Abstract
Background
Sarcopenia might function as an indicator for frailty, and as such as a risk factor for the development of postoperative complications. The aim of this study was to meta‐analyse the relation between preoperative sarcopenia and the development of severe postoperative complications in patients undergoing oncological surgery.
Methods
PubMed and Embase databases were systematically searched from inception until May 2018. Included were studies reporting on the incidence of severe postoperative complications and radiologically determined preoperative sarcopenia. Studies reporting the skeletal muscle as a continuous variable only were excluded. Data were extracted independently by two reviewers. Random effect meta‐analyses were applied to estimate the pooled odds ratio (OR) with 95% confidence intervals (95% CI) for severe postoperative complications, defined as Clavien‐Dindo grade ≥3, including 30‐day mortality. Heterogeneity was evaluated with I 2 testing. Analyses were performed overall and stratified by measurement method, tumour location and publication date.
Results
A total of 1924 citations were identified, and 53 studies (14 295 patients) were included in the meta‐analysis. When measuring the total skeletal muscle area, 43% of the patients were sarcopenic, versus 33% when measuring the psoas area. Severe postoperative complications were present in 20%, and 30‐day mortality was 3%. Preoperative sarcopenia was associated with an increased risk of severe postoperative complications (ORpooled: 1.44, 95% CI: 1.24–16.8, P<0.001, I 2=55%) and 30‐day mortality (ORpooled: 2.15, 95% CI: 1.46–3.17, P<0.001, I 2=14%). A low psoas mass was a stronger predictor for severe postoperative complications compared with a low total skeletal muscle mass (ORpooled: 2.06, 95% CI: 1.37–3.09, ORpooled: 1.32, 95% CI: 1.14–1.53, respectively) and 30‐day mortality [ORpooled: 6.17 (95% CI: 2.71–14.08, ORpooled: 1.80 (95% CI: 1.24–2.62), respectively]. The effect was independent of tumour location and publication date.
Conclusions
The presence of low psoas mass prior to surgery, as an indicator for sarcopenia, is a common phenomenon and is a strong predictor for the development of postoperative complications. The presence of low total skeletal muscle mass, which is even more frequent, is a less informative predictor for postoperative complications and 30‐day mortality. The low heterogeneity indicates that the finding is consistent over studies. Nevertheless, the value of sarcopenia relative to other assessments such as frailty screening is not clear. Research is needed in order to determine the place of sarcopenia in future preoperative risk stratification.
Keywords: Sarcopenia, Postoperative complications, Radiology, Surgery
1. Introduction
Surgery is part of the multimodality treatment of most solid tumours. Though very effective, surgical treatment may lead to postoperative complications.1, 2 Especially in frail patients, these complications can lead to permanent functional loss and negatively influence survival and long‐term quality of life.3, 4 The benefits of surgery should therefore be carefully weighed against these negative sequelae.5, 6 For this purpose, there has been increasing attention for methods to identify frail patients in recent years.4 In some cases, the presence of factors associated with frailty may lead to the decision not to perform a certain surgical procedure. In other cases, interventions may be undertaken to improve a patient's performance preoperatively in order to undergo surgery under the most optimal circumstances.
The presence of preoperative sarcopenia can be a possible method for detecting frail patients. Sarcopenia describes the loss of skeletal muscle mass associated with increased age, so called primary sarcopenia, or secondary to systemic conditions such as cancer or an inflammatory state.7, 8. The advantage of the use of sarcopenia over other screening methods for frailty, such as extensive questionnaires and assessments, is its objective, quantitative and relatively quick nature. Furthermore, the presence of sarcopenia can be determined on images routinely obtained in the oncological workup, and does therefore not require additional testing of the patient. Sarcopenia can be diagnosed with the use of clinical tests and/or measurement of the skeletal muscle mass.8 A common method for the detection of sarcopenia is the CT‐based estimation of the lean skeletal muscle mass.9, 10
Both a negative and a positive effect of the presence of sarcopenia on the development of severe complications have been reported. 11, 12, 13, 14, 15, 16, 17, 18 Therefore, we performed a systematic review and meta‐analysis of all the studies that recorded incidences of severe postoperative complications in patients with or without sarcopenia, undergoing oncological surgery for any type of solid tumour.
2. Methods
2.1. Search strategy
The Preferred Reporting Items for Systematic Review and Meta‐analysis (PRISMA) and the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed in this systematic review and meta‐analysis.19, 20
We searched the PubMed and Embase databases for studies reporting on sarcopenia and postoperative complications published form the inception of each database to May 1, 2018. The search terms were ‘sarcopenia’, ‘postoperative complications’, ‘neoplasms’, and ‘surgery’, with synonyms for each (see Supplementary File S1 for search strategy). No filters or restrictions were applied.
2.2. Selection criteria
The following studies were included: Studies should report on the development of severe postoperative complications in the first 30 days after surgery in adult patients. Patients had to undergo surgical resection of any type of malignancy. Only studies were included that applied a CT‐based assessment of skeletal muscle mass with use of the skeletal muscle area (SMI) and psoas area (TPI) on the level of the third lumbar vertebra. Furthermore, only studies were included when the presence or absence of sarcopenia was based on a clearly defined cut‐off. Studies were excluded when skeletal muscle mass was reported as a continuous variable. Studies describing only a specific type of postoperative complication or studies that did not distinguish severe complications from less severe or overall complications were excluded. Non‐English studies were excluded. When different publications described the same set of patients, the most recent study was included in the here presented meta‐analysis.
2.3. Endpoints
The primary endpoint was the presence of severe postoperative complications within the first 30 days after surgery. Severe complications were classified with use of the Clavien‐Dindo scale; complications with score of ≥3 were considered severe postoperative complications.21 The 30‐day mortality was considered separately. Data about the 30‐day mortality was obtained in two different ways: firstly, as the 30‐day mortality apart from the severe complications in studies that reported them separately and secondly, as a grade V complication on the Clavien‐Dindo scale, being a subgroup of the total number of postoperative complications. Therefore, in the latter studies, the data about patients with grade V complications were used in both the analysis regarding the severe postoperative complications and in the analysis regarding the 30‐day mortality.
2.4. Data extraction and quality assessment
The first author, year of publication, total study population, median age, gender, location of the malignancy, number of patients with and without sarcopenia and the number of patients with postoperative complications was extracted from each study.
We assessed the methodological quality of the included studies using the Newcastle‐Ottawa scale for cohort studies.22 This scale assesses the patient selection, comparability and outcomes. The outcomes of the assessments were converted in order to classify each study as having a good, fair or poor methodological quality. Two reviewers (L. W. and A. H.) independently performed the process of study selection, data extraction and quality assessment. Disagreements were resolved by consensus.
2.5. Statistical analysis
For each study. we calculated odds ratios (ORs) and 95% confidence intervals (95% CI) for postoperative complications in patients with and without preoperative sarcopenia. A random‐effect model was used for all analyses as we expected considerable variation in types of cancer and cut‐off values for the definition of sarcopenia. Heterogeneity was assessed with use of the I 2 statistics and was interpreted as follows: 0–40% low heterogeneity, 30–60% moderate heterogeneity, 50–90% substantial heterogeneity and 75–100% considerable heterogeneity.23 To explore sources of heterogeneity, we performed subgroup analysis with studies stratified by: measurement method of sarcopenia (total skeletal muscle mass versus psoas mass), tumour location and publication date. The stratification was performed for both severe postoperative complications and 30‐day mortality separately. We only included strata considering two or more studies. The null hypothesis, that the relationship between sarcopenia and postoperative complications or 30‐day mortality is equal across the defined strata, was tested with a X 2 test. All analyses were performed with use of Review Manager (RevMan) 5.3. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
3. Results
3.1. Included studies
A total of 1924 articles was identified by our search. A total of 140 studies met the eligibility criteria and of those studies 53 were included in the meta‐analysis (Figure 1). Cross reference search did not provide additional studies.
Figure 1.
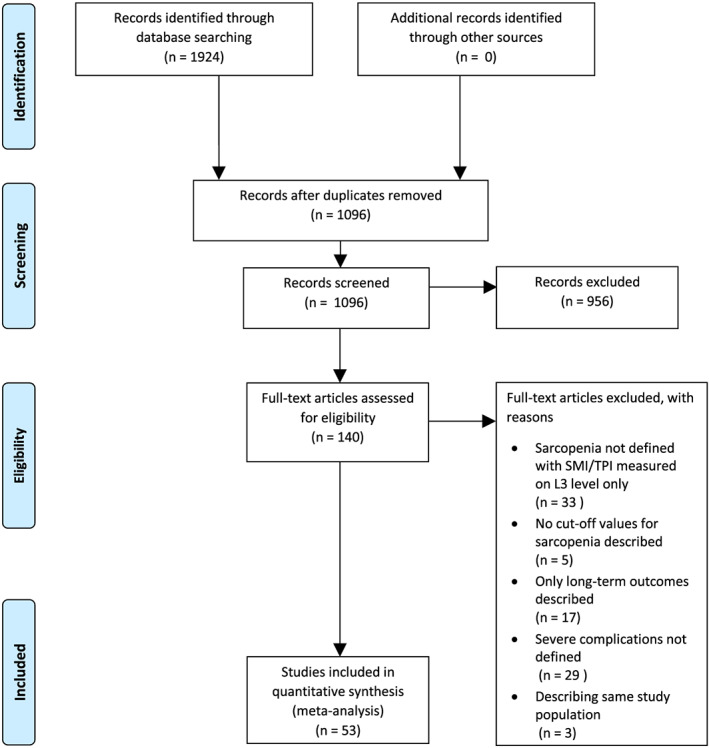
Flowchart of included studies
3.2. Quality of the included studies
A summary of the methodological quality of the included studies is presented in Table 1. A total of 20 studies (38%) did have a good methodological quality. In the remaining 33 studies (62%), the methodological quality was poor, due to a low score on the comparability domain.
Table 1.
Methodological quality of the included studies with regard to our study question
| Study | Selection | Comparabilitya | Outcome | Overall quality |
|---|---|---|---|---|
| Amini, 201524 |

|

|

|

|
| Banaste, 201716 |

|

|

|

|
| Chemama, 201625 |

|

|

|

|
| Choi, 201826 |

|

|

|

|
| Coelen, 201527 |

|

|

|

|
| Elliot, 201728 |

|

|

|

|
| Grotenhuis, 201629 |

|

|

|

|
| Harada, 201530 |

|

|

|

|
| Harimoto, 201331 |

|

|

|

|
| Higashi, 201532 |

|

|

|

|
|
Jarvinen, 201833 |

|

|

|

|
| Jones, 201534 |

|

|

|

|
| Kudou, 201735 |

|

|

|

|
| Kuwada, 201836 |

|

|

|

|
| Levolger, 201537 |

|

|

|

|
| Lodewick, 201538 |

|

|

|

|
| Malietzis, 201639 |

|

|

|

|
| Mason, 201740 |

|

|

|

|
| Mayr, 201841 |

|

|

|

|
| Nakamura, 201842 |

|

|

|

|
| Nakashima, 201843 |

|

|

|

|
| Nakanishi, 201844 |

|

|

|

|
| Nimomiya, 201745 |

|

|

|

|
| Nishida, 201646 |

|

|

|

|
| Nishigori, 201647 |

|

|

|

|
| Okumura, 2016 48 |

|

|

|

|
| Okumura, 2015a49 |

|

|

|

|
| Okumura, 2015b50 |

|

|

|

|
| Otsuji, 201551 |

|

|

|

|
| Ouchi, 201652 |

|

|

|

|
| Pędziwiatr, 201618 |

|

|

|

|
| Peng, 201153 |

|

|

|

|
| Peyton, 201654 |

|

|

|

|
| Rutten, 201755 |

|

|

|

|
| Saeki, 201856 |

|

|

|

|
| Sakurai, 201715 |

|

|

|

|
| Da Silva, 201857 |

|

|

|

|
| Smith, 201458 |

|

|

|

|
| Sui, 201759 |

|

|

|

|
| Takagi, 2016 60 |

|

|

|

|
| Takagi, 2017a61 |

|

|

|

|
| Takagi, 2017b62 |

|

|

|

|
| Takeda, 201863 |

|

|

|

|
| Tegels, 201564 |

|

|

|

|
| Umetsu, 201865 |

|

|

|

|
| Valero, 201566 |

|

|

|

|
| Van der Kroft, 201867 |

|

|

|

|
| Van Vught, 201568 |

|

|

|

|
| Van Vught, 201769 |

|

|

|

|
| Van Vught, 201870 |

|

|

|

|
| Voron, 201571 |

|

|

|

|
| Wagner, 201872 |

|

|

|

|
| Zhuang, 201673 |

|

|

|

|
Studies were considered of good quality on the comparability domain if either selected cohorts or a multivariate analysis on the effect of sarcopenia on the development of postoperative complications were present.
3.3. Study characteristics
The included studies concerned 14 295 patients. The most reported tumour location was hepatiopancreaticobiliary (22/53 studies), followed by upper gastro intestinal (GI) (12/53 studies), and lower GI (12/53 studies). The estimated pooled median age of the included studies was 61 years. A total of 25 (47%) studies reported a median age ≥65 years. The majority of the patients, 78%, in the included studies was male. Half (26/53) of the included studies were published before 2017 and 27 studies in 2017 and 2018. Characteristics of the included studies are displayed in Table 2.
Table 2.
Characteristics of included studies
| Study | Population (N) | Agea | Males | Location of malignancyb | SMI/TPI | Sarcopenia | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Amini, 201524 | 763 | 67 (58–74) | 418 | 55 | HPB | TPI | 192 | 25.1 |
| Banaste, 201716 | 214 | 59 (24–78) | 105 | 49 | Lower GI | SMI | 90 | 42.1 |
| Chemama, 201625 | 97 | 53 (46–62) | 37 | 38 | Lower GI | SMI | 39 | 40.0 |
| Choi, 201826 | 180 | 64 ± 9 | 98 | 54 | HPB | SMI | 60 | 33.3 |
| Coelen, 201527 | 100 | 62 ±9 | 64 | 64 | HPB | SMI | 42 | 42.0 |
| Elliot, 201728 | 192 | 62 ± 9 | 156 | 81 | Upper GI | SMI | 49 | 25.5 |
| Grotenhuis, 201629 | 120 | 62 (19–78) | 88 | 73 | Upper GI | SMI | 54 | 45.0 |
| Harada, 201530 | 256 | NRc | 234d | 92 | Upper GI | TPI | 84 | 32.8 |
| Harimoto, 201331 | 186 | 67 ± 11 | NR | NR | HPB | SMI | 75 | 40.3 |
| Higashi, 201532 | 144 | 65 ± 10 | 108 | 75 | HPB | SMI | 72 | 50.0 |
| Jarvinen, 201833 | 115 | 63 ±9 | 86 | 75 | Upper GI | SMI | 92 | 80.0 |
| Jones, 201534 | 100 | 69 ±10 | 60 | 60 | Lower GI | TPI | 15 | 15.0 |
| Kudou, 201735 | 148 | NR | NR | NR | Upper GI | SMI | 42 | 28.4 |
| Kuwada, 201836 | 491 | ~71e | 348 | 71 | Upper GI | SMI | 123 | 25.1 |
| Levolger, 201537 | 90 | 62 (22–86) | 63 | 70 | HPB | SMI | 52 | 57.8 |
| Lodewick, 201538 | 171 | 64 (24–86) | 104 | 61 | HPB | SMI | 80 | 46.8 |
| Malietzis, 201639 | 805 | 69 (961–77) | 472 | 59 | Lower GI | SMI | 485 | 60.2 |
| Mason, 201740 | 698 | 62 ±7 | 698 | 100 | Urogenital | SMI | 388 | 55.6 |
| Mayr, 201841 | 327 | 70 (63–75) | 262 | 80 | Urogenital | SMI | 108 | 33.0 |
| Nakamura, 201842 | 328 | 71 (38–87) | 195 | 59 | Other | TPI | 183 | 55.8 |
| Nakashima, 201843 | 341 | NR | 289 | 85 | Upper GI | SMI | 171 | 50.1 |
| Nakanishi, 201844 | 494 | 66 ± 12 | 298 | 60 | Lower GI | SMI | 298 | 60.3 |
| Nimomiya, 201745 | 265 | 65 ± 10 | 164 | 62 | HPB | SMI | 170 | 64.2 |
| Nishida, 201646 | 266 | 69 (27‐87) | 181 | 68 | HPB | SMI | 132 | 49.6 |
| Nishigori, 201647 | 199 | ~65e | 164 | 82 | Upper GI | SMI | 149 | 74.8 |
| Okumura, 2016 48 | 207 | ~67e | 111 | 54 | HPB | TPI | 71 | 32.4 |
| Okumura, 2015a49 | 230 | 67 (32–87) | 124 | 54 | HPB | TPI | 64 | 32.1 |
| Okumura, 2015b50 | 109 | 68 (63–74) | 67 | 62 | HPB | SMI | 69 | 63.3 |
| Otsuji, 201551 | 256 | 67 (34–85) | 162 | 63 | HPB | TPI | 85 | 33.2 |
| Ouchi, 201652 | 60 | 69 (43–88) | 35 | 58 | Lower GI | TPI | 20 | 33.3 |
| Pędziwiatr, 201618 | 124 | 66 (range NR) | 73 | 59 | Lower GI | SMI | 34 | 27.4 |
| Peng, 201153 | 259 | 58 ± 12 | 155 | 60 | HPB | TPI | 41 | 15.8 |
| Peyton, 201654 | 128 | 63 (31–85) | 85 | 66 | Urogenital | TPI | 32 | 25.0 |
| Rutten, 201755 | 216 | 63 (16–85) | 0 | 0 | Urogenital | SMI | 70 | 32.4 |
| Saeki, 201856 | 157 | 65 (range NR) | 122 | 78 | Upper GI | SMI | 85 | 54.1 |
| Sakurai, 201715 | 569 | 67 ± 11 | 396 | 70 | Upper GI | SMI | 142 | 24.9 |
| Da Silva, 201857 | 250 | NR | 0 | 0 | Urogenital | SMI | 56 | 22.4 |
| Smith, 201458 | 200 | 66 ± 12 | 141 | 71 | Urogenital | TPI | 77 | 38.5 |
| Sui, 201759 | 354 | 70 ± 11 | 203 | 57 | HPB | SMI | 87 | 24.6 |
| Takagi, 2016 60 | 254 | 66 ± 11 | 207 | 82 | HPB | SMI | 118 | 46.5 |
| Takagi, 2017a61 | 154 | 65 ± 13 | 90 | 58 | HPB | SMI | 38 | 24.7 |
| Takagi, 2017b62 | 219 | 66 ±12 | 143 | 65 | HPB | SMI | 55 | 25.1 |
| Takeda, 201863 | 144 | ~61e | 102 | 71 | Lower GI | SMI | 37 | 25.7 |
| Tegels, 201564 | 152 | 70 (37–88) | 87 | 57 | Upper GI | SMI | 86 | 56.6 |
| Umetsu, 201865 | 65 | 72 (31–81) | 47 | 72 | HPB | TPI | 48 | 73.8 |
| Valero, 201566 | 96 | 62 ± 12 | 59 | 61 | HPB | TPI | 47 | 49.0 |
| Van der Kroft, 201867 | 63 | NR | 39 | 64 | Lower GI | SMI | 33 | 52.4 |
| Van Vught, 201568 | 206 | ~61e | 100 | 49 | Lower GI | SMI | 90 | 43.7 |
| Van Vught, 201769 | 452 | 65 (58–71) | 278 | 62 | Lower GI | SMI | 206 | 45.6 |
| Van Vught, 201870 | 816 | ~70e | 440 | 54 | Lower GI | SMI | 412 | 50.5 |
| Voron, 201571 | 109 | 62 ±13 | 92 | 84 | HPB | SMI | 59 | 54.1 |
| Wagner, 201872 | 424 | 63 (19–87) | 203 | 48 | HPB | TPI | 145 | 34.2 |
| Zhuang, 201673 | 937 | 64 ± 15 | 730 | 78 | Upper GI | SMI | 389 | 41.5 |
Mean ± SD, median (range), a: interquartile range.
HPB: liver, pancreas, bile ducts; Upper GI: esophagus, stomach; Lower GI: colon, rectum, peritoneal carcinomatosis; urogenital: kidney, prostate, bladder, ovary, endometrium; Other.
NR: not reported.
Estimated, based on both included and excluded patients.
Estimated, calculation based on mean/median age in different groups.
3.4. Sarcopenia
In 40/53 studies (75%), the total skeletal muscle mass was used to determine sarcopenia. The psoas mass was used in 13/53 (25%) of the studies. The cut‐off values used to determine low total skeletal muscle mass and low psoas mass, respectively, are listed in Table S1, ranging from 40.5 to 71.6 in male patients and 33.5 to 55.3 in female patients for low total skeletal mass and ranging 4.3–7.8 in males and 3.8–6.4 in females for low psoas mass. A total of 5938 patients (42%) were sarcopenic. In studies using the total skeletal muscle mass, a total of 4849 patients (43%) were considered sarcopenic, versus 1089 patients (33%) in studies measuring the psoas area.
3.5. Severe postoperative complications
A total of 2920 patients (20%) with severe postoperative complications were recorded. A total of 1398 patients with sarcopenia (24%) and 1522 patients without sarcopenia (18%) developed a severe postoperative complication (see Figure 2). ORpooled: 1.44 (95% CI 1.24–16.8, P<0.001).
Figure 2.
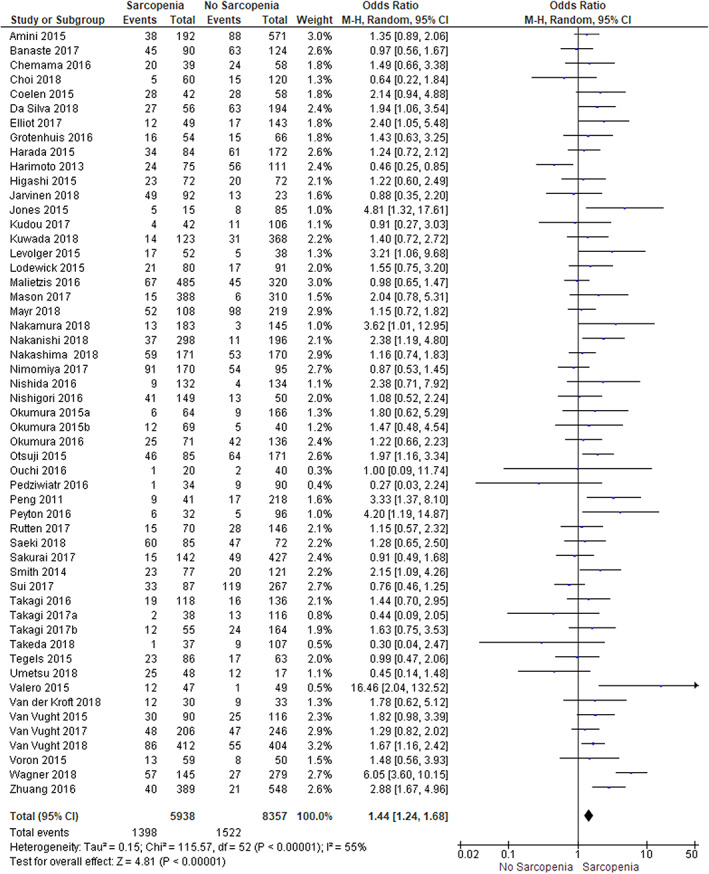
Sarcopenia and the development of severe postoperative complications
The I 2 was 55%, representing moderate heterogeneity. The outcomes of the analysis did not change when only studies with good methodological quality were included in the analysis (Figure S1). Subgroup analysis on the effect of the use of total skeletal muscle mass or psoas mass showed a significant difference between the subgroups (P 0.04). The pooled OR for the effect of the presence of sarcopenia on the development of severe postoperative complications was 1.32 (95% CI 1.14–1.53) in studies using total skeletal muscle mass and 2.06 (95% CI 1.37–3.09) in studies using the psoas mass (Figure 3a). Subgroup analysis based on tumour location or date of publication showed no differences between the subgroups. (Figures S2 and S3).
Figure 3.
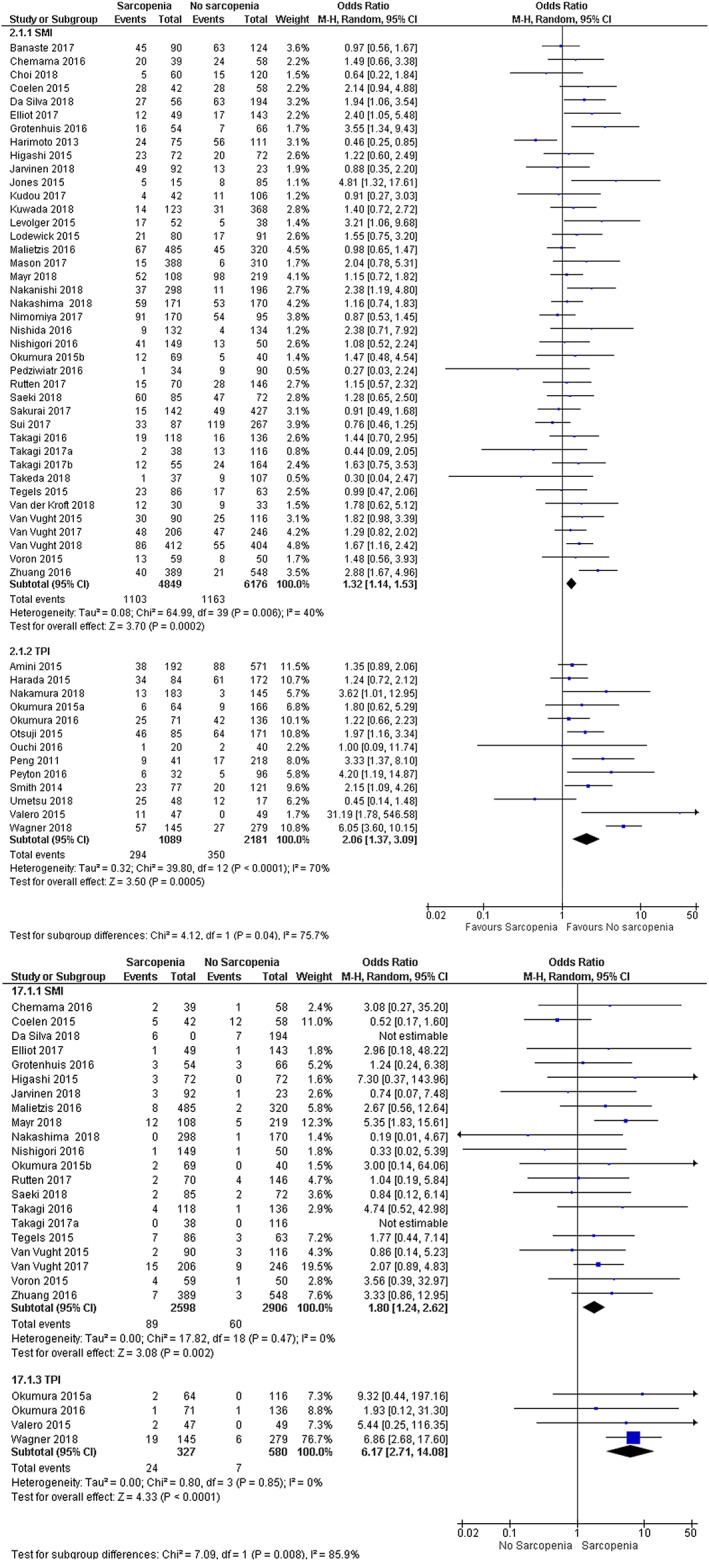
Predictive value of total skeletal muscle mass and total psoas mass (a) Severe postoperative complications (b) 30‐day mortality
3.6. Thirty‐day mortality
A number of 25 studies with a total of 6411 patients reported on 30‐day mortality. Sarcopenia was present in 46% of the patients, and the pooled 30‐day mortality was 3%. In patients with sarcopenia, 30‐day mortality was 4% and in patients without sarcopenia 2% [ORpooled: 2.15 (95% CI 1.46–3.17, P<0.001)] (Figure 4). The I 2 was 14%, indicating little heterogeneity.
Figure 4.
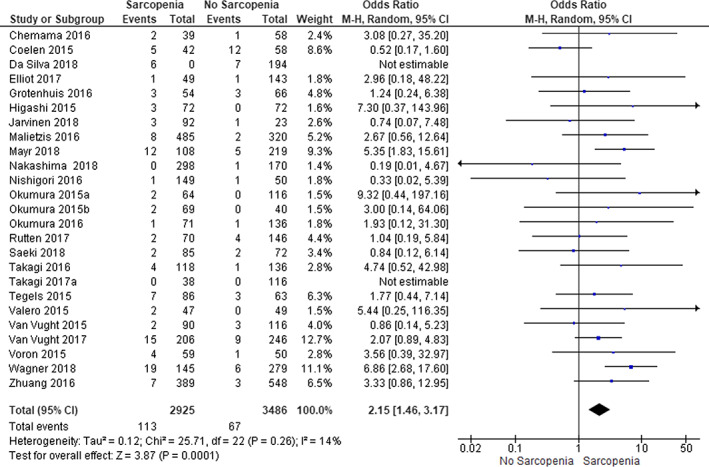
Sarcopenia and 30 day mortality
Subgroup analysis for the effect of the use of the total skeletal muscle mass or the psoas mass for the detection of sarcopenia showed a significant difference between the subgroups. The pooled OR for the effect of sarcopenia on the 30‐day mortality in studies using the total skeletal muscle mass was 1.80 (95% CI 1.24–2.62) versus 6.17 (95% CI 2.71–14.08) in studies using the psoas mass, P<0.001 (Figure 3b).
The subgroup analyses on the effect of tumour location and date of publication showed no significant differences between the subgroups. Subgroup analyses are presented in Figures S2 and S3.
4. Discussion
In this meta‐analysis 14 295 surgical oncological patients were included from 53 studies, where 20/53 were good quality studies. Results did not change when only good quality studies were included. Most studies (22/53) concerned patients diagnosed with a hepaticopancreaticobiliary malignancy. The prevalence of radiologically determined sarcopenia is relatively high, with a prevalence of 43% when sarcopenia was based on total skeletal mass and 33% for sarcopenia based on psoas mass. Preoperative sarcopenia was associated with an increased risk of severe postoperative complications (ORpooled: 1.44, 95% CI: 1.24–16.8, P<0.001, I 2=55%) and 30‐day mortality (ORpooled: 2.15, 95% CI: 1.46–3.17, P<0.001, I 2=14%). A low psoas mass was a stronger predictor for severe postoperative complications (ORpooled: 2.06, 95% CI: 1.37–3.09, ORpooled: 1.32, 95% CI: 1.14–1.53, respectively) and 30‐day mortality compared with a low total skeletal muscle mass [ORpooled: 6.17 (95% CI: 2.71–14.08), ORpooled: 1.80 (95% CI: 1.24‐2.62), respectively]. The effect on the risk of severe complications was independent of tumour location and publication date.
Our analysis showed that the presence of low psoas mass prior to surgery is a stronger predictor for the development of postoperative complications when compared with the presence of low total skeletal muscle mass. This finding has not been reported earlier. This makes the measurement of the psoas mass a more adequate risk factor than the measurement of the total skeletal muscle mass in the selection of patients with an increased risk of developing postoperative complications. A possible explanation for the greater effect of a low total psoas mass compared with a low total skeletal muscle mass might be that the quantification of the psoas muscles reflects the physical condition of a patient better than the total skeletal muscle area. The relation between a low psoas mass and a decreased physical performance has been discussed in literature.74, 75 The psoas muscles are only active during standing, bending, and lifting, representing an active lifestyle.76 A decreased psoas mass could therefore indicate that the patient is less active, has a decreased physical condition, and might subsequently be more frail and vulnerable for the development of postoperative complications. Furthermore, when a postoperative complication does occur, the impact of the complication could possibly be less well compensated, which can lead to a higher mortality risk in the postoperative period. Another aspect is that low psoas mass is also related to longer term postoperative mortality among different groups of patients.24, 77, 78 Thus suggesting that low psoas mass might not only reflect a decreased physical condition on the short term but also represent physical changes with effects lasting over time.
The use of radiologically determined sarcopenia in preoperative risk stratification has some restrictions. Radiologically assessed sarcopenia is present in a large number of patients. When measured with total skeletal muscle mass, sarcopenia is present in almost half of the study population, and when measured with psoas mass in one‐third of the patients. The lower prevalence of the low psoas mass emphasizes its better suitability over the total skeletal muscle mass to select those patients that are at an increased risk for the development of postoperative complications. The widespread presence of sarcopenia reduces its discriminative value between those at an increased risk and those not at an increased risk for the development of postoperative complications. In addition, in this work, the reported value of a low psoas mass as predictor for the development of postoperative complications is outperformed by tests incorporating strength and coordination, i.e. the Timed Up and Go (TUG) (OR: 3.43, 95% CI 1.14–10.35 versus ORpooled: 2.06, 95% CI: 1.37–3.09, respectively).79 A less clear picture is present when the predictive value of a low psoas mass is compared with the presence of frailty. Different frailty assessments yield different ORs for the prediction of postoperative complications, with ORs ranging 1.80–6.40.3, 4 The OR for low psoas mass is in the lower range of this spectrum. This indicates that the value of the assessment of the psoas mass as a replacement for frailty screening is limited. Nevertheless, sarcopenia is a cause of physical frailty, and assessment of sarcopenia might help to select those patients that are physical frail.80 Therefore, despite the limited value of radiologically assessed sarcopenia as a stand‐alone risk factor, screening on sarcopenia with use of the psoas mass might be an addition to existing multi‐domain assessments.
An important strength of this meta‐analysis is the quality of the studies used in this analysis. Next to the 17 studies marked as having a good methodological quality, a total of 25 other studies did perform a multivariate analysis for other outcomes. With the presence of a multivariate analysis in itself as an indicator of quality, almost 80% of the studies included in this meta‐analysis can be considered as having a good methodological quality. Another strong point is the fairly high homogeneity in this meta‐analysis, thus showing that the results are consistent across studies and can be used in the general population of patients with abdominal malignancies. Furthermore, this research showed that there is no selection bias for patients undergoing surgical treatment for a malignancy based on ‘eyeballing’ for the presence of sarcopenia. The number of patients with sarcopenia undergoing oncologic surgery remains relatively stable over the years. If the selection bias was present, a decrease in the number of patients with sarcopenia who underwent major oncological surgery should be expected. This study also has some limitations. First, all included studies were retrospective cohort studies, with known disadvantages regarding risk of bias and potential missing data. Secondly, the used cut‐off values for total skeletal muscle mass and psoas mass in the studies included in this analysis varied greatly. In the studies included in this meta‐analysis, a total of 37 different cut‐off values, divided in 24 different values for studies using the total skeletal muscle mass and 13 different values for studies using the psoas mass, were used, limiting the degree of comparability between the outcomes of the different studies. Furthermore, the number of studies reporting on the psoas mass is relatively small. However, with over 3000 patients included in these studies, the number of included patients is deemed large enough to reliably determine the differences between the psoas mass and the total skeletal muscle mass as a risk factor for postoperative morbidity. Another limitation is the exclusion of studies investigating the influence of the total skeletal muscle mass as a continuous variable (N=5), without cut‐off values to determine sarcopenia, on the development of sarcopenia. The exclusion of these studies does lead to missing of data, but evaluation of these studies showed the same trend in the relationship between skeletal muscle mass and sarcopenia as was described in this meta‐analysis.
Based on the results of this meta‐analysis, several subjects for future research arise. An important subject is combining the radiologically assessed psoas mass with tools assessing muscle strength and functioning, and possible also including factors as coordination and cognition. Assessment of the psoas mass adds quantification of the lean skeletal muscle mass to the existing information. This might lead to a better assessment of the patient physical condition and might subsequently further improve preoperative risk stratification. Furthermore, the position of sarcopenia relative to frailty in the preoperative risk stratification should be evaluated. As mentioned, sarcopenia can contribute to frailty, but it does not equal frailly.80 Therefore, it is not clear whether patients who are deemed frail are the same patients who are considered sarcopenic. Research is needed to explore the agreements and differences between those patient groups in order to determine the place of sarcopenia in future preoperative risk stratification. Another topic is the standardization of cut‐off values for both total psoas mass and total skeletal muscle mass. As mentioned above, use of standardized cut‐off values improves the comparability between studies. Furthermore, with standardization, it becomes more clear, which patients are considered sarcopenic and have increased risk of developing postoperative complications, improving the utility of radiologically assessed in clinical practice. An entirely other subject of future research is the preoperative ‘treatment’ of sarcopenia. Literature showed that resistance training can be effective to improve muscle strength, skeletal muscle mass and physical function.81 However, the effects of resistance training on postoperative outcomes is unclear. Furthermore, the benefits of resistance training are not limited to sarcopenic patients alone but to all elderly, regardless of whether or not they have sarcopenia,82 which advocates for the encouragement of physical activity in all elderly, not limited to those suffering from sarcopenia.
In summary, radiologically assessed preoperative sarcopenia is associated with the development of postoperative complications. The presence of low psoas mass surpasses the presence of low skeletal muscle mass as a risk factor for the development of severe postoperative complications, and even more so as a risk factor for 30‐day mortality, in patients undergoing surgery for a solid malignancy. The addition of assessment of the total psoas mass to existing screening tools focussing on muscle strength and coordination could lead to further improvement of preoperative risk stratification in surgical oncology.
Conflict of interest
None declared.
Supporting information
Data S1. Supporting Information
Figure S1. Comparison of studies with a good versus a poor methodological quality
Figure S1a. Severe postoperative complications
Figure S1b. 30‐day mortality
Figure S2. Stratification by tumour location
Figure S2a. Severe postoperative complications: grouped tumour location
Figure S2b. Severe postoperative complications: specific tumour location
Figure S2c. 30‐day mortality: grouped tumour location
Figure S2d. 30‐day mortality: specific tumour location
Figure S3a. Severe postoperative complications
Figure S3b. 30‐day mortality
Acknowledgements
There were no sources of funding. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.83
Weerink L. B. M., van der Hoorn A., van Leeuwen B. L., and de Bock G. H. (2020) Low skeletal muscle mass and postoperative morbidity in surgical oncology: a systematic review and meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 11, 636–649. 10.1002/jcsm.12529.
References
- 1. Szakmany T, Ditai J, Kirov M, Protsenko D, Osinaike B, Venara A, et al. In‐hospital clinical outcomes after upper gastrointestinal surgery: data from an international observational study. Eur J Surg Oncol. 2017;43:2324–2332. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4:5‐9493‐4‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watt J, Tricco AC, Talbot‐Hamon C, Pham B, Rios P, Grudniewicz A, et al. Identifying older adults at risk of harm following elective surgery: a systematic review and meta‐analysis. BMC Med. 2018;16:2‐017‐0986‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huisman MG, Kok M, de Bock GH, van Leeuwen BL. Delivering tailored surgery to older cancer patients: preoperative geriatric assessment domains and screening tools—a systematic review of systematic reviews. Eur J Surg Oncol. 2017;43:1–14. [DOI] [PubMed] [Google Scholar]
- 5. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, et al. Determinants of long‐term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341, discussion 341‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weerink LBM, Gant CM, van Leeuwen BL, de Bock GH, Kouwenhoven EA, Faneyte IF. Long‐term survival in octogenarians after surgical treatment for colorectal cancer: prevention of postoperative complications is key. Ann Surg Oncol. 2018;25:3874–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 10. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 11. Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–1458. [DOI] [PubMed] [Google Scholar]
- 12. Jones K, Gordon‐Weeks A, Coleman C, Silva M. Radiologically determined sarcopenia predicts morbidity and mortality following abdominal surgery: a systematic review and meta‐analysis. World J Surg. 2017;41:2266–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol. 2015;112:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta‐analysis. Ann Surg. 2018. Jul;268:58–69. [DOI] [PubMed] [Google Scholar]
- 15. Sakurai K, Kubo N, Tamura T, Toyokawa T, Amano R, Tanaka H, et al. Adverse effects of low preoperative skeletal muscle mass in patients undergoing gastrectomy for gastric cancer. Ann Surg Oncol. 2017;24:2712–2719. [DOI] [PubMed] [Google Scholar]
- 16. Banaste N, Rousset P, Mercier F, Rieussec C, Valette P, Glehen O, et al. Preoperative nutritional risk assessment in patients undergoing cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for colorectal carcinomatosis. Int J Hyperthermia. 2017;1–6. [DOI] [PubMed] [Google Scholar]
- 17. Choi B, Lee Y, An S, Lee S, Lee E, Noh G. Population pharmacokinetics and analgesic potency of oxycodone. Br J Clin Pharmacol. 2017;83:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedziwiatr M, Pisarska M, Major P, Grochowska A, Matlok M, Przeczek K, et al. Enhanced Recovery after Surgery Protocol (ERAS) combined with laparoscopic colorectal surgery diminishes the negative impact of sarcopenia on short‐term outcomes. Clin Nutr ESPEN. 2016;12:e49. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 21. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 07/01 2018.
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amini N, Gupta R, Margonis GA, Kim Y, Spolverato G, Rezaee N, et al. Impact of sarcopenia on short‐and long‐term outcomes in patients undergoing curative intent resection for pancreatic adenocarcinoma: a new tool. Gastroenterology. 2015;148:S1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chemama S, Bayar MA, Lanoy E, Ammari S, Stoclin A, Goéré D, et al. Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol. 2016;23:3891–3898. [DOI] [PubMed] [Google Scholar]
- 26. Choi MH, Yoon SB, Lee K, Song M, Lee IS, Lee MA, et al. Preoperative sarcopenia and post‐operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coelen RJS, Wiggers JK, Nio CY, Besselink MG, Busch ORC, Gouma DJ, et al. Preoperative computed tomography assessment of skeletal muscle mass is valuable in predicting outcomes following hepatectomy for perihilar cholangiocarcinoma. HPB. 2015;17:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elliott JA, Murphy CF, Docherty NG, Doyle SL, Gallardo AL, Gahan C, et al. Pathophysiologic gut hormone and bile acid signalling after oesophagectomy: implications for appetite, postprandial hypoglycaemia and nutritional status in survivorship. Int J Surg. 2017;47:S91. [Google Scholar]
- 29. Grotenhuis BA, Shapiro J, van Adrichem S, de Vries M, Koek M, Wijnhoven BP, et al. Sarcopenia/muscle mass is not a prognostic factor for short‐ and long‐term outcome after esophagectomy for cancer. World J Surg. 2016;40:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harada K, Ida S, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 2016. Aug;29:627–633. [DOI] [PubMed] [Google Scholar]
- 31. Harimoto N, Shirabe K, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. [DOI] [PubMed] [Google Scholar]
- 32. Higashi T, Hayashi H, Taki K, Sakamoto K, Kuroki H, Nitta H, et al. Sarcopenia, but not visceral fat amount, is a risk factor of postoperative complications after major hepatectomy. Int J Clin Oncol. 2016;21:310–319. [DOI] [PubMed] [Google Scholar]
- 33. Jarvinen T, Ilonen I, Kauppi J, Salo J, Rasanen J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2018;16:27‐018‐1327‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones K, Doleman B, Shore S, Clarke J, Lund J, Williams J. Impact of sarcopenia on postoperative morbidity in colorectal cancer patients undergoing curative resection. Br J Anaesth. 2014;112:192P. [Google Scholar]
- 35. Kudou K, Saeki H, Nakashima Y, Edahiro K, Korehisa S, Taniguchi D, et al. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann Surg Oncol. 2017;24:1804–1810. [DOI] [PubMed] [Google Scholar]
- 36. Kuwada K, Kuroda S, Kikuchi S, Yoshida R, Nishizaki M, Kagawa S, et al. Sarcopenia and comorbidity in gastric cancer surgery as a useful combined factor to predict eventual death from other causes. Ann Surg Oncol. 2018;25:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levolger S, Van Vledder MG, Muslem R, Koek M, Niessen WJ, De Man RA, et al. Sarcopenia impairs survival in patients with potentially curable hepatocellular carcinoma. J Surg Oncol. 2015;112:208–213. [DOI] [PubMed] [Google Scholar]
- 38. Lodewick TM, Van Nijnatten TJA, Van Dam RM, Van Mierlo K, Dello SAWG, Neumann UP, et al. Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB. 2015;17:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malietzis G, Johns N, Al Hassi HO, Knight SC, Kennedy RH, Fearon KCH, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surger y for colorectal cancer. Ann Surg. 2016;263:320–325. [DOI] [PubMed] [Google Scholar]
- 40. Mason RJ, Boorjian SA, Bhindi B, Rangel L, Frank I, Karnes RJ, et al. The association between sarcopenia and oncologic outcomes after radical prostatectomy. Clin Genitourin Cancer. 2018;16:e629–e636. [DOI] [PubMed] [Google Scholar]
- 41. Mayr R, Gierth M, Zeman F, Reiffen M, Seeger P, Wezel F, et al. Sarcopenia as a comorbidity‐independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle. 2018. Jun;9:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura R, Inage Y, Tobita R, Yoneyama S, Numata T, Ota K, et al. Sarcopenia in resected non‐small cell lung cancer: effect on postoperative outcomes. J Thorac Oncol. 2018. Jul;13:895–903. [DOI] [PubMed] [Google Scholar]
- 43. Nakashima Y, Saeki H, Nakanishi R, Sugiyama M, Kurashige J, Oki E, et al. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. 2018;267:1100–1104. [DOI] [PubMed] [Google Scholar]
- 44. Nakanishi R, Oki E, Sasaki S, Hirose K, Jogo T, Edahiro K, et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today. 2018;48:151–157. [DOI] [PubMed] [Google Scholar]
- 45. Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg. 2017;39:45–51. [DOI] [PubMed] [Google Scholar]
- 46. Nishida Y, Kato Y, Kudo M, Aizawa H, Okubo S, Takahashi D, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. 2016;20:1586–1594. [DOI] [PubMed] [Google Scholar]
- 47. Nishigori T, Tsunoda S, Shinohara H, Hosogi H, Hisamori S, Sakai Y. Sarcopenia as a predictor of prognosis after curative resection for advanced gastric cancer. J Am Coll Surg. 2016;223:e47–e48. [Google Scholar]
- 48. Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Yagi S, Iida T, et al. Impact of preoperative sarcopenia on outcomes after resection of biliary cancer. Hepatol Int. 2016;10:S70. [Google Scholar]
- 49. Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088–1098. [DOI] [PubMed] [Google Scholar]
- 50. Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, et al. Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24:1037–1045. [DOI] [PubMed] [Google Scholar]
- 51. Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, et al. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg. 2015;39:1494–1500. [DOI] [PubMed] [Google Scholar]
- 52. Ouchi A, Asano M, Aono K, Watanabe T, Oya S. Laparoscopic colorectal resection in patients with sarcopenia: a retrospective case‐control study. J Laparoendosc Adv Surg Tech A. 2016;26:366–370. [DOI] [PubMed] [Google Scholar]
- 53. Peng P, Van Vledder M, Tsai S, De Jong M, Ng J, Kamel I, et al. Sarcopenia negatively impacts short‐term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB. 2011;13:52–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peyton CC, Heavner MG, Rague JT, Krane LS, Hemal AK. Does sarcopenia impact complications and overall survival in patients undergoing radical nephrectomy for stage III and IV kidney cancer. J Endourol. 2016;30:229–236. [DOI] [PubMed] [Google Scholar]
- 55. Rutten IJG, van Dijk DPJ, Kruitwagen RFPM, Beets‐Tan RGH, Olde Damink SWM, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saeki H, Nakashima Y, Kudou K, Sasaki S, Jogo T, Hirose K, et al. Neoadjuvant chemoradiotherapy for patients with cT3/nearly T4 esephageal cancer: is sarcopenia correlated with postoperative complications and prognosis? World J Surg. 2018. Sep;42:2894–2290. [DOI] [PubMed] [Google Scholar]
- 57. Paula SD, Aguiar Bruno K, Azevedo Aredes M, Villa╟⌡a Chaves G. Sarcopenia and skeletal muscle quality as predictors of postoperative complication and early mortality in gynecologic cancer. Int J Gynecol Cancer. 2018;28:412–420. [DOI] [PubMed] [Google Scholar]
- 58. Smith AB, Deal AM, Yu H, Boyd B, Matthews J, Wallen EM, et al. Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol. 2014;191:1714–1720. [DOI] [PubMed] [Google Scholar]
- 59. Sui K, Okabayshi T, Iwata J, Morita S, Sumiyoshi T, Iiyama T, et al. Correlation between the skeletal muscle index and surgical outcomes of pancreaticoduodenectomy. Surg Today. 2018;48:545–551. [DOI] [PubMed] [Google Scholar]
- 60. Takagi K, Yagi T, Yoshida R, Shinoura S, Umeda Y, Nobuoka D, et al. Sarcopenia and American Society of Anesthesiologists Physical Status in the assessment of outcomes of hepatocellular carcinoma patients undergoing hepatectomy. Acta Med Okayama. 2016;70:363–370. [DOI] [PubMed] [Google Scholar]
- 61. Takagi K, Yagi T, Yoshida R, Umeda Y, Nobuoka D, Kuise T, et al. Sarcopenia predicts postoperative infection in patients undergoing hepato‐biliary‐pancreatic surgery. Intl J Surg. 2017;6:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takagi K, Yoshida R, Yagi T, Umeda Y, Nobuoka D, Kuise T, et al. Radiographic sarcopenia predicts postoperative infectious complications in patients undergoing pancreaticoduodenectomy. BMC Surg. 2017;17:64‐017‐0261‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takeda Y, Akiyoshi T, Matsueda K, Fukuoka H, Ogura A, Miki H, et al. Skeletal muscle loss is an independent negative prognostic factor in patients with advanced lower rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS One. 2018;13:e0195406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tegels JJW, Van Vugt JLA, Reisinger KW, Hulsewé KWE, Hoofwijk AGM, Derikx JPM, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112:403–407. [DOI] [PubMed] [Google Scholar]
- 65. Umetsu S, Wakiya T, Ishido K, Kudo D, Kimura N, Miura T, et al. Effect of sarcopenia on the outcomes after pancreaticoduodenectomy for distal cholangiocarcinoma. ANZ J Surg. 2018. Sep;88:E654–E658. [DOI] [PubMed] [Google Scholar]
- 66. Valero V, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Kroft G, Bours DMJL, Janssen‐Heijnen DM, van Berlo DCLH, Konsten DJLM. Value of sarcopenia assessed by computed tomography for the prediction of postoperative morbidity following oncological colorectal resection: a comparison with the malnutrition screening tool. Clin Nutr ESPEN. 2018;24:114–119. [DOI] [PubMed] [Google Scholar]
- 68. van Vugt JLA, Braam HJ, van Oudheusden TR, Vestering A, Bollen TL, Wiezer MJ, et al. Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2015;22:3625–3631. [DOI] [PubMed] [Google Scholar]
- 69. Van Vugt J, Buettner S, Levolger S, Van Den Braak RC, Suker M, Gaspersz M, et al. Sarcopenia is associated with hospital expenditure in patients undergoing cancer surgery of the alimentary tract. Ann Surg Oncol. 2017;24:S159–S160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van Vugt JLA, Coebergh Van Den Braak RRJ, Lalmahomed ZS, Vrijland WW, Dekker JWT, Zimmerman DDE, et al. Impact of low skeletal muscle mass and density on short and long‐term outcome after resection of stage I‐III colorectal cancer: results from a prospective multicenter observational cohort study. J Cachexia Sarcopenia Muscle 2017;8:1015–1016. [Google Scholar]
- 71. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short‐ and long‐term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015;261:1173–1183. [DOI] [PubMed] [Google Scholar]
- 72. Wagner D, Marsoner K, Tomberger A, Haybaeck J, Haas J, Werkgartner G, et al. Low skeletal muscle mass outperforms the Charlson Comorbidity Index in risk prediction in patients undergoing pancreatic resections. Eur J Surg Oncol. 2018;44:658–663. [DOI] [PubMed] [Google Scholar]
- 73. Zhuang C, Huang D, Pang W, Zhou C, Wang S, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long‐term survival after radical gastrectomy for gastric cancer: Analysis from a large‐scale cohort. Medicine. 2016;95:e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zuckerman J, Ades M, Mullie L, Trnkus A, Morin J, Langlois Y, et al. Psoas Muscle Area and Length of Stay in Older Adults Undergoing Cardiac Operations. Ann Thorac Surg. 2017;103:1498–1504. [DOI] [PubMed] [Google Scholar]
- 75. Sur MD, Namm JP, Hemmerich JA, Buschmann MM, Roggin KK, Dale W. Radiographic sarcopenia and self‐reported exhaustion independently predict NSQIP serious complications after pancreaticoduodenectomy in older adults. Ann Surg Oncol. 2015;22:3897–3904. [DOI] [PubMed] [Google Scholar]
- 76. Hansen L, de Zee M, Rasmussen J, Andersen TB, Wong C, Simonsen EB. Anatomy and biomechanics of the back muscles in the lumbar spine with reference to biomechanical modeling. Spine (Phila Pa 1976) 2006;31:1888–1899. [DOI] [PubMed] [Google Scholar]
- 77. Rangel EL, Rios‐Diaz AJ, Uyeda JW, Castillo‐Angeles M, Cooper Z, Olufajo OA, et al. Sarcopenia increases risk of long‐term mortality in elderly patients undergoing emergency abdominal surgery. J Trauma Acute Care Surg. 2017;83:1179–1186. [DOI] [PubMed] [Google Scholar]
- 78. Thurston B, Pena GN, Howell S, Cowled P, Fitridge R. Low total psoas area as scored in the clinic setting independently predicts midterm mortality after endovascular aneurysm repair in male patients. J Vasc Surg. 2018;67:460–467. [DOI] [PubMed] [Google Scholar]
- 79. Huisman MG, van Leeuwen BL, Ugolini G, Montroni I, Spiliotis J, Stabilini C, et al. “Timed Up & Go”: a screening tool for predicting 30‐day morbidity in onco‐geriatric surgical patients? A multicenter cohort study. PLoS One. 2014;9:e86863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dent E, Morley JE, Cruz‐Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging. 2018;22:1148–1161. [DOI] [PubMed] [Google Scholar]
- 82. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy‐Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017. J Cachexia Sarcopenia Muscle. 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Figure S1. Comparison of studies with a good versus a poor methodological quality
Figure S1a. Severe postoperative complications
Figure S1b. 30‐day mortality
Figure S2. Stratification by tumour location
Figure S2a. Severe postoperative complications: grouped tumour location
Figure S2b. Severe postoperative complications: specific tumour location
Figure S2c. 30‐day mortality: grouped tumour location
Figure S2d. 30‐day mortality: specific tumour location
Figure S3a. Severe postoperative complications
Figure S3b. 30‐day mortality


