’ to specifically name this condition characterized by age‐related decrease in muscle mass, with increased risk of physical disability and poor quality of life.148, 150, 151, 152 The pathophysiology of sarcopenia is complex, and multiple mechanisms contribute to its development including a down‐regulation of anabolic hormones like insulin, sexual steroids and growth hormone, an increased apoptotic activity in muscle cells and increased circulating pro‐inflammatory cytokines.35, 153, 154, 155
Aging is associated with a chronic state of systemic low‐grade inflammation, characterized by increased plasma levels of pro‐inflammatory mediators like TNF‐α and IL‐6, which are able to stimulate proteolysis mainly via UPR, as previously described for cancer cachexia.154, 156, 157, 158, 159, 160, 161, 162 However, the role of UPR in sarcopenia is still contentious.163, 164, 165 Some studies have described an up‐regulation of components of the UPR in sarcopenia while other have shown a down‐regulation or no differences.166, 167, 168, 169, 170
Enhanced production of reactive oxygen species is thought to promote sarcopenia by destabilizing mitochondria in skeletal muscle fibres and thus increasing their susceptibility to apoptotic stimuli while also down‐regulating pathways associated with mitochondrial biogenesis.157, 163, 171 Oxidative stress may also promote other sarcopenia‐associated processes, such as proteolysis, up‐regulation of TNF‐α levels and inhibition of muscle cell differentiation.163, 171, 172, 173
In fact, the apoptosis of satellite cells contributes to the reduction of muscle mass and function associated with age.174, 175 During aging, the regenerative potential of skeletal muscle is lost, because of impairment of satellite cells, which lose their capacity to regenerate and to self‐renewal.174, 175
Additionally, a loss of motoneurons, neuromuscular remodelling, denervation and fibre‐type switch may also contribute to reduced numbers of muscle fibres and the overall muscle mass.155, 163, 176, 177, 178
Dietary changes leading to lower and/or deficient intake of energy and protein may contribute to loss of muscle mass and function.35 Immobility and reduced physical inactivity may also contribute to the onset of sarcopenia in older people.35, 148
Finally, cancer cachexia (often occurring in older patients) may co‐occur with sarcopenia.35, 148 In fact, these two syndromes overlap considerably, especially in older patients.35, 155 Most cachectic individuals are also sarcopenic; however, most sarcopenic individuals are not considered as being cachectic, so sarcopenia can be considered as a component of cachexia.12, 35, 148, 179 Sarcopenia is not associated with weight loss, in opposition to cachexia that is associated with significant weight loss, that leads to a reduction of both fat and fat‐free mass.12, 180 Furthermore, although both processes are associated with systemic inflammation, cachexia is characterized by more intense inflammatory processes in contrast with sarcopenic patients that often show a slight or undetectable systemic inflammation.35, 155, 180, 181 Sarcopenia is mediated by a number of factors, while in cachexia, the activation of proinflammatory cytokines has a major and direct effect on disrupting muscle metabolism.155
To help clarify the diagnosis of sarcopenia, the European Working Group on Sarcopenia in Older People (EWGSOP) initially recommended the use of two criteria, namely low muscle mass and low muscle function (strength or performance).148
A recent update by EWGSOP2 designated low muscle strength as the primary criterion for diagnosing sarcopenia. Presently, muscle strength is the most reliable measure of muscle function.182 Presence of low muscle strength (criterion 1 – grip strength <30kg or <20kg for men and women, respectively) supports a diagnosis of probable sarcopenia. Additional documentation of criterion 2, low muscle quantity or quality (appendicular lean mass adjusted for height: ALM/height2 <7.23 or <5.67kg/m2 for men and women, respectively) can confirm the diagnosis of sarcopenia. If criteria 1 and 2 are present together with low physical performance (considered a third criterion 3 – gait speed ≤0.8m/s), sarcopenia is considered severe.182
Multiple groups published different operational criteria to define sarcopenia.183, 184, 185, 186 In 2011, the International Working Group (IWG) proposed a definition of sarcopenia that includes low physical performance (gait speed <1.0m/s) and low muscle mass (ALM/height2 ≤7.23 or ≤5.67kg/m2 for men and women, respectively).184 The Foundation for the National Institutes of Health (FNIH) Sarcopenia Project presented distinct criteria to define sarcopenia185 on the basis of low appendicular lean mass adjusted for body mass index (ALM/BMI <0.789 for men or <0.512 for women), in the presence of low muscle strength (grip strength <26 kg for men or <16 kg for women).185 The FNIH definition is more restrictive than the EWGSOP and IWG criteria, which may result in sarcopenia being diagnosed less frequently.183
The Asian Working Group for Sarcopenia (AWGS) agreed with the low muscle mass plus low muscle strength and/or low physical performance criteria.187 The AWGS recommended cutoff values for muscle mass measurements (<7.0kg/m2 for men and <5.4kg/m2 or 5.7kg/m2 for women, using X‐ray absorptiometry or bioimpedance analysis, respectively), grip strength (<26kg for men and <18kg for women) and gait speed (<0.8m/s).187 Of all these definitions, the most widely used criteria for diagnosing sarcopenia is the one proposed by EWGSOP/EWGSOP2.148, 182
Sarcopenia can also be classified according to its causes and by disease stages.148, 182, 188 Sarcopenia is considered primary or age‐related when no other specific cause is evident but aging itself, or secondary when causal factors other than (or in addition to) aging are evident, such as disease, inactivity, or poor nutrition.148, 182 The EWGSOP also proposed a sarcopenia staging scheme to help guide the clinical management of this syndrome, defining the stages of ‘presarcopenia’ characterized by low muscle mass without impact on muscle strength or performance, ‘sarcopenia’ with low muscle mass plus low muscle strength or low physical performance and ‘severe sarcopenia’, when all the three criteria meet.148
Regarding the possible therapeutic options for sarcopenia, the primary intervention should be physical exercise.155, 189, 190 The advantages of exercise have been demonstrated convincingly and resistance exercise training was shown to increase muscle mass and strength, improving muscle protein accretion.190, 191 It has been suggested that a combination of several exercises into a programme may achieve better results than a highly focused exercise regimen.189, 190, 192
Nutrition is another important component to consider.190, 192 However, clinical trials using standardized interventions with single or complex nutritional supplementation are still missing.190, 192 The use of protein (like some milk‐based proteins) or essential amino acid supplementation (particularly leucine) has shown only minor effects on muscle recovery.192, 193 The efficacy of vitamin D supplementation to ameliorate sarcopenia is still a matter of debate.190, 192, 193 The use of anabolic hormones also continues to show limited efficacy in treating sarcopenia.155, 190 Overall, no drug is currently approved for sarcopenia treatment and a combined strategy with nutritional supplementation and exercise programs seems to be the most promising approach for sarcopenia.189, 190, 193
More research is necessary and encouraged in the sarcopenia's field in order to prevent or delay adverse outcomes that arouse heavy burden for patients' health, dependence and economics as well for healthcare systems.35, 182
4. Anorexia
Defined as the loss of appetite, anorexia is considered to be an important component of cachexia and also may play a role in the pathogenesis of sarcopenia.2, 23, 194, 195, 196, 197 However, it is important to emphasize that anorexia can be distinct from both syndromes and can occur independently from either.12, 17, 196, 198 With anorexia, the wasting of skeletal muscle that characterizes cachexia does not occur.199 Also, nutritional supplement cannot replenish the loss of body mass in cachexia, contrary to what happens in anorexia.199 Moreover, anorexia alone cannot explain and cause all the metabolic changes that happens during cachexia.7, 84, 198 Anorexia occurs in other conditions such as psychiatric problems, the use of certain medications, depression, aging that leads to an appetite decrease, gastro‐intestinal problems and a variety of alterations in central neurotransmitters.12, 195, 200
Anorexia may be categorized in two categories, in accordance with the alterations of the volitional or nonvolitional control of eating.201 If it occurs because of an altered body image perception leading to the volitional refuse of eating, it is defined as primary anorexia (or anorexia nervosa), and if it happens as a consequence of a higher and/or persistent inflammatory response secondary to chronic or acute diseases, it is defined as secondary anorexia (or disease‐specific anorexia).201 In anorexia nervosa, the suppression of appetite is because of the psychiatric need to adjust body image to unrealistic models.201, 202 In fact, a part of patients suffering from anorexia nervosa may not lack appetite.203 In secondary anorexia, the suppression of the appetite is because of the function disruption of the neuronal pathways that regulate the physiological eating behaviour.201, 204
Anorexia nervosa is thought to be triggered by a combination of multiple biological and social factors.203 This condition is closely related to social pressure and media exposure and is not easy to treat.203
Anorexia of aging includes a combination of physical and social changes associated with aging (e.g. decline in smell in taste, reduced appetite, delayed gastric emptying, dementia, depression, solitude and poverty).194, 205 Anorexia of aging is thought to develop through two general mechanisms: the reduced drive to eat resulting from lower energy requirements and earlier satiety signals.205 Still, the exact mechanisms underlying anorexia of aging are still to be clarified.205 It is important to emphasize that this type of anorexia may occur even in otherwise healthy adults.205 Furthermore, anorexia of aging can be considered a direct risk factor for weight loss and consequent sarcopenia.194
Secondary anorexia is frequently observed in cancer patients and is associated with limited food intake, worse disease outcomes, increased morbidity and mortality and reduced quality of life.206, 207 Anorexia is present in up to 50% of newly diagnosed cancer patients and is the fourth most common symptom (after pain, fatigue and weakness) in patients with advanced cancer stages.7, 207, 208 Several factors contribute for anorexia in cancer patients: chemotherapy/drugs, depression, constipation, emesis, decreased gastric emptying, dysphagia, mucositis/stomatitis, reduced or altered taste, pain and tumour products and host factors.17
Chronic anorexia in wasting syndromes implies that the adaptative feeding response fails.209 Tumour‐released substances such as proinflammatory cytokines, lactate, or PTHRP contribute decisively to anorexia.195 Many cytokines (IL‐1α, IL‐1β, IL‐6, IL‐8 and TNF‐α) have a known effect on appetite by modulating central nervous system neurotransmitter cascades.195, 199, 204, 210, 211, 212, 213, 214, 215 It was also hypothesized that cancer anorexia may be the end result of alterations in the neurohormonal signals (central and peripheral) that govern appetite.216
Leptin is an adipokine that acts on specific hypothalamic receptors, promoting satiety via downstream neuropeptides such as NPY.140, 195, 209 Cytokines such as IL‐1 may induce anorexia by stimulating the expression and release of leptin and by mimicking leptin's hypothalamic effect.73, 140, 195, 209, 213 Lactate inhibits food intake via hypothalamic activation of the adenosine monophosphate kinase/malonyl‐CoA signalling pathway.195, 217 Therefore, cancer anorexia is strongly associated with functional damage of the hypothalamic mechanisms that normally control the eating behaviour.201, 218 Additionally, cancer treatments as chemotherapy and radiation, which cause nauseas and vomiting, may contribute to anorexia.7, 199
One of the challenges of anorexia assessment in patients is to characterize all the distinct contributors and then provide a correct and multimodal approach to the treatment.17 Therefore, in order to accurately diagnose anorexia, it is important to have valid and reliable methods.207 The assessment of anorexia is based in visual analogue scales, numerical scales, verbal descriptors, or individual questionnaires, introducing significant subjectivity into the process of diagnosis.17 In clinical practice, a ‘yes/no’ questionnaire (‘do you experience a decreased appetite?’) or the anorexia symptom scale of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)‐C30 (third version)219 is most often used.207 Lately, two other methods have been proposed for specifically diagnosing anorexia associated with cachexia: the Anorexia/Cachexia Subscale of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT‐A/CS) questionnaire220 and the visual analogue scale for appetite, but the validation of the cut‐offs is still lacking.35, 206, 207 Therefore, nowadays, no gold standard exists for diagnosing anorexia in patients with cancer.207
Concerning possible pharmacological treatments for anorexia, a number of specific orexigenics have been developed.195 Megestrol was approved to treat anorexia in patients with AIDS and was also shown to improve weight gain and ameliorate anorexia in children with cancer.195, 221 Cannabinoids also seem to increase appetite by enhancing NPY in the hypothalamus.195 Ghrelin and related substances also have an impact on food intake by anorexic patients.195, 222
5. Asthenia
Asthenia is a universal feature of advanced malignancy, and it is another prominent and frequent manifestation of advanced cancer.17, 223 Most advanced cancer patients show a combination of cachexia and asthenia, but they can also occur independently.7, 17, 224 It is important to emphasize that cachexia is not a requisite for asthenia to occur.224, 225 Asthenia comes from the Greek ‘asthenos’ and means absence of strength, loss of muscle force and muscle weakness.3, 5, 223 It is characterized physically by profound tiredness after usual or small efforts, accompanied by an unpleasant and anticipatory sensation of generalized weakness and fatigue, and loss of muscle strength. Mentally, asthenia is associated with decreased capacity for intellectual work, impaired concentration, loss of memory and emotional lability.7, 17, 223, 226
Although some authors may refer to asthenia with the term ‘fatigue’, it is important to emphasize that fatigue is only one dimension and symptom of asthenia.227 In fact, fatigue is defined by tiredness or exhaustion as result of physical or mental effort, while asthenia is characterized by tiredness or exhaustion in the absence of physical or mental effort.228 Some authors also describe an asthenia‐fatigue syndrome or cancer‐related fatigue (CRF), when asthenia in associated with cancer.229, 230, 231, 232
Even though asthenia has a complex pathophysiology, it seems to be mediated by a combination of factors released by the tumour, host responses (cytokines) and direct consequences of the tumour presence.223, 225, 229 The brain (associated with fatigue) and the muscle (associated with weakness) seem to be the two major organs that should be address to understand the mechanisms underlying asthenia.225 More studies are necessary to obtain a deeper understanding of asthenia and its mechanisms.
Metabolic abnormalities involved in the development of cachexia also generally lead to the development of asthenia, and the loss of muscle resulting from progressive cachexia provides a rationale for asthenia.17, 224, 225, 228 However, it is important to emphasize that muscle wasting does not occur because of asthenia itself.17, 224 In addition to cachexia, there are numerous other factors that contributing to asthenia in cancer patients including anaemia, infection, muscle abnormalities/immobility, chemotherapy and/or radiotherapy, metabolic problems, cytokines, psychological distress and pain/drug side effects.17, 229
Asthenia is frequently underdiagnosed or neglected in cancer patients, accepting the fatigue as a ‘normal’ symptom.229 However, asthenia and the fatigue associated have an important effect in the life quality of patients.233 Even so, because asthenia is a subjective sensation for the patients, its assessment is hard and difficult.17, 223 The most common approaches to the assessment of asthenia were reviewed by Bruera and Sweeney in 2000.17 The first approach is to assess the patient's functional capacity in a standard/normal task such as walking in a treadmill or with simple and daily tasks.17 Another approach employs scales to rate the patient's functional abilities and is the most used in oncology.17 The most commonly used scales are the Eastern Cooperative Oncology Group – Performance Status (ECOG‐PS) and Karnofsky Performance Status (KPS) scales.17, 234, 235, 236 The last approach is the subjective assessment of fatigue employing questionnaires.17, 237, 238 Despite these efforts, it is currently accepted that self‐assessment should be the ‘gold standard’, because asthenia is essentially a subjective sensation.17
Berger et al., in 2015, presented Standards of Care for CRF Management using the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines).232 These standards help managing CRF and should give useful guidance for professionals, by proposing a treatment algorithm.232
Corticosteroids have been suggested to decrease asthenia in advanced cancer patients,17, 239 but their long‐term use is associated with important side effects.17, 239 The use of psychostimulants seems to be effective only in patients with asthenia having an opioid toxicity.17 Megestrol acetate was also associated with clinical benefits in asthenic patients.17 Importantly, physical exercise, physiotherapy and occupational therapies seem to improve asthenia.17, 239
6. Conclusion
Although cachexia, sarcopenia, anorexia and asthenia can be defined as distinct clinical conditions, they share multiple important common points and a certain degree of overlap (Figure 4 and Table 1). The two features that better demonstrate this overlap are inflammation and weakness (Figure 4 ). These features may be observed not only in cachectic or sarcopenic patients but also in patients suffering from anorexia or asthenia. Because weakness/fatigue and inflammation are features transversal to several conditions, clinicians cannot exclusively use these features to distinguish between the four conditions presented. Clinicians should look for features (or combination of features) that make each condition unique (Figure 4 ) and to evaluate them in the context of each patient's clinical history and personal profile (e.g. earlier diseases, presence of mental conditions, age).
Figure 4.

Links and overlaps between related conditions. Inflammation, weakness and fatigue are features of cachexia, sarcopenia, anorexia and asthenia. *Cachexia can occur with or without loss of appetite and fat mass
Table 1.
Summary of the definitions, features and diagnosis criteria of the four conditions
| Condition | Definition | Features | Diagnosis/assessment criteria |
|---|---|---|---|
| Cachexia | Multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and to progressive functional impairment2 |
‐ Loss of weight (with or without loss of fat mass) ‐ With or without loss of appetite ‐ Inflammation ‐ Negative protein and energy balance ‐ Skeletal muscle wasting ‐ Lipolysis and browning of adipose tissue ‐ Impaired regeneration of muscle cells ‐ Mitochondrial dysfunction ‐ Disruption of neuronal pathways ‐ Acute‐phase response ‐ Malabsorption |
‐ Weight loss >5% over past 6 months (in absence of simple starvation); or ‐ Body mass index <20 and any degree of weight loss >2%; or ‐ Appendicular skeletal muscle index consistent with sarcopenia (male patients <7,26kg/m2, female patients <5,45kg/m2) and any degree of weight loss >2% (According to international consensus by Fearon et al., 2011)2 |
| Sarcopenia | Syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death148, 182 |
‐ Reduction of anabolic hormones ‐ Increased apoptotic activities in the muscle ‐ Systemic low‐grade inflammation ‐ Mitochondrial dysfunction ‐ Impaired regeneration of muscle cells |
‐ Criterion 1: Low muscle strength (assessed by grip strength; chair stand test); ‐ Criterion 2: Low muscle quantity or quality (ASMM by DXA; whole‐body SMM or ASMM by BIA; lumbar muscle cross‐sectional area by CT/MRI); ‐ Criterion 3: Low physical performance (Gait speed; SPPB; TUG; 400‐meter walk). (Probable sarcopenia is identified by Criterion 1. Diagnosis is confirmed by additional documentation of Criterion 2. If all the three criteria met, sarcopenia is considered severe.) |
| Anorexia | Loss of apetite195 |
‐ Loss of weight and fat mass, that can be reversed by nutritional support ‐ Higher and/or persistent inflammatory response ‐ Disruption of neuronal pathways that regulates eating behaviour |
Based in visual analogue scales, numerical scales, verbal descriptors or individual questionnaires (EORTC).17, 207 Two methods have been suggested: FAACT‐A/CS and VAS.207, 220 |
| Asthenia | Condition defined as absence of strength, weakness and reduced vital power224 |
‐ Generalized weakness and fatigue ‐ Loss of muscle force, muscle weakness ‐ Profound tiredness after usual or small effort ‐ Decreased in intellectual work ‐ Impaired concentration and loss of memory ‐ Emotional lability ‐ Inflammation (cytokines) ‐ In cancer patients: combination of factors released by the tumour and direct consequences of the tumour presence |
Assessment hard, difficult and subjective. ‐ Assess the functional capacity through the capacity of do standard tasks. ‐ Assessment of performance status by scales to rate the functional abilities. The most two used scales are ECOG‐PS and KPS. ‐ Subjective assessment of fatigue through questionnaires |
Several diagnostic criteria have been developed for these conditions. While the diagnosis of sarcopenia relies on the evaluation of muscle mass and function, diagnosing cachexia requires an assessment of skeletal muscle and total body mass. While cachexia and sarcopenia have been well studied and some degree of consensus has been reached concerning both, secondary anorexia and asthenia have been largely forgotten or ignored.
The terms used for designating and characterizing these conditions have evolved over the years (e.g. asthenia and fatigue), which may cause some degree of confusion. In fact, in a general way, the terms to designate the conditions must be simple and unanimous.
The importance of better understanding the differences between these conditions resides in the fact that it can allow clinicians to accurately diagnose the patients. The molecular pathways that are involved in these conditions and syndromes also must be better explored in order to improve diagnosis and identify therapeutic targets. An early and accurate diagnosis can make the difference in the treatment options as well in the patients' outcome and quality of life.
Conflict of interest
None declared.
The manuscript does not contain clinical studies or patient data.
Acknowledgements
This study was funded by Portuguese League Against Cancer – Regional Nucleus of the North (Liga Portuguesa Contra o Cancro – Núcleo Regional do Norte) and by the Research Center of the Portuguese Oncology Institute of Porto (project no. PI127‐CI‐IPOP‐118‐2019). Joana M.O. Santos is supported by a PhD fellowship (SFRH/BD/135871/2018) from FCT – Fundação para a Ciência e a Tecnologia.
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.240
da Silva S. P., Santos J. M. O., Costa e Silva M. P., Gil da Costa R. M., and Medeiros R. (2020) Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia, Journal of Cachexia, Sarcopenia and Muscle, 11, 619–635. 10.1002/jcsm.12528.
References
- 1. Katz AM, Katz PB. Diseases of the heart in the works of Hippocrates. Br Heart J. 1962;24:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 3. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–762. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt SF, Rohm M, Herzig S, Berriel DM. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer. 2018;4:849–860. [DOI] [PubMed] [Google Scholar]
- 5. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. [DOI] [PubMed] [Google Scholar]
- 6. Camargo RG, Quintas Teixeira Ribeiro H, Geraldo MV, Matos‐Neto E, Neves RX, Carnevali LC Jr, et al. Cancer Cachexia and MicroRNAs. Mediators Inflamm 2015;2015:367561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myrianthefs PM, Batistaki C. Cancer cachexia and immunomodulation. J Buon. 2005;10:181–188. [PubMed] [Google Scholar]
- 8. Santo RCE, Fernandes KZ, Lora PS, Filippin LI, Xavier RM. Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2018;9:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Von Roenn JH, Roth EL, Craig R. HIV‐related cachexia: potential mechanisms and treatment. Oncology. 1992;49:50–54. [DOI] [PubMed] [Google Scholar]
- 10. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang SW, Pan WS, Lozano Beltran D, Oleyda Baldelomar L, Solano MA, Tuero I, et al. Gut hormones, appetite suppression and cachexia in patients with pulmonary TB. PLoS One. 2013;8:e54564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 13. Argiles JM, Stemmler B, Lopez‐Soriano FJ, Busquets S. Inter‐tissue communication in cancer cachexia. Nat Rev Endocrinol. 2018;15:9–20. [DOI] [PubMed] [Google Scholar]
- 14. Argiles JM, Lopez‐Soriano FJ, Busquets S. Counteracting inflammation: a promising therapy in cachexia. Crit Rev Oncog. 2012;17:253–262. [DOI] [PubMed] [Google Scholar]
- 15. Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsoli M, Robertson G. Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol Metab. 2013;24:174–183. [DOI] [PubMed] [Google Scholar]
- 17. Bruera E, Sweeney C. Cachexia and asthenia in cancer patients. Lancet Oncol. 2000;1:138–147. [DOI] [PubMed] [Google Scholar]
- 18. Der‐Torossian H, Wysong A, Shadfar S, Willis MS, McDunn J, Couch ME. Metabolic derangements in the gastrocnemius and the effect of Compound A therapy in a murine model of cancer cachexia. J Cachexia Sarcopenia Muscle. 2013;4:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanders KJ, Kneppers AE, van de Bool C, Langen RC, Schols AM. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle. 2016;7:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scherbakov N, Doehner W. Cachexia as a common characteristic in multiple chronic disease. J Cachexia Sarcopenia Muscle. 2018;9:1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fearon KC, Baracos VE. Cachexia in pancreatic cancer: new treatment options and measures of success. HPB (Oxford). 2010;12:323–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124–1132. [DOI] [PubMed] [Google Scholar]
- 24. Rowland KM Jr, Loprinzi CL, Shaw EG, Maksymiuk AW, Kuross SA, Jung SH, et al. Randomized double‐blind placebo‐controlled trial of cisplatin and etoposide plus megestrol acetate/placebo in extensive‐stage small‐cell lung cancer: a North Central Cancer Treatment Group study. J Clin Oncol. 1996;14:135–141. [DOI] [PubMed] [Google Scholar]
- 25. Bauer J, Capra S, Ferguson M. Use of the scored Patient‐Generated Subjective Global Assessment (PG‐SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–785. [DOI] [PubMed] [Google Scholar]
- 26. Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer‐related cachexia. Nutrition. 2001;17:S1–S20. [DOI] [PubMed] [Google Scholar]
- 27. Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF. Estimation of cachexia among cancer patients based on four definitions. J Oncol. 2009;2009:693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loumaye A, Thissen JP. Biomarkers of cancer cachexia. Clin Biochem. 2017;50:1281–1288. [DOI] [PubMed] [Google Scholar]
- 30. Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer Cachexia: Beyond Weight Loss. J Oncol Pract. 2016;12:1163–1171. [DOI] [PubMed] [Google Scholar]
- 31. Yaxley A, Miller MD. The challenge of appropriate identification and treatment of starvation, sarcopenia, and cachexia: a survey of Australian dietitians. J Nutr Metab. 2011;2011:603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Argiles JM, Lopez‐Soriano FJ, Stemmler B, Busquets S. Novel targeted therapies for cancer cachexia. Biochem J. 2017;474:2663–2678. [DOI] [PubMed] [Google Scholar]
- 33. Hemming L, Maher D. Understanding cachexia and excessive weight loss in cancer. Br J Community Nurs. 2005;10:492–495. [DOI] [PubMed] [Google Scholar]
- 34. Sadeghi M, Keshavarz‐Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91–104. [DOI] [PubMed] [Google Scholar]
- 35. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics.”. Clin Nutr. 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 36. Zhou T, Wang B, Liu H, Yang K, Thapa S, Zhang H, et al. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J Cachexia Sarcopenia Muscle. 2018;9:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Argiles JM, Betancourt A, Guardia‐Olmos J, Pero‐Cebollero M, Lopez‐Soriano FJ, Madeddu C, et al. Validation of the CAchexia SCOre (CASCO). Staging Cancer Patients: The Use of miniCASCO as a Simplified Tool. Front Physiol. 2017;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Argiles JM, Lopez‐Soriano FJ, Toledo M, Betancourt A, Serpe R, Busquets S. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle. 2011;2:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the consensus‐definition for cancer cachexia and evaluation of a classification model—a study based on data from an international multicentre project (EPCRC‐CSA). Ann Oncol. 2014;25:1635–1642. [DOI] [PubMed] [Google Scholar]
- 40. Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75:188–198. [DOI] [PubMed] [Google Scholar]
- 41. Dev R, Wong A, Hui D, Bruera E. The evolving approach to management of cancer cachexia. Oncology (Williston Park). 2017;31:23–32. [PubMed] [Google Scholar]
- 42. Karagianni VT, Papalois AE, Triantafillidis JK. Nutritional status and nutritional support before and after pancreatectomy for pancreatic cancer and chronic pancreatitis. Indian J Surg Oncol. 2012;3:348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jager‐Wittenaar H, Dijkstra PU, Dijkstra G, Bijzet J, Langendijk JA, van der Laan B, et al. High prevalence of cachexia in newly diagnosed head and neck cancer patients: an exploratory study. Nutrition. 2017;35:114–118. [DOI] [PubMed] [Google Scholar]
- 44. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 45. Kwon M, Kim RB, Roh JL, Lee SW, Kim SB, Choi SH, et al. Prevalence and clinical significance of cancer cachexia based on time from treatment in advanced‐stage head and neck squamous cell carcinoma. Head Neck. 2017;39:716–723. [DOI] [PubMed] [Google Scholar]
- 46. Matsuyama T, Ishikawa T, Okayama T, Oka K, Adachi S, Mizushima K, et al. Tumor inoculation site affects the development of cancer cachexia and muscle wasting. Int J Cancer. 2015;137:2558–2565. [DOI] [PubMed] [Google Scholar]
- 47. Barata AT, Santos CA, Fonseca J., Nutrição. In: Cancro do Pâncreas. Algés: Grupo de Investigação do Cancro Digestivo, 2017, pp 104‐120. [Google Scholar]
- 48. Moreira‐Pais A, Ferreira R, Gil da Costa R. Platinum‐induced muscle wasting in cancer chemotherapy: mechanisms and potential targets for therapeutic intervention. Life Sci. 2018;208:1–9. [DOI] [PubMed] [Google Scholar]
- 49. Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC. Chemotherapy‐induced muscle wasting: association with NF‐kappaB and cancer cachexia. Eur J Transl Myol. 2018;28:7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Argilés JM, López‐Soriano FJ, Busquets S. Mediators of cachexia in cancer patients. Nutrition. 2019. [DOI] [PubMed]
- 51. Torti FM, Dieckmann B, Beutler B, Cerami A, Ringold GM. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985;229:867–869. [DOI] [PubMed] [Google Scholar]
- 52. Cerami A, Ikeda Y, Le Trang N, Hotez PJ, Beutler B. Weight loss associated with an endotoxin‐induced mediator from peritoneal macrophages: the role of cachectin (tumor necrosis factor). Immunol Lett. 1985;11:173–177. [DOI] [PubMed] [Google Scholar]
- 53. Argiles JM, Lopez‐Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev. 1999;19:223–248. [DOI] [PubMed] [Google Scholar]
- 54. Hou YC, Wang CJ, Chao YJ, Chen HY, Wang HC, Tung HL, et al. Elevated serum interleukin‐8 level correlates with cancer‐related cachexia and sarcopenia: an indicator for pancreatic cancer outcomes. J Clin Med. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Song B, Zhang D, Wang S, Zheng H, Wang X. Association of interleukin‐8 with cachexia from patients with low‐third gastric cancer. Comp Funct Genomics 2009; 10.1155/2009/212345:212345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Patel HJ, Patel BM. TNF‐alpha and cancer cachexia: molecular insights and clinical implications. Life Sci. 2017;170:56–63. [DOI] [PubMed] [Google Scholar]
- 57. Han Y, Weinman S, Boldogh I, Walker RK, Brasier AR. Tumor necrosis factor‐alpha‐inducible Ikappa B alpha proteolysis mediated by cytosolic m‐calpain. A mechanism parallel to the ubiquitin‐proteasome pathway for nuclear factor‐kappa b activation. J Biol Chem. 1999;274:787–794. [DOI] [PubMed] [Google Scholar]
- 58. Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. Biomed Res Int. 2014;2014:168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Giordano A, Calvani M, Petillo O, Carteni M, Melone MR, Peluso G. Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem. 2003;90:170–186. [DOI] [PubMed] [Google Scholar]
- 60. Busquets S, Sanchis D, Alvarez B, Ricquier D, Lopez‐Soriano FJ, Argiles JM. In the rat, tumor necrosis factor alpha administration results in an increase in both UCP2 and UCP3 mRNAs in skeletal muscle: a possible mechanism for cytokine‐induced thermogenesis? FEBS Lett. 1998;440:348–350. [DOI] [PubMed] [Google Scholar]
- 61. Schcolnik‐Cabrera A, Chavez‐Blanco A, Dominguez‐Gomez G, Duenas‐Gonzalez A. Understanding tumor anabolism and patient catabolism in cancer‐associated cachexia. Am J Cancer Res. 2017;7:1107–1135. [PMC free article] [PubMed] [Google Scholar]
- 62. Tijerina AJ. The biochemical basis of metabolism in cancer cachexia. Dimens Crit Care Nurs. 2004;23:237–243. [DOI] [PubMed] [Google Scholar]
- 63. Peterson JM, Feeback KD, Baas JH, Pizza FX. Tumor necrosis factor‐alpha promotes the accumulation of neutrophils and macrophages in skeletal muscle. J Appl Physiol (1985) 2006;101:1394–1399. [DOI] [PubMed] [Google Scholar]
- 64. Chiba F, Soda K, Yamada S, Tokutake Y, Chohnan S, Konishi F, et al. The importance of tissue environment surrounding the tumor on the development of cancer cachexia. Int J Oncol. 2014;44:177–186. [DOI] [PubMed] [Google Scholar]
- 65. Penafuerte CA, Gagnon B, Sirois J, Murphy J, MacDonald N, Tremblay ML. Identification of neutrophil‐derived proteases and angiotensin II as biomarkers of cancer cachexia. Br J Cancer. 2016;114:680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Owen CA, Campbell EJ. Angiotensin II generation at the cell surface of activated neutrophils: novel cathepsin G‐mediated catalytic activity that is resistant to inhibition. J Immunol. 1998;160:1436–1443. [PubMed] [Google Scholar]
- 67. Sukhanov S, Semprun‐Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, et al. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342:143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cabello‐Verrugio C, Cordova G, Salas JD. Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci. 2012;13:560–569. [DOI] [PubMed] [Google Scholar]
- 69. Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petruzzelli M, Schweiger M, Schreiber R, Campos‐Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer‐associated cachexia. Cell Metab. 2014;20:433–447. [DOI] [PubMed] [Google Scholar]
- 71. Argiles JM, Busquets S, Lopez‐Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care. 2003;6:401–406. [DOI] [PubMed] [Google Scholar]
- 72. Mantovani G, Maccio A, Mura L, Massa E, Mudu MC, Mulas C, et al. Serum levels of leptin and proinflammatory cytokines in patients with advanced‐stage cancer at different sites. J Mol Med (Berl). 2000;78:554–561. [DOI] [PubMed] [Google Scholar]
- 73. Laviano A, Meguid MM, Yang ZJ, Gleason JR, Cangiano C, Rossi FF. Cracking the riddle of cancer anorexia. Nutrition. 1996;12:706–710. [DOI] [PubMed] [Google Scholar]
- 74. Albrecht JT, Canada TW. Cachexia and anorexia in malignancy. Hematol Oncol Clin North Am. 1996;10:791–800. [DOI] [PubMed] [Google Scholar]
- 75. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers. 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 76. Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miyamoto Y, Hanna DL, Zhang W, Baba H, Lenz HJ. Molecular Pathways: Cachexia Signaling‐A Targeted Approach to Cancer Treatment. Clin Cancer Res. 2016;22:3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Petruzzelli M, Wagner EF. Mechanisms of metabolic dysfunction in cancer‐associated cachexia. Genes Dev. 2016;30:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Han J, Meng Q, Shen L, Wu G. Interleukin‐6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bo S, Dianliang Z, Hongmei Z, Xinxiang W, Yanbing Z, Xiaobo L. Association of interleukin‐8 gene polymorphism with cachexia from patients with gastric cancer. J Interferon Cytokine Res. 2010;30:9–14. [DOI] [PubMed] [Google Scholar]
- 81. Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Penna F, Ballaro R, Beltra M, De Lucia S, Costelli P. Modulating metabolism to improve cancer‐induced muscle wasting. Oxid Med Cell Longev. 2018;2018:7153610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP‐ubiquitin‐proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995;268:E996–E1006. [DOI] [PubMed] [Google Scholar]
- 84. Mendes MC, Pimentel GD, Costa FO, Carvalheira JB. Molecular and neuroendocrine mechanisms of cancer cachexia. J Endocrinol. 2015;226:R29–R43. [DOI] [PubMed] [Google Scholar]
- 85. Op den Kamp CM, Langen RC, Snepvangers FJ, de Theije CC, Schellekens JM, Laugs F, et al. Nuclear transcription factor kappa B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr. 2013;98:738–748. [DOI] [PubMed] [Google Scholar]
- 86. Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM. Ca(2+)‐dependent proteolysis in muscle wasting. Int J Biochem Cell Biol. 2005;37:2134–2146. [DOI] [PubMed] [Google Scholar]
- 87. Argiles JM, Busquets S, Moore‐Carrasco R, Figueras M, Almendro V, Lopez‐Soriano FJ. Targets in clinical oncology: the metabolic environment of the patient. Front Biosci. 2007;12:3024–3051. [DOI] [PubMed] [Google Scholar]
- 88. Argiles JM, Orpi M, Busquets S, Lopez‐Soriano FJ. Myostatin: more than just a regulator of muscle mass. Drug Discov Today. 2012;17:702–709. [DOI] [PubMed] [Google Scholar]
- 89. He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, Thomas‐Ahner J, et al. NF‐kappaB‐mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest. 2013;123:4821–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Eley HL, Russell ST, Tisdale MJ. Mechanism of activation of dsRNA‐dependent protein kinase (PKR) in muscle atrophy. Cell Signal. 2010;22:783–790. [DOI] [PubMed] [Google Scholar]
- 91. Michalak KP, Mackowska‐Kedziora A, Sobolewski B, Wozniak P. Key roles of glutamine pathways in reprogramming the cancer metabolism. Oxid Med Cell Longev. 2015;2015:964321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yoshida S, Kaibara A, Ishibashi N, Shirouzu K. Glutamine supplementation in cancer patients. Nutrition. 2001;17:766–768. [DOI] [PubMed] [Google Scholar]
- 93. Argiles JM, Fontes‐Oliveira CC, Toledo M, Lopez‐Soriano FJ, Busquets S. Cachexia: a problem of energetic inefficiency. J Cachexia Sarcopenia Muscle. 2014;5:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Miura S, Tomitsuka E, Kamei Y, Yamazaki T, Kai Y, Tamura M, et al. Overexpression of peroxisome proliferator‐activated receptor gamma co‐activator‐1alpha leads to muscle atrophy with depletion of ATP. Am J Pathol. 2006;169:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Barkhudaryan A, Scherbakov N, Springer J, Doehner W. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail. 2017;4:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med. 1975;6:61–64. [PubMed] [Google Scholar]
- 97. Olivan M, Springer J, Busquets S, Tschirner A, Figueras M, Toledo M, et al. Theophylline is able to partially revert cachexia in tumour‐bearing rats. Nutrition & Metabolism. 2012;9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer‐induced cachexia in mice. Int J Oncol. 2010;37:347–353. [DOI] [PubMed] [Google Scholar]
- 99. Wysong A, Couch M, Shadfar S, Li L, Rodriguez JE, Asher S, et al. NF‐kappaB inhibition protects against tumor‐induced cardiac atrophy in vivo. Am J Pathol. 2011;178:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zimmers TA, Jiang Y, Wang M, Liang TW, Rupert JE, Au ED, et al. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res Cardiol. 2017;112:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vaitkus JA, Celi FS. The role of adipose tissue in cancer‐associated cachexia. Exp Biol Med (Maywood) 2017;242:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Daas SI, Rizeq BR, Nasrallah GK. Adipose tissue dysfunction in cancer cachexia. J Cell Physiol. 2018;234:13–22. [DOI] [PubMed] [Google Scholar]
- 103. Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. [DOI] [PubMed] [Google Scholar]
- 104. Bouillaud F, Villarroya F, Hentz E, Raimbault S, Cassard AM, Ricquier D. Detection of brown adipose tissue uncoupling protein mRNA in adult patients by a human genomic probe. Clin Sci (Lond). 1988;75:21–27. [DOI] [PubMed] [Google Scholar]
- 105. Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, et al. Adipose triglyceride lipase contributes to cancer‐associated cachexia. Science. 2011;333:233–238. [DOI] [PubMed] [Google Scholar]
- 106. Dalal S. Lipid metabolism in cancer cachexia. Ann Palliat Med. 2019;8:13–23. [DOI] [PubMed] [Google Scholar]
- 107. Shaw JH, Wolfe RR. Fatty acid and glycerol kinetics in septic patients and in patients with gastrointestinal cancer. The response to glucose infusion and parenteral feeding. Ann Surg. 1987;205:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, et al. Zinc‐alpha2‐glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up‐regulated in mice with cancer cachexia. Proc Natl Acad Sci U S A. 2004;101:2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nomura K, Noguchi Y, Matsumoto A. Stimulation of decreased lipoprotein lipase activity in the tumor‐bearing state by the antihyperlipidemic drug bezafibrate. Surg Today. 1996;26:89–94. [DOI] [PubMed] [Google Scholar]
- 110. Batista ML Jr, Neves RX, Peres SB, Yamashita AS, Shida CS, Farmer SR, et al. Heterogeneous time‐dependent response of adipose tissue during the development of cancer cachexia. J Endocrinol. 2012;215:363–373. [DOI] [PubMed] [Google Scholar]
- 111. Dong M, Lin J, Lim W, Jin W, Lee HJ. Role of brown adipose tissue in metabolic syndrome, aging, and cancer cachexia. Front Med. 2018;12:130–138. [DOI] [PubMed] [Google Scholar]
- 112. Elattar S, Dimri M, Satyanarayana A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 2018;32:4727–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour‐derived PTH‐related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Narsale AA, Enos RT, Puppa MJ, Chatterjee S, Murphy EA, Fayad R, et al. Liver inflammation and metabolic signaling in ApcMin/+ mice: the role of cachexia progression. PLoS One. 2015;10:e0119888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zimmers TA, McKillop IH, Pierce RH, Yoo JY, Koniaris LG. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology. 2003;38:326–334. [DOI] [PubMed] [Google Scholar]
- 116. Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One. 2011;6:e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bosaeus I. Nutritional support in multimodal therapy for cancer cachexia. Support Care Cancer. 2008;16:447–451. [DOI] [PubMed] [Google Scholar]
- 118. Holroyde CP, Gabuzda TG, Putnam RC, Paul P, Reichard GA. Altered glucose metabolism in metastatic carcinoma. Cancer Res. 1975;35:3710–3714. [PubMed] [Google Scholar]
- 119. Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole‐body energy demands. Am J Clin Nutr. 2009;89:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Berriel Diaz M, Krones‐Herzig A, Metzger D, Ziegler A, Vegiopoulos A, Klingenspor M, et al. Nuclear receptor cofactor receptor interacting protein 140 controls hepatic triglyceride metabolism during wasting in mice. Hepatology. 2008;48:782–791. [DOI] [PubMed] [Google Scholar]
- 121. Teli MR, James OF , Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow‐up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 122. van Norren K, Dwarkasing JT, Witkamp RF. The role of hypothalamic inflammation, the hypothalamic‐pituitary‐adrenal axis and serotonin in the cancer anorexia‐cachexia syndrome. Curr Opin Clin Nutr Metab Care. 2017;20:396–401. [DOI] [PubMed] [Google Scholar]
- 123. Burfeind KG, Michaelis KA, Marks DL. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol. 2016;54:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dwarkasing JT, Boekschoten MV, Argiles JM, van Dijk M, Busquets S, Penna F, et al. Differences in food intake of tumour‐bearing cachectic mice are associated with hypothalamic serotonin signalling. J Cachexia Sarcopenia Muscle. 2015;6:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dwarkasing JT, Witkamp RF, Boekschoten MV, Ter Laak MC, Heins MS, van Norren K. Increased hypothalamic serotonin turnover in inflammation‐induced anorexia. BMC Neurosci. 2016;17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Melichar B, Zezulova M. The significance of altered gastrointestinal permeability in cancer patients. Curr Opin Support Palliat Care. 2011;5:47–54. [DOI] [PubMed] [Google Scholar]
- 127. Klein GL, Petschow BW, Shaw AL, Weaver E. Gut barrier dysfunction and microbial translocation in cancer cachexia: a new therapeutic target. Curr Opin Support Palliat Care. 2013;7:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc (Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta. 1812;2011:1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target? Int J Biochem Cell Biol. 2013;45:2186–2190. [DOI] [PubMed] [Google Scholar]
- 130. Potgens SA, Brossel H, Sboarina M, Catry E, Cani PD, Neyrinck AM, et al. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci Rep‐Uk. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bindels LB, Beck R, Schakman O, Martin JC, De Backer F, Sohet FM, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS One. 2012;7:e37971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Garcia JM, Garcia‐Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer‐induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–2926. [DOI] [PubMed] [Google Scholar]
- 133. Karapanagiotou EM, Polyzos A, Dilana KD, Gratsias I, Boura P, Gkiozos I, et al. Increased serum levels of ghrelin at diagnosis mediate body weight loss in non‐small cell lung cancer (NSCLC) patients. Lung Cancer. 2009;66:393–398. [DOI] [PubMed] [Google Scholar]
- 134. Takahashi M, Terashima M, Takagane A, Oyama K, Fujiwara H, Wakabayashi G. Ghrelin and leptin levels in cachectic patients with cancer of the digestive organs. Int J Clin Oncol. 2009;14:315–320. [DOI] [PubMed] [Google Scholar]
- 135. Wang HS, Oh DS, Ohning GV, Pisegna JR. Elevated serum ghrelin exerts an orexigenic effect that may maintain body mass index in patients with metastatic neuroendocrine tumors. J Mol Neurosci. 2007;33:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sheriff S, Kadeer N, Joshi R, Friend LA, James JH, Balasubramaniam A. Des‐acyl ghrelin exhibits pro‐anabolic and anti‐catabolic effects on C2C12 myotubes exposed to cytokines and reduces burn‐induced muscle proteolysis in rats. Mol Cell Endocrinol. 2012;351:286–295. [DOI] [PubMed] [Google Scholar]
- 137. Mano‐Otagiri A, Iwasaki‐Sekino A, Nemoto T, Ohata H, Shuto Y, Nakabayashi H, et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul Pept. 2010;160:81–90. [DOI] [PubMed] [Google Scholar]
- 138. Zeng X, Chen S, Yang Y, Ke Z. Acylated and unacylated ghrelin inhibit atrophy in myotubes co‐cultured with colon carcinoma cells. Oncotarget. 2017;8:72872–72885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Reano S, Graziani A, Filigheddu N. Acylated and unacylated ghrelin administration to blunt muscle wasting. Curr Opin Clin Nutr Metab Care. 2014;17:236–240. [DOI] [PubMed] [Google Scholar]
- 140. Inui A. Cancer anorexia‐cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002;52:72–91. [DOI] [PubMed] [Google Scholar]
- 141. Mueller TC, Bachmann J, Prokopchuk O, Friess H, Martignoni ME. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia—can findings from animal models be translated to humans? BMC Cancer. 2016;16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Argiles JM, Lopez‐Soriano FJ, Stemmler B, Busquets S. Therapeutic strategies against cancer cachexia. Eur J Transl Myol. 2019;29:7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Schlemmer M, Suchner U, Schapers B, Duerr EM, Alteheld B, Zwingers T, et al. Is glutamine deficiency the link between inflammation, malnutrition, and fatigue in cancer patients? Clin Nutr. 2015;34:1258–1265. [DOI] [PubMed] [Google Scholar]
- 144. Malik JS, Yennurajalingam S. Prokinetics and ghrelin for the management of cancer cachexia syndrome. Ann Palliat Med. 2019;8:80–85. [DOI] [PubMed] [Google Scholar]
- 145. Solheim TS, Laird BJA, Balstad TR, Bye A, Stene G, Baracos V, et al. Cancer cachexia: rationale for the MENAC (Multimodal‐Exercise, Nutrition and Anti‐inflammatory medication for Cachexia) trial. BMJ Support Palliat Care. 2018;8:258–265. [DOI] [PubMed] [Google Scholar]
- 146. Lira FS, Antunes Bde M, Seelaender M, Rosa Neto JC. The therapeutic potential of exercise to treat cachexia. Curr Opin Support Palliat Care. 2015;9:317–324. [DOI] [PubMed] [Google Scholar]
- 147. Maddocks M, Hopkinson J, Conibear J, Reeves A, Shaw C, Fearon KC. Practical multimodal care for cancer cachexia. Curr Opin Support Palliat Care. 2016;10:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:5‐8. [DOI] [PubMed] [Google Scholar]
- 150. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. [DOI] [PubMed] [Google Scholar]
- 151. Rosenberg IH. Summary comments. The American Journal of Clinical Nutrition. 1989;50:1231–1233. [Google Scholar]
- 152. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. [DOI] [PubMed] [Google Scholar]
- 153. Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA. Role of endocrine‐immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol Aspects Med. 2005;26:181–201. [DOI] [PubMed] [Google Scholar]
- 154. Dalle S, Rossmeislova L, Koppo K. The Role of Inflammation in Age‐Related Sarcopenia. Front Physiol. 2017;8:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. [DOI] [PubMed] [Google Scholar]
- 156. Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin‐6 and tumor necrosis factor‐alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. [DOI] [PubMed] [Google Scholar]
- 157. Zembron‐Lacny A, Dziubek W, Rogowski L, Skorupka E, Dabrowska G. Sarcopenia: monitoring, molecular mechanisms, and physical intervention. Physiol Res. 2014;63:683–691. [DOI] [PubMed] [Google Scholar]
- 158. Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL‐6 and TNF‐alpha. Eur J Med Res. 2017;22:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Puzianowska‐Kuznicka M, Owczarz M, Wieczorowska‐Tobis K, Nadrowski P, Chudek J, Slusarczyk P, et al. Interleukin‐6 and C‐reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing. 2016;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age‐related proinflammatory state. Blood. 2005;105:2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor‐alpha (TNF‐alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Paik JK, Chae JS, Kang R, Kwon N, Lee SH, Lee JH. Effect of age on atherogenicity of LDL and inflammatory markers in healthy women. Nutr Metab Cardiovasc Dis. 2013;23:967–972. [DOI] [PubMed] [Google Scholar]
- 163. McCormick R, Vasilaki A. Age‐related changes in skeletal muscle: changes to life‐style as a therapy. Biogerontology. 2018;19:519–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Little RD, Prieto‐Potin I, Perez‐Baos S, Villalvilla A, Gratal P, Cicuttini F, et al. Compensatory anabolic signaling in the sarcopenia of experimental chronic arthritis. Sci Rep. 2017;7:6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gaugler M, Brown A, Merrell E, DiSanto‐Rose M, Rathmacher JA, Reynolds TH. PKB signaling and atrogene expression in skeletal muscle of aged mice. J Appl Physiol (1985) 2011;111:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy‐related ubiquitin ligases, atrogin‐1 and MuRF1 are up‐regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev. 2006;127:794–801. [DOI] [PubMed] [Google Scholar]
- 168. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407–1412. [DOI] [PubMed] [Google Scholar]
- 169. Bossola M, Pacelli F, Costelli P, Tortorelli A, Rosa F, Doglietto GB. Proteasome activities in the rectus abdominis muscle of young and older individuals. Biogerontology. 2008;9:261–268. [DOI] [PubMed] [Google Scholar]
- 170. Deruisseau KC, Kavazis AN, Powers SK. Selective downregulation of ubiquitin conjugation cascade mRNA occurs in the senescent rat soleus muscle. Exp Gerontol. 2005;40:526–531. [DOI] [PubMed] [Google Scholar]
- 171. Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. [DOI] [PubMed] [Google Scholar]
- 172. Sandiford SD, Kennedy KA, Xie X, Pickering JG, Li SS. Dual oxidase maturation factor 1 (DUOXA1) overexpression increases reactive oxygen species production and inhibits murine muscle satellite cell differentiation. Cell Commun Signal. 2014;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Scherz‐Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Paris ND, Soroka A, Klose A, Liu W, Chakkalakal JV. Smad4 restricts differentiation to promote expansion of satellite cell derived progenitors during skeletal muscle regeneration. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Park SS, Kwon ES, Kwon KS. Molecular mechanisms and therapeutic interventions in sarcopenia. Osteoporos Sarcopenia. 2017;3:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol. 1993;18:331–358. [DOI] [PubMed] [Google Scholar]
- 177. Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sheth KA, Iyer CC, Wier CG, Crum AE, Bratasz A, Kolb SJ, et al. Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol Aging. 2018;67:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Barreto SG. Pancreatic cancer: let us focus on cachexia, not just sarcopenia! Future Oncol. 2018;14:2791–2794. [DOI] [PubMed] [Google Scholar]
- 180. Cruz‐Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7. [DOI] [PubMed] [Google Scholar]
- 181. Jensen GL. Inflammation: roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. 2008;32:656–659. [DOI] [PubMed] [Google Scholar]
- 182. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, et al. An evidence‐based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sim M, Prince RL, Scott D, Daly RM, Duque G, Inderjeeth CA, et al. Sarcopenia Definitions and Their Associations With Mortality in Older Australian Women. J Am Med Dir Assoc. 2019;20:76–82, e72. [DOI] [PubMed] [Google Scholar]
- 187. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 188. Carmeli E, Imam B, Merrick J. The relationship of pre‐sarcopenia (low muscle mass) and sarcopenia (loss of muscle strength) with functional decline in individuals with intellectual disability (ID). Arch Gerontol Geriatr. 2012;55:181–185. [DOI] [PubMed] [Google Scholar]
- 189. Woo J. Sarcopenia. Clin Geriatr Med. 2017;33:305–314. [DOI] [PubMed] [Google Scholar]
- 190. Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, et al. Sarcopenia: an overview. Aging Clin Exp Res. 2017;29:11–17. [DOI] [PubMed] [Google Scholar]
- 191. Landi F, Marzetti E, Martone AM, Bernabei R, Onder G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:25–31. [DOI] [PubMed] [Google Scholar]
- 192. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Barillaro C, Liperoti R, Martone AM, Onder G, Landi F. The new metabolic treatments for sarcopenia. Aging Clin Exp Res. 2013;25:119–127. [DOI] [PubMed] [Google Scholar]
- 194. Sanford AM. Anorexia of aging and its role for frailty. Curr Opin Clin Nutr Metab Care. 2017;20:54–60. [DOI] [PubMed] [Google Scholar]
- 195. Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle. 2015;6:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Morley JE. Anorexia of ageing: a key component in the pathogenesis of both sarcopenia and cachexia. J Cachexia Sarcopenia Muscle. 2017;8:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Amitani M, Asakawa A, Amitani H, Inui A. Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol. 2013;45:2179–2185. [DOI] [PubMed] [Google Scholar]
- 198. Coletti D. Chemotherapy‐induced muscle wasting: an update. Eur J Transl Myol. 2018;28:7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tisdale MJ. Cancer anorexia and cachexia. Nutrition. 2001;17:438–442. [DOI] [PubMed] [Google Scholar]
- 200. Morley JE. Pathophysiology of the anorexia of aging. Curr Opin Clin Nutr Metab Care. 2013;16:27–32. [DOI] [PubMed] [Google Scholar]
- 201. Laviano A, Koverech A, Seelaender M. Assessing pathophysiology of cancer anorexia. Curr Opin Clin Nutr Metab Care. 2017;20:340–345. [DOI] [PubMed] [Google Scholar]
- 202. Erskine HE, Whiteford HA, Pike KM. The global burden of eating disorders. Curr Opin Psychiatry. 2016;29:346–353. [DOI] [PubMed] [Google Scholar]
- 203. Yoshimura M, Uezono Y, Ueta Y. Anorexia in human and experimental animal models: physiological aspects related to neuropeptides. J Physiol Sci. 2015;65:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Nicolini A, Ferrari P, Masoni MC, Fini M, Pagani S, Giampietro O, et al. Malnutrition, anorexia and cachexia in cancer patients: A mini‐review on pathogenesis and treatment. Biomed Pharmacother. 2013;67:807–817. [DOI] [PubMed] [Google Scholar]
- 205. Wysokinski A, Sobow T, Kloszewska I, Kostka T. Mechanisms of the anorexia of aging‐a review. Age (Dordr) 2015;37:9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Laviano A, Meguid MM, Rossi‐Fanelli F. Cancer anorexia: clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol. 2003;4:686–694. [DOI] [PubMed] [Google Scholar]
- 207. Blauwhoff‐Buskermolen S, Ruijgrok C, Ostelo RW, de Vet HCW, Verheul HMW, de van der Schueren MAE, et al. The assessment of anorexia in patients with cancer: cut‐off values for the FAACT‐A/CS and the VAS for appetite. Support Care Cancer. 2016;24:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175–179. [DOI] [PubMed] [Google Scholar]
- 209. Inui A. Neuropeptide Y: a key molecule in anorexia and cachexia in wasting disorders? Mol Med Today. 1999;5:79–85. [DOI] [PubMed] [Google Scholar]
- 210. Sonti G, Ilyin SE, Plata‐Salaman CR. Neuropeptide Y blocks and reverses interleukin‐1 beta‐induced anorexia in rats. Peptides. 1996;17:517–520. [DOI] [PubMed] [Google Scholar]
- 211. Plata‐Salaman CR. Anorexia induced by activators of the signal transducer gp 130. Neuroreport. 1996;7:841–844. [DOI] [PubMed] [Google Scholar]
- 212. Turrin NP, Ilyin SE, Gayle DA, Plata‐Salaman CR, Ramos EJ, Laviano A, et al. Interleukin‐1beta system in anorectic catabolic tumor‐bearing rats. Curr Opin Clin Nutr Metab Care. 2004;7:419–426. [DOI] [PubMed] [Google Scholar]
- 213. Gayle D, Ilyin SE, Plata‐Salaman CR. Central nervous system IL‐1 beta system and neuropeptide Y mRNAs during IL‐1 beta‐induced anorexia in rats. Brain Res Bull. 1997;44:311–317. [DOI] [PubMed] [Google Scholar]
- 214. Plata‐Salaman CR, Ilyin SE, Gayle D. Brain cytokine mRNAs in anorectic rats bearing prostate adenocarcinoma tumor cells. Am J Physiol. 1998;275:R566–R573. [DOI] [PubMed] [Google Scholar]
- 215. Sonti G, Ilyin SE, Plata‐Salaman CR. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol. 1996;270:R1394–R1402. [DOI] [PubMed] [Google Scholar]
- 216. Davis MP, Dreicer R, Walsh D, Lagman R, LeGrand SB. Appetite and cancer‐associated anorexia: a review. J Clin Oncol. 2004;22:1510–1517. [DOI] [PubMed] [Google Scholar]
- 217. Cha SH, Lane MD. Central lactate metabolism suppresses food intake via the hypothalamic AMP kinase/malonyl‐CoA signaling pathway. Biochem Biophys Res Commun. 2009;386:212–216. [DOI] [PubMed] [Google Scholar]
- 218. Dwarkasing JT, Marks DL, Witkamp RF, van Norren K. Hypothalamic inflammation and food intake regulation during chronic illness. Peptides. 2016;77:60–66. [DOI] [PubMed] [Google Scholar]
- 219. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 220. Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, et al. Re‐validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res. 2000;9:1137–1146. [DOI] [PubMed] [Google Scholar]
- 221. Cuvelier GD, Baker TJ, Peddie EF, Casey LM, Lambert PJ, Distefano DS, et al. A randomized, double‐blind, placebo‐controlled clinical trial of megestrol acetate as an appetite stimulant in children with weight loss due to cancer and/or cancer therapy. Pediatr Blood Cancer. 2014;61:672–679. [DOI] [PubMed] [Google Scholar]
- 222. Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo‐controlled phase 2 study. Cancer. 2012;118:4785–4794. [DOI] [PubMed] [Google Scholar]
- 223. Watanabe S, Bruera E. Anorexia and cachexia, asthenia, and lethargy. Hematol Oncol Clin North Am. 1996;10:189–206. [DOI] [PubMed] [Google Scholar]
- 224. Cleary JF. The reversible causes of asthenia in cancer patients In Portenoy RK, Bruera E, eds. Topics in Palliative Care, Vol. 2 Oxford University Press; 1998. p 183–202. [Google Scholar]
- 225. Neuenschwander H, Bruera E. Pathophysiology of Cancer Asthenia In Portenoy RK, Bruera E, eds. Topics in Palliative Care, Vol. 2 Oxford University Press; 1998. p 171–182. [Google Scholar]
- 226. Theologides A. Anorexins, asthenins, and cachectins in cancer. Am J Med. 1986;81:696–698. [DOI] [PubMed] [Google Scholar]
- 227. Narayanan V, Koshy C. Fatigue in cancer: a review of literature. Indian J Palliat Care. 2009;15:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hinshaw DB, Carnahan JM, Johnson DL. Depression, anxiety, and asthenia in advanced illness. J Am Coll Surg. 2002;195:271–277, discussion 277‐278. [DOI] [PubMed] [Google Scholar]
- 229. Aparicio LM, Pulido EG, Gallego GA. Sunitinib‐induced asthenia: from molecular basis to clinical relief. Cancer Biol Ther. 2011;12:765–771. [DOI] [PubMed] [Google Scholar]
- 230. Scialla SJ, Cole RP, Bednarz L. Redefining cancer‐related asthenia‐fatigue syndrome. J Palliat Med. 2006;9:866–872. [DOI] [PubMed] [Google Scholar]
- 231. Bower JE. Cancer‐related fatigue‐‐mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Berger AM, Mooney K, Alvarez‐Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer‐related fatigue, Version 2. J Natl Compr Canc Netw. 2015;13:1012–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Gonçalves F., Cuidados Paliativos. In: Cancro do Pâncreas. Algés: Grupo de Investigação do Cancro Digestivo, 2017; pp 258‐274. [Google Scholar]
- 234. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. [DOI] [PubMed] [Google Scholar]
- 235. Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J Oncol. 2016;2016:6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 237. Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. [DOI] [PubMed] [Google Scholar]
- 238. Chuang LL, Lin KC, Hsu AL, Wu CY, Chang KC, Li YC, et al. Reliability and validity of a vertical numerical rating scale supplemented with a faces rating scale in measuring fatigue after stroke. Health Qual Life Outcomes. 2015;13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Gerber LH. Cancer‐related fatigue: persistent, pervasive, and problematic. Phys Med Rehabil Clin N Am. 2017;28:65–88. [DOI] [PubMed] [Google Scholar]
- 240. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle. 2019. Oct; 10(5): 1143‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


