Abstract
Coronavirus disease-19 (COVID-19)-related severe acute respiratory distress syndrome can lead to acute cor pulmonale. We report a case of acute cor pulmonale secondary to severe COVID-19 acute respiratory distress syndrome diagnosed with transesophageal echocardiography. Almitrine infusion allowed rapid enhancement of right ventricular function as well as improvement in oxygenation. (Level of Difficulty: Intermediate.)
Key Words: acute cor pulmonale, almitrine, ARDS, COVID-19, SARS-CoV-2
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; ACP, acute cor pulmonale; ARDS, acute respiratory distress syndrome; CI, cardiac index; COVID-19, coronavirus disease-19; CT, computed tomography; Fio2, fraction of inspired oxygen; Pao2, partial pressure of oxygen; RV, right ventricle; RVSWI, right ventricular stroke work index; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; TEE, transesophageal echocardiography
Graphical abstract
Coronavirus disease-19 (COVID-19)-related severe acute respiratory distress syndrome can lead to acute cor pulmonale. We report a case of acute…
History of Presentation
A 57-year-old woman was admitted at the Amiens University Hospital intensive care unit for severe acute respiratory distress syndrome (ARDS) 12 days following onset of cough, dyspnea, and fever. Real-time reverse transcriptase polymerase chain reaction of nasopharyngeal swab was positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Chest computed tomography (CT) scan revealed peripheral ground glass-like opacities with superimposed interlobular and intralobular septal thickening (crazy paving). The patient’s condition deteriorated rapidly, leading to mechanical ventilation with lung-protective settings, prone positioning, deep sedation, neuromuscular blockade, and inhaled nitric oxide at 10 ppm. Despite these measures, she remained hypoxemic with a partial pressure of oxygen (Pao2)-to-fraction of inspired oxygen (Fio2) ratio of 70. After starting mechanical ventilation and sedation, mean arterial pressure dropped to 60 mm Hg. Norepinephrine was administered at the dose of 0.3 μg/kg/min–1 to achieve a mean arterial pressure target higher than 65 mm Hg. Blood pressure rapidly reached 115/50 (71) mm Hg. For patients in severe ARDS associated with hemodynamic impairment, local guidelines recommend close monitoring with a pulmonary artery catheter and transesophageal echocardiography (TEE). A pulmonary artery catheter and TEE revealed acute cor pulmonale (ACP) with pulmonary hypertension.
Learning Objectives
-
•
To diagnose ACP in patients with SARS-CoV-2–related ARDS.
-
•
To appreciate potential role of almitrine in improving oxygenation and RV function.
-
•
To understand SARS-CoV-2–related atypical type of ARDS.
Past Medical History
Medical history included only an overweight with a body mass index of 38.9 kg/m2.
Differential Diagnosis
The differential diagnosis included pulmonary embolism and right ventricular (RV) infarction, which were discounted later due to the CT pulmonary angiogram not showing pulmonary embolism and normal electrocardiography and normal high-sensitivity troponin. D-dimer was 3.69 μg/ml–1.
Investigations
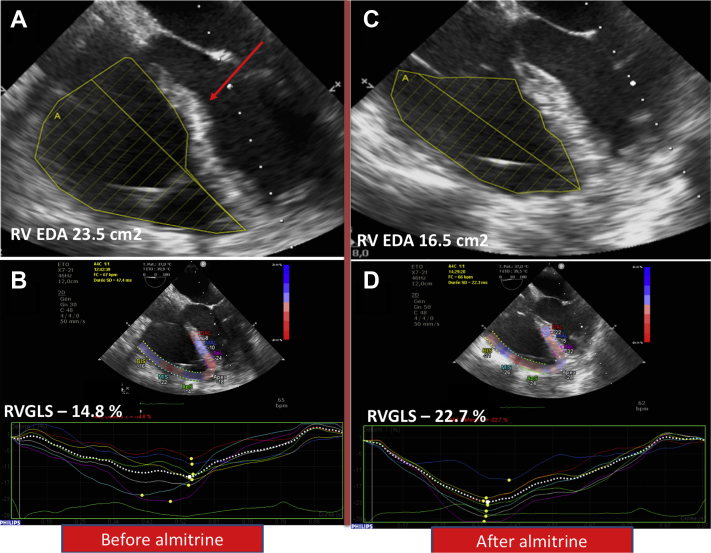
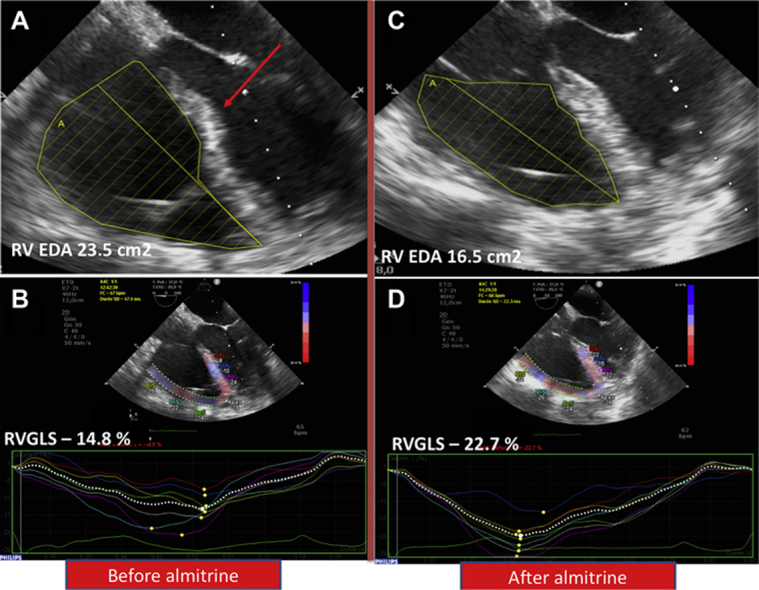
Prior to commencing almitrine, arterial blood gas sample showed a Pao2 of 63 mm Hg, Paco2 of 46.6 mm Hg, pH at 7.4, Pao2/Fio2 ratio of 70, and lactate at 2.3 mmol/l–1. Pulmonary artery catheter showed pulmonary artery pressure of 57/32 (42) mm Hg with pulmonary vascular resistance at 5.9 WU and pulmonary artery occlusion pressure of 7 mm Hg. RV stroke work index (RVSWI) was 19.7 g/m/beat/m2 (normal range 8 to 10 g/m/beat/m2). TEE showed an ACP: the RV-to-left ventricular area ratio was of 1:1 with septal dyskinesia (Figure 1A). In 2-dimensional conventional TEE, tricuspid annular plane systolic excursion was 22 mm, tricuspid lateral annulus systolic velocity (S’-wave) was 15.2 cm/s–1, and RV fractional area change was 43%. Speckle tracking–based RV 2-dimensional strain was performed (Figure 1B). The RV global longitudinal strain was –14.8% and RV free wall longitudinal strain was –17.3% (RV global longitudinal strain normal value <–20%). Left ventricular ejection fraction was 65% without any valvular disease. Cardiac index (CI) was 2.6 l/min–1/m–2.
Figure 1.
Transesophageal Echocardiography Before and After Almitrine Infusion
(A) Two-dimensional image before almitrine infusion showing dilated right ventricle (RV) with an RV-to-left ventricular area ratio of 1:1 with septal dyskinesia (red arrow). (B) Speckle tracking–based RV 2-dimensional strain showing impaired RV global longitudinal strain (RVGLS) of –14.8% (normal value: <–20%). (C) Two-dimensional image after almitrine infusion showing RV-to-left ventricular area ratio of 0.75 and normal septal shape. (D) Speckle tracking–based RV 2-dimensional strain showing improvement of RVGLS to –22.7%. EDA = end-diastolic area.
Management
Severe and prolonged hypoxemia can lead to organ dysfunction. Thus, the need for venovenous extracorporeal membrane oxygenation to improve oxygenation was considered for this patient with a very low Pao2/Fio2 ratio of 70. As inhibition of hypoxic vasoconstriction was considered as a key point to explain severe hypoxemia related to coronavirus disease-2019 (COVID-19), the decision was made to test almitrine treatment before starting extracorporeal membrane oxygenation (2). However, because almitrine may increase RV afterload in a patient who already has an ACP, close RV monitoring was performed. A low dose of almitrine was given at an infusion rate of 4 μg/kg/min via a central venous catheter. Complete hemodynamic, echocardiographic, and biological assessment was repeated at 1, 2, and 12 h following almitrine initiation (Table 1). TEE video loops before and after almitrine infusion are shown in Videos 1 and 2. Oxygenation improved significantly in the few hours following almitrine treatment (Table 2). TEE showed an improvement of RV function: RV dilatation decreased (ratio at 0.75) and septal dyskinesia disappeared (Figure 1C). Moreover, RV global longitudinal strain improved from –14.8% to –22.7% (Figure 1D). Twelve hours following almitrine infusion, RVSWI decreased from 19.7 to 17.5 g/m/beat/m2.
Table 1.
Pulmonary Artery Catheter Parameters Before and After Almitrine Infusion
| Before | H1 | H2 | H12 | |
|---|---|---|---|---|
| Mean PAP, mm Hg | 42 | 42 | 37 | 36 |
| Systolic PAP, mm Hg | 57 | 65 | 52 | 50 |
| PAOP, mm Hg | 7 | 8 | 8 | 6 |
| Diastolic PAP, mm Hg | 32 | 29 | 30 | 28 |
| CI, l/min–1/m–2 | 2.6 | 2.6 | 3.9 | 3 |
| PVR, Wood units | 5.9 | 5.9 | 3.4 | 4.5 |
CI = cardiac index; H1 = 1 h following almitrine infusion; H2 = 2 h following almitrine infusion; H12 = 12 h following almitrine infusion; PAOP = pulmonary arterial occlusion pressure; PAP = pulmonary artery pressure; PEEP = positive end-expiratory pressure; PVR = pulmonary vascular resistance.
Online Video 1.
Transesophageal echocardiography before almitrine infusion.
Online Video 2.
Transesophageal echocardiography after almitrine infusion.
Table 2.
Respiratory Parameters Before and After Almitrine Infusion
| Before | H1 | H2 | H12 | |
|---|---|---|---|---|
| RR/min | 30 | 30 | 30 | 30 |
| PEEP, cm H2O | 12 | 12 | 12 | 12 |
| Tidal volume, ml | 420 | 420 | 420 | 420 |
| Plateau pressure, cm H2O | 31 | 30 | 29 | 28 |
| Respiratory system compliance, ml/cm H2O–1) | 22 | 23 | 24 | 26 |
| Driving pressure | 19 | 18 | 18 | 18 |
| Pao2, mm Hg | 63 | 67 | 130 | 170 |
| Fio2 | 90 | 80 | 60 | 60 |
| Pao2/Fio2 ratio | 70 | 84 | 216 | 283 |
Fio2 = fraction of inspired oxygen; Pao2 = partial pressure of oxygen; RR = respiratory rate; other abbreviations as in Table 1.
Discussion
The clinical spectrum of SARS-CoV-2–related cardiovascular complication includes myocarditis, pericarditis, vasoplegia, RV failure, and acute coronary syndromes (1,2).
In this case, we highlight the RV dysfunction related to SARS-CoV-2 infection. Pathophysiology of cardiovascular dysfunction related to SARS-CoV-2 remains unclear. One explanation is the recognition of human angiotensin-converting enzyme 2 (ACE2) by SARS-CoV-2. ACE2 is used by the virus as a functional receptor to enter the cell (3). Moreover, ACE2 receptor is not only present in lung alveolar cells but also in many extra pulmonary tissues especially heart, and vascular endothelium. Hence, direct viral effect on the heart and vessels may lead to RV dysfunction. Another explanation is the impact of ARDS and mechanical ventilation on the RV. ACP is a well-known complication of ARDS despite a protective ventilation, with an incidence of 25%. Hence, ACP may be related to a high driving pressure, leading to an increased RV afterload (4). Moreover, the patient was on norepinephrine, which may increase RV afterload. Another explanation is that hypoxia could lead to RV dysfunction in its own right (5).
SARS-CoV-2–related ARDS seems to be a nontypical ARDS, as several patients had a preserved lung mechanics with high compliance despite severe hypoxemia. Some authors have hypothesized that SARS-CoV-2–related hypoxemia is due the impairment of hypoxic pulmonary vasoconstriction and dysregulation of pulmonary blood flow, leading to an intrapulmonary shunt responsible for the severe hypoxemia (6). In a recently published report on a critically ill patient with SARS-CoV-2 infection, the authors used dual-energy chest CT. They found “considerable” pulmonary vessels dilatation and increased pulmonary blood flow surrounding areas of consolidation (7).
Almitrine, a selective pulmonary vessel vasoconstrictor, was shown to increase arterial oxygenation via redistribution of pulmonary blood flow from shunt areas to pulmonary units, with normal ventilation-to-perfusion ratio (8). Moreover, previous studies in the 1990s showed that at a low dose, the deleterious effect on pulmonary vascular resistance was negligible, especially when associated with nitric oxide (9). Hence, we hypothesized that almitrine use in the case of SARS-CoV-2 atypical ARDS might be useful.
In the present case, almitrine infusion was associated with RV function improvement and decrease in pulmonary vascular resistance. This is probably due not only to a better oxygenation, but also to a better distribution of pulmonary vascular flow to aerated lung areas. Before almitrine infusion, we observed a high RVSWI with a normal-to-low range of CI, suggesting a hemodynamic disconnection between the RV and left ventricle. The reduction in RV afterload by almitrine infusion resulted in an improvement in this disconnection (decreased RVSWI and improved CI).
As almitrine infusion could induce reversible lactic acidosis and hepatic dysfunction (10), careful monitoring of lactate and screening test for hepatic function were performed. We did not observe those side effects.
To our knowledge, this is first report of almitrine use for SARS-CoV-2–related ARDS showing an improvement in oxygenation. Nevertheless, monitoring of pulmonary artery pressure and RV function are important for safe use of almitrine. Before clinical use, well-conducted studies with sufficient sample sizes are needed to demonstrate the efficacy of almitrine in SARS-CoV-2–associated severe ARDS. A randomized controlled trial is underway to address this issue and will investigate the efficacy of intravenous almitrine in reducing the need for mechanical ventilation in patients with hypoxemic acute respiratory failure due to COVID-19-related pneumonia (NCT04357457).
Follow-Up
Almitrine was administered to the patient for 4 days, resulting in consistent improvement in oxygenation and ventilator parameters. RV function remained stable. Weaning from mechanical ventilation occurred on day 16. The patient was discharged from the intensive care unit on day 26.
Conclusions
ACP may occur in patients suffering from SARS-CoV-2–related ARDS. Echocardiography is the main tool to diagnose ACP in this context. In the present case, almitrine infusion was effective in improving oxygenation and RV function.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Creel-Bulos C., Hockstein M., Amin N., Melhem S., Truong A., Sharifpour M. Acute cor pulmonale in critically ill patients with COVID-19. N Engl J Med. 2020;382:e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augoustides J.G. Cardiovascular consequences and considerations of coronavirus infection – perspectives for the cardiothoracic anesthesiologist and intensivist during the coronavirus crisis. J Cardiothorac Vasc Anesth. 2020;34:1713–1716. doi: 10.1053/j.jvca.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekontso Dessap A., Boissier F., Charron C. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 5.Netzer N.C., Strohl K.P., Högel J., Gatterer H., Schilz R. Right ventricle dimensions and function in response to acute hypoxia in healthy human subjects. Acta Physiol (Oxf) 2017;219:478–485. doi: 10.1111/apha.12740. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang M., Som A., Mendoza D.P. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020 Apr 30 doi: 10.1016/S1473-3099(20)30367-4. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roch A., Hraiech S., Dizier S., Papazian L. Pharmacological interventions in acute respiratory distress syndrome. Ann Intensive Care. 2013;3:20. doi: 10.1186/2110-5820-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallart L., Lu Q., Puybasset L., Umamaheswara Rao G.S., Coriat P., Rouby J.J. Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. The NO Almitrine Study Group. Am J Respir Crit Care Med. 1998;158:1770–1777. doi: 10.1164/ajrccm.158.6.9804066. [DOI] [PubMed] [Google Scholar]
- 10.B’chir A., Mebazaa A., Losser M.R., Romieu M., Payen D. Intravenous almitrine bismesylate reversibly induces lactic acidosis and hepatic dysfunction in patients with acute lung injury. Anesthesiology. 1998;89:823–830. doi: 10.1097/00000542-199810000-00005. [DOI] [PubMed] [Google Scholar]