Abstract
Context
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has resulted in a global health emergency, the like of which has never been seen before. Prostate cancer (PCa) services across the globe have been on hold due to changing medical and surgical priorities. There is also epidemiological evidence that PCa patients have increased incidence and mortality from SARS-CoV-2 infection due to gender differences, age, and higher propensity for risk factors (eg, respiratory disease, obesity, hypertension, and smoking status).
Objective
To contribute to the emerging body of knowledge on the risks of SARS-CoV-2 infection to PCa patients and, in the face of PCa treatment delays, provide evidence-based recommendations for ongoing management of specific PCa patient groups.
Evidence acquisition
A literature search was performed using all sources (MEDLINE, EMBASE, ScienceDirect, Cochrane Libraries, and Web of Science) as well as the media to harness emerging data on the SARS-CoV-2 pandemic and its influence on PCa. Eligibility criteria were originality of data and relevance to PCa management. The authors note that during these unprecedented times, retrospective data are constantly being updated from multiple sources globally.
Evidence synthesis
A total of 72 articles and data sources were found initially. Owing to repetition, lack of originality, or nonrelevance, six articles were rejected, leaving 23 retrospective studies, seven basic science research articles, 15 societal and journal guidelines, and 21 epidemiological data sources, from countries at different stages of SARS-CoV-2 pandemic. These were analyzed qualitatively to produce evidence-based guidelines for the management of PCa patients at different stages of the patient journey, with strategies to reduce the risk of viral spread.
Conclusions
PCa patients may have an increased risk of SARS-CoV-2 infection as well as morbidity and mortality if infected. Once appropriately triaged, and to reduce viral spread, PCa patients can have surveillance by telemedicine, and institute lifestyle changes and social quarantining measures. If risk stratification suggests that treatment should be planned, androgen deprivation therapy can be started, or potentially surgery or radiation therapy is possible on a case-by-case basis.
Patient summary
Prostate cancer patients can be followed up remotely until the severe acute respiratory syndrome coronavirus 2 pandemic resolves, but higher-risk cases may have treatment expedited to limit any negative impact on prostate cancer outcomes.
Keywords: COVID-19 pandemic, Infection risks, Prostate cancer, Treatment delays, Triage
Take Home Message
Prostate cancer (PCa) patients may have an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and mortality. Most can be followed up remotely until the pandemic resolves, but, once appropriately triaged, higher-risk cases can have treatment expedited to limit any negative impact on PCa outcomes.
1. Introduction
Since December 2019, the number of individuals infected with the novel zoonotic coronavirus (CoV) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) disease is increasing daily, creating a global health crisis. While this disease affects every gender, age, and ethnicity, it tends to be more lethal in men of older age group, overlapping with the prostate cancer (PCa) population [1]. Furthermore, along with other comorbidities, a cancer diagnosis itself may increase the mortality of SARS-CoV-19 [2]. Every year, over 1.2 million new patients are added to the survivorship of PCa patients worldwide, and based on the pandemic's expansion across the globe, millions of men with PCa will likely become infected before the end of the year. Prior experience of CoV epidemics, with SARS-CoV affecting Asia, the Americas, and Europe in 2003, and Middle East respiratory syndrome coronavirus (MERS-CoV) in the Arabian Peninsula in 2012, did not have such a global impact on PCa patients.
At the time of writing, hospital systems are swamped with SARS-CoV-2 admissions, placing elective PCa diagnostic and surgical services on hold due to lack of beds, reduced anesthetic cover and staffing levels, and policies designed to reduce the risk of viral spread [3]. Helpful recommendations from urological societies have clarified triage systems for useful guidance on which more urgent urological and PCa surgeries should be performed [4], but it is unknown how long the block will last and whether it will return. This review aims to examine the impact of COVID-19 on clinical cancer care, patient management, disease outcomes, and healthcare provision for patients with PCa, and present evidence-based management recommendations for each stage of the PCa journey amid the SARS-CoV-2 pandemic with emphasis on the role of telemedicine.
2. Evidence acquisition
Medline, Medline in process, Embase, Science Direct, Cochrane Library, Web of Science, and media searches were performed for COVID-19, SARS-CoV-2, urology, and PCa until April 2, 2020. Guidelines and recommendations as well as global, international, and regional infection, hospitalization, intensive care unit (ICU) admission, and mortality data were retrieved from the Center for Disease Control (CDC) and emerging COVID-19 resources on reputable societal and journal websites up to the same date (eg, American Urological Association, Society of American Gastrointestinal and Endoscopic Surgeons [SAGES], Royal College of Surgeons [RCS], Association of Operative Registered Nurses [AORN], European Association of Urology Robotic Urology Society [ERUS], European Urology, JAMA, Nature, New England Journal of Medicine, and The Lancet). A total of 72 articles and data sources were found initially. Eligibility criteria were originality of data and relevance to PCa management. Owing to repetition, lack of originality, or nonrelevance, six articles were rejected, leaving 23 retrospective studies, seven basic science research articles, 15 societal and journal guidelines, and 21 epidemiological data sources. These were analyzed qualitatively in order to generate evidence-based guidelines for the management of PCa patients at different stages of the patient journey. As supporting evidence, specific infection, hospitalization, ICU admission, and mortality rates were subdivided and presented in a table form according to geography, ethnicity, and comorbidities (see Table 1, Table 2, Table 3).
Table 1.
Gender distribution with incidence and mortality rates due to COVID-19 [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70].
| Countries | Sex | Incidence rate (%) | Mortality rate (%) |
|---|---|---|---|
| Italy | Male | 58.3 | 71 |
| Female | 41.7 | 29 | |
| China | Male | 5 | 64 |
| Female | 48.6 | 36 | |
| South Korea | Male | 38.58 | 54 |
| Female | 61.42 a | 46 | |
| United States | Male | 48 | 17.32 |
| Female | 40 | 9.13 |
CDC = Center for Disease Control; COVID-19 = coronavirus disease 2019.
Korea's CDC says a woman who became the country's 31st confirmed patient on February 18 had attended services held by a religious group called the Shincheonji Church of Jesus, The Temple of the Tabernacle of the Testimony. More than half of those cases involve members of, or those somehow linked to, the religious sect the Shincheonji Church of Jesus, where a so-called superspreader infected at least 37 people on February 23, 2020.
Table 2.
High cancer mortality rate in SARS-CoV-2–infected patients [32], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80].
| Comorbidity | Italy | China | Overall (48% had comorbidity) | Mortality rate |
|---|---|---|---|---|
| Ischemic heart disease (%) | 30 | 5.8 | 8 | 10.5 |
| Diabetes (%) | 35.5 | 16.2 | 19 | 7.3 |
| Hypertension (%) | 76 | 23.7 | 22.2–26.9 | 6 |
| Active cancer (%) | 20.3 | 28 | 39 | 28.6 |
COVID-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Summary of data from (just in time) studies comparing demographic, clinical, treatment, and laboratory data from electronic medical health records between those who survived and those who succumbed to the disease. The analyses also tested serial samples for viral DNA. Overall, 91 (48%) of the 191 patients had comorbidities.
Table 3.
Hospitalization, intensive care unit (ICU) admission, and case fatality percentages for reported COVID-19, by age group and gender in the USA [70], [81], [82].
| Factors | Hospitalization | ICU admission | Case fatality |
|---|---|---|---|
| Age (yr) | |||
| 65–74 | 28.6 | 8.1 | 2.7 |
| 75–84 | 30.5 | 10.5 | 4.3 |
| >85 | 31.3 | 6.3 | 10.4 |
| Male | 22% | NA | 17.2 |
| Female | 18% | NA | 9.3 |
COVID-19 = coronavirus disease 2019; NA = not available.
3. Evidence synthesis
3.1. Virology of SARS-CoV-2
First identified in December 2019, the novel zoonotic CoV SARS-CoV-2 belongs to the subfamily Coronavirinae, which contains positive-sense single-stranded RNA, and genus betacoronavirus [5], [6], [7]. CoVs are pathogenic in humans and animals, causing disease syndromes of varying severity affecting mainly respiratory, but also the liver, gastrointestinal, and central nervous systems [7]. Other notable CoVs were SARS-CoV, which caused a human epidemic of severe acute respiratory syndrome in 2003, with a patient mortality rate of 10% [8], and MERS-CoV, which impacted the Middle East in 2012, with a mortality rate of approximately 35% [9].
SARS-CoV-2 most likely arose from bats, based on genetic sequencing similarities [7], with SARS-CoV-2 sharing 79.5% genetic sequence with SARS-CoV but 96.2% with a bat CoV (BatCoV RaTG13) [5]. As yet, the intermediate host from the bats to humans is not known [7]. Structurally, the virion has an envelope spike (S) protein that drives entry of SARS-CoV-2 into target host cells by engaging the cellular receptor angiotensin-converting enzyme-2 (ACE2) [10], [11]. This step is accompanied by activation of cellular TMPRSS2, a type-II transmembrane serine protease [12]. The TMPRSS2 protein interaction is of major mechanistic significance in PCa, as the androgenically regulated TMPRRS2/ERG fusion has been found in approximately 50% of PCa patients.
The incubation period for SARS-CoV-2 is around 5 d, varying from 1 to 14 d, and the main mode of transmission is human to human, via direct contact or aerosol spread, or through fomites [13], [14]. According to the CDC, infected patients have a low risk of being contagious until the onset of symptoms, most commonly in the 2nd week of illness, but as a precaution, it recommends limiting contact until 10 d after the illness has resolved [15]. Examining the aerosol and surface stability, in comparison with SARS-CoV, SARS-CoV-2 remained stable in the aerosol drops for hours and on surfaces for days (depending on the amount of virus shed), the worst surfaces being stainless steel and plastic [14]. This means that the virus can spread at large-scale events, nosocomially exacerbating its pandemic potential [14].
3.2. Epidemiology
The important epidemiological impact of the SARS-CoV-2 pandemic presents challenging questions to urologists and PCa patients as to how long it will take for services to recover, and what is the ongoing risk of infection in aging men. At the time of writing, with urological services on hold in many hospital systems around the world, there is much to be learnt from the epidemic timelines in countries that are now hopefully in recovery phase. The World Health Organization (WHO) was first informed of a cluster of 41 patients with a viral pneumonia of unknown etiology from the Hunan Seafood Market on December 31, 2019. Wuhan was placed under quarantine on January 23, 2019, with Hubei Province following suit days after [7], [16], and only recently did China announce plans to lift the lockdown on April 8, 2020 [17]. The presumption will be that once “lockdown” is lifted and social quarantining restrictions relaxed, hospitals will resume normal services. This timeline is in keeping with a statistical model recently developed by Zhao and Chen [16], who predicted an end to the Wuhan and Hubei epidemic at the end of March 2020. Based on this timeline, it seems unlikely that normal urological services will resume in affected countries until 3–6 mo following the onset of government restrictions. This means that PCa patients awaiting biopsy or treatment may be in a triage system for up to ≥6 mo, with possibly starting androgen deprivation therapy (ADT) to defer treatment until the pandemic is resolved.
Compelling epidemiological evidence suggests that PCa patients have increased susceptibility to SARS-CoV-2 based on their male gender, age, and associated comorbidities. Gender disparity has been reported for the SARS-CoV-2 pandemic, with some sources quoting twice as many men as women affected, although there is clear geographical variation (Table 1, Table 2). In China, as of February 11, 2020, examination of 44 672 confirmed cases found that the male to female ratio was from 0.99 to 1 in Wuhan, 1.04 to 1 in Hubei, and 1.06 to 1 in the country [18]. Another Chinese study with a smaller cohort of 1099 confirmed cases has shown that 58% of those infected were men [19]. Beyond Asia, for the Italian outbreak, Livingston and Bucher [20] also found that 58% of 22 512 confirmed cases were men (Table 1). Further evidence suggests that morbidity and mortality rates in confirmed cases are worse for men than for women [1], with reports in Chinese cohorts, albeit with small numbers, showing that 73% of patients are admitted to hospital [21], 67% of patients are admitted to intensive treatment unit (ITU), and 66% of mortalities in ITU occur in male patients [22] (Table 3). Similarly, another Chinese cohort found that of 99 patients developing pneumonia, 67% were male [23]. In keeping with previous SARS and MERS outbreaks, older men with chronic morbidities and impaired immunity seem to be at maximum risk, once infection has taken hold [24] (summarized in Table 2, Table 3). Smoking rate, which is also associated with worse outcomes, may be a factor in male preponderance, and certainly in China, smoking is significantly more common in men than in women [23]. The potential role of ACE2 in gender and ethnic disparity in SARS-CoV-2 infection has also been suggested, and although ACE2 expression is similar in Asians, Caucasians, men, women, old, and young, it is much higher in Asian smokers than in non-Asian smokers [23].
3.3. Impact of SARS-CoV-2 diagnosis on clinical management of PCa
3.3.1. Clinical features
The clinical syndrome of SARS-CoV-2 infection ranges from asymptomatic disease to pneumonia, multiorgan failure, and death. Published retrospective studies, mainly from China, report symptoms in decreasing order of frequency as fever, cough, fatigue, arthralgia and myalgia, headache, hemoptysis, and diarrhea [19], [21]. In a Chinese retrospective study, 78.7% of patients had mild disease, while 15.7% progressed to severe disease and 6.1% required ITU admission [19]. There is also geographical variation, based on more virulent strains of SARS-CoV-2, with the Italian epidemic showing higher rates of more severe disease [20].
3.3.2. Urological complications
ACE2 expression (potential for SARS-CoV-2 infectivity) has been shown in 4% of proximal convoluted cells in the kidney and 2% of bladder urothelium [25]. This may cause acute kidney injury (AKI) in 3–9% of cases [26], [27]. Other urological abnormalities such as hematuria and proteinuria may be seen in up to 44% of admitted patients, as well as abnormal serum creatinine in 15.5% and blood urea in 14.1% [26], [27]. Significantly, the development of an AKI has been an independent risk factor for fatality [26], [27].
3.3.3. Diagnosis
A clinical diagnosis may be made in a patient who has coryzal symptoms with fever, dyspnea, and signs of pneumonia [28]. Chest x-ray may show interstitial consolidation, more common at the lung periphery, seen more clearly on a computed tomography (CT) scan [28]. Laboratory diagnosis hinges on viral nucleic acid identification and viral isolation in respiratory tract specimens as well as in blood and feces [28], primarily by reverse transcription polymerase chain reaction (RT-PCR) [7], with detection rates improving with additional SARS-CoV-2 IgG and IgM serology [29].
3.4. PCa diagnosis and treatment during the SARS-CoV-2 pandemic
The guidelines to urologists for the general approaches and clinical management of PCa patients during the COVID-19 pandemic are summarized in Table 4.
Table 4.
Summary guidelines for the management of PCa during COVID-19 pandemic.
| Patient group | Clinician recommendation for surge phase | Rationale for clinician recommendation |
|---|---|---|
| Prediagnosis (awaiting biopsy) | Triage according to DRE, age-adjusted and absolute PSA, 4K test, genomics (eg, Select Mdx or Confirm Mx if prior negative biopsy), MRI PIRADS score, and clinical T stage with assessed risk of finding NCCN high- and very–high-risk disease | Risk of delaying treatment in NCCN high- and very-high-risk cases [36], [39], [49] |
| Active surveillance | Telemedicine consults Triage for repeat biopsy for those already in AS programs according to DRE, PSA, genomics (eg, Oncotype MDx), MRI PIRADS score, and clinical T stage, with assessed risk of finding NCCN high- and very-high-risk disease |
Risk of delaying treatment in NCCN high- and very-high-risk cases [36], [39], [49] Telemedicine [57], [58] |
| Newly diagnosed patients and patients whose treatment is delayed (see Table 5) | Assessment of age and comorbidities Triage according to NCCN risk and time of diagnosis See Table 5 Consider ADT for NCCN high- and very–high-risk cases on individual basis If planning treatment, assess risk of ongoing ADT, multiple visits required for RT, short stay of surgery, and local urology service capacity For those going through surgery, consider same day discharge |
Risk of delaying treatment in NCCN high and very high risk cases [36], [39], [49] Unfavorable intermediate- and high-risk cases can potentially wait 6 mo from diagnosis [39] No evidence comparing COVID-19 risk of multiple visits for RT versus overnight stay of surgery Same-day discharge may mitigate COVID-19 risk [44] |
| PSA surveillance after treatment | Telemedicine consults. | Telemedicine [57], [58] |
| Biochemical recurrence | Triage men with BCR according to surgical pathology Gleason score, absolute PSA > 0.5, PSA doubling time < 7.5 mo, and solid tumor genomics (eg, Decipher score) for risk of disease progression and metastasis Consider restaging weighed against COVID-19 risk of hospital visits Consider starting ADT |
Surgical pathology Gleason score, absolute PSA > 0.5, and PSA doubling time < 7.5 mo predictive of metastasis-free survival for BCR PCa patients [83] Guidelines for consideration of RT for BCR [49] |
| Metastatic disease | Continue ADT if stable, monitoring for complications Alternative hormonal manipulation if mCRPC Chemotherapy according to risk of delay vs risk of SARS-CoV Immunotherapy (sipuleucel, ipilumab, and nivolumab) according to risk of delay vs risk of COVID-19 Liaise with medical oncology |
As yet no clear guidance, so determined on individual case basis in collaboration with medical oncology |
ADT = androgen deprivation therapy; AS = active surveillance; BCR = biochemical recurrence; COVID-19 = coronavirus disease 2019; DRE = digital rectal examination; mCRPC = metastatic castration-resistant prostate cancer; MRI = magnetic resonance imaging; NCCN = National Comprehensive Cancer Network; PCa = prostate cancer; PIRADS = Prostate Imaging Reporting and Data System; PPE = personal protective equipment; PSA = prostate-specific antigen; RT = radiotherapy; SARS-CoV = severe acute respiratory syndrome coronavirus.
There is emerging evidence that patients with cancer have increased susceptibility to SARS-CoV-2 infection and potentially worse outcomes [84], [85]. General recommendations for PCa patients waiting for treatment, under AS, or after treatment should exercise PPE, social distancing, and quarantining according to local guidelines [85]. Smoking cessation and optimization of comorbidities to reduce risk and severity of infection should be recommended to all [30], [31], [32]. Lifestyle changes, physical exercise, mindfulness, as well as dietary changes and supplements, for example, POMMI-T or Zyflammend, as part of comprehensive lifestyle program are increasingly recommended to patients under AS and after treatment, and should be emphasized to these patient groups during the pandemic, as well as to those awaiting diagnosis and treatment [86]. Other specific recommendations with rationale for each patient group are given in the table.
3.4.1. Smoking and comorbidities
Smoking is associated with chronic obstructive pulmonary disease), which increases the risk and severity of SARS-CoV-2 infection. Furthermore, smoking with vaping may independently increase SARS-CoV-2 infection risk and morbidity as well [30]. Smokers have a 1.4 × increased risk of SARS-CoV-2 infection and a 2.4 × increased risk of ICU admission, mechanical ventilation, and death [31]. A meta-analysis of 4202 smokers out of 22 549 PCa patients revealed that the smoking status was associated with an increased risk of biochemical recurrence (BCR), progression to metastasis, and PCa-specific mortality [31]. As yet, there is no evidence on whether the combined effects of smoking and SARS-CoV-2 in PCa patients will have a cumulative deleterious effect, but clearly a sensible recommendation on both counts is smoking cessation. Retrospective studies from China revealed that patients with diabetes or hypertension were more likely to be admitted to ITU [22], and ACE2 expression is increased in diabetics and also in patients treated with ACE inhibitors and angiotensin receptor blockers (ARBs) [32]. As shown in Table 2, PCa patients with either diabetes or hypertension should seek advice from their physicians to optimize their treatment, especially if this includes ACE inhibitors or ARBs [32], to reduce their risk of SARS-CoV-2 infection and morbidity.
3.4.2. Prostate-specific antigen
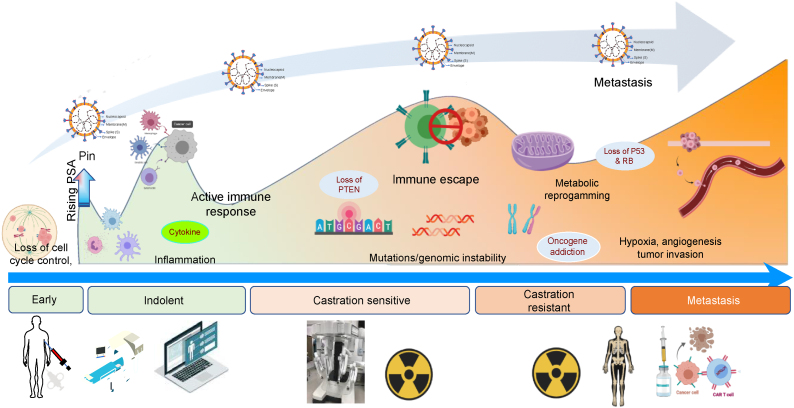
Currently, there is no evidence on the effect of SARS-CoV-2 on prostate-specific antigen (PSA) levels (Fig. 1); the literature is sparse on the effects of any systemic viral infection on PSA, including other CoVs and influenza viruses. A study in a cohort of young military patients affected by infectious mononucleosis (the Epstein-Barr virus from the human herpes family) showed a higher likelihood of a PSA rise than controls, but the absolute change was small (only 5.9% with a rise of >0.50 ng/ml). They also found that patients with nonspecific systemic viral infections were more likely to have a PSA rise than controls, but only >0.2 ng/ml [33]. In addition to the specific viral prostate infection, the effects of a viral immune response with systemic inflammation may affect local vascular permeability or prostate epithelium, resulting in PSA elevation. Thus, a systemic viral infection may produce a small rise in PSA in PCa patients in active surveillance or watchful waiting programs, but no such inference can be made on post–radical prostatectomy patients.
Fig. 1.
Prostate cancer management during COVID-19 pandemic. Schema of potential impact of SARS-CoV-2 on prostate tumor progression to lethal disease (upper panel); the virus may differentially contribute at the biological and mechanistic level to the tumor's journey during the various stages toward metastasis. Illustration of the impact of COVID-19 infection on prostate cancer patient clinical visits, biopsy and MRI, video consultations and projected delays in surgery and RT, and with possible ADT until pandemic resolves (lower panel). For patients with recurrent (CRPC) and metastatic disease, delays in restaging investigations (mpMRI prostate, PET CT scan of the bone) are projected, as well as well as chemotherapy and adoptive T-cell immunotherapy. Moreover, there is a lack of access to clinical trials that are on hold for treatment of lethal prostate cancer. ADT = androgen deprivation therapy; COVID-19 = coronavirus disease 2019; CRPC = castration-resistant prostate cancer; CT = computed tomography; mpMRI = multiparametric MRI; MRI = magnetic resonance imaging; PET = positron emission tomography; RT = radiotherapy; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
3.4.3. Prostate biopsy
For those urologists who perform transrectal biopsies, there is evidence that SARS-CoV-2 may also be transmitted by fecal-oral route. A clinical study reported that over half of patients with SARS-CoV-2 infection had viral genome in the stool, and in just under a quarter, this viral shedding continued beyond when the virus was cleared from the respiratory tract, potentially as a result of ACE2 expression within the gastrointestinal tract [34]. As discussed earlier, both renal proximal convoluted tubular cells and bladder urothelium express ACE2 [25]; however, reports of viral urinary shedding are conflicting. One study found urine samples to be negative for SARS-CoV-2 viral RNA [35], but another showed that 6.9% of infected patients had viral RNA in their urine, which stayed positive until after their throat swabs were cleared [35]. If biopsy is recommended due to risk assessment, SARS-CoV-2 infection risk to patients and providers requires standardized hospital protocols for infection control and personal protective equipment (PPE).
3.4.4. Surgery
The SARS-CoV-2 pandemic has caused widespread cancellations of PCa surgeries, due to reduced anesthesiology cover, bed and resource availability, staffing levels, and policies aimed at reducing viral spread [3]. At the authors’ Institution, there is a triage system in place, where patients awaiting robotic-assisted radical prostatectomy (RARP) for National Comprehensive Cancer Network (NCCN) high-risk PCa are assessed on a case-by-case basis, depending on wait time prior to suspension of surgery and individual disease parameters (summarized in Table 5). A number of recent studies have examined the influence of delay between diagnosis of PCa and surgical treatment on outcomes [36–39). A single-institution study of over 2600 patients undergoing radical prostatectomy with an average of 56 mo of follow-up found, despite a trend toward higher BCR rates after surgery, that this was significant for only NCCN high-risk groups beyond 12 mo [36]. Gupta et al [39] examined nearly 1250 patients with unfavorable intermediate- and high-risk PCa, and found no difference in outcomes between groups waiting for <3 or 3–6 mo after diagnosis, with up to 10 yr of follow-up. Loeb et al [37] studied delaying surgery for patients with Gleason 6 PCa, who under current guidelines will be in active surveillance programs, and Park et al [38] evaluated open and minimally invasive radical prostatectomy performed before and after 4 wk following diagnosis of prostate biopsy, which is too short a time frame to influence the decision for expedited surgery in this context. Optimization of operating room (OR) times means that procedures should be performed by experienced prostatectomists with no training for residents or fellows during this period, and the number of trained OR staff in the room should be kept to a bare minimum [40].
Table 5.
Recommendations for prostate cancer treatment during SARS-CoV-2 pandemic.
| Risk group according to NCCN guidelines | Recommendations |
|---|---|
| Low risk | Treatment with active surveillance is recommended. Telemedicine consultations are recommended for follow-up and triage for those already in AS programs for repeat biopsy by DRE, PSA, genomics (eg, Oncotype MDx), MRI PIRADS score, and clinical T stage. |
| Intermediate risk | Patients with ISUP 2 PCa are recommended to have active monitoring. In men with ISUP 3 PCa, ADT may be considered. |
| High risk | Radical prostatectomy or curative radiation therapy can be performed if capacity is available. In case of deferred treatment, patients can be given ADT. |
ADT = androgen deprivation therapy; AS = active surveillance; DRE = digital rectal examination; ISUP = International Society of Urological Pathology; MRI = magnetic resonance imaging; NCCN = National Comprehensive Cancer Network; PCa = prostate cancer; PIRADS = Prostate Imaging Reporting and Data System; PSA = prostate-specific antigen; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
3.4.5. Anesthesia and OR equipment
Patients being considered for surgery should have a SARS-CoV-2–negative nasal swab PCR and potentially a CT thorax to rule out parenchymal lung infection, in case of a false negative nasal swab PCR. Studies have shown that the PCR test is affected by technique and timing within disease course, with a false negative rate of 15–25%, and there are reports of negatively tested patients transmitting the disease [41]. At the authors’ institution, nasal swab PCR is mandatory, but CT thorax is not; however, given the possibility of false negative results, full precautions should apply regardless. The primary risk of anesthesia relates to intubation as an aerosol-generating procedure, increasing the risk of acute respiratory virus infection in the staff [42]. For those involved, environmental and PPE control protocols are important, and intubation should be performed, if possible, under airborne isolation conditions and ORs with negative pressure environments [43].
In patients for whom RARP is planned, OR recommendations for minimally invasive surgery are available from a number of sources including ERUS, RCS, RCOG, SAGES, American Association of Gynecologic Laparoscopists (AAGL), and AORN [44]. The putative risk is viral presence in the CO2 plume, putting OR staff at risk, based on prior evidence of HPV, hepatitis B, and hepatitis A smoke contamination [45]; without evidence, this also applies to the SARS-CoV-2 virus. Nevertheless, the prudent approach is a cautious one, with the procedural aim of limiting CO2 and smoke release into the environment. This can be achieved by the lowest possible intra-abdominal pressure and closed systems with ultra-low penetrating air filters for plume evacuation (eg, the Airseal system in smoke evacuation mode [Conmed CONMED Corporate Headquarters 525 French Road Utica, NY 13502 USA] filters smoke particles up to 0.01 μm in size; SARS-CoV-2 size is 0.06–0.14 μm) [43]. In addition, the ports should be closed when possible, instrument movement should be limited, intra-abdominal smoke should be kept to a minimum by suction, and smoke production should be reduced by avoiding the use of ultrasonic sealing and using low electrocautery power and bipolar vessel sealing, and sutures and clips whenever possible [40], [46], [47]. OR personnel should also wear full protective equipment including masks with goggles or visors, and the console should be cleaned between cases [40], [46], [47].
3.4.6. Radiation therapy
Radiation reduces white cell counts, but whether this increases the risk of SARS-CoV-2 for patients is not clear [48]. Recently, a radiation oncology panel (from the USA and UK) published a systematic review with recommendations on how to manage PCa patients safely during SARS-CoV-2 pandemic, applying a “RADS framework” referring to remote visits, avoidance, deferment, and shortening of radiotherapy (RT) protocols [49]. For PCa patients with NCCN low-risk and favorable intermediate-risk disease, avoidance is recommended until the pandemic resolves (Table 4, Table 5). For NCCN unfavorable intermediate-risk, high-risk, and locally advanced disease, treatment can be deferred with the start of ADT, depending on how the pandemic develops; however, if ADT cannot be given, the risks of treatment for cure should be weighed against the risks of SARS-CoV-2 infection [49]. Patients considered for adjuvant RT after surgery should have salvage RT instead, and the “RADS framework” will also apply to patients referred for palliative RT (Fig. 1).
3.4.7. Hormone therapy
Presently, there is no evidence that ADT directly increases the risk of viral infection, so patients could be treated safely with luteinizing hormone-releasing hormone agonists, abiraterone, or enzalutamide [50], unless convincing evidence emerges to the contrary. However, ADT may exacerbate comorbidities such as obesity, diabetes, and hypertension, which may increase the risk of SARS-CoV-2 infection and complications [32], [51]. If present, patients should be advised regarding lifestyle changes and should be assessed by their internists on ADT to optimize diabetic and hypertensive treatments (Table 5). SARS-CoV-2 requires TMPRSS2 as well as ACE2 for cellular uptake, and it is known that upregulation of TMPRSS2 is caused by androgenic hormones in PCa cells [12], [52]. Given that SARS-CoV-2 has this novel mechanism of pathogenicity, further research is required to determine accurately the risk of SARS-CoV-2 infection in patients on ADT. In the meantime, we must optimize comorbidities and monitor for ADT complications, while adhering to government regulations to limit the risk of SARS-CoV-2 infection during progression to metastatic disease (Fig. 1).
3.4.8. Chemotherapy
Advanced PCa patients on chemotherapy treatments such as Taxotere, carboplatin, or cabazitaxel may have an increased risk of SARS-CoV-2 infection. Cancer patients undergoing chemotherapy, including taxanes, have been shown to have an increased risk of viral infections, specifically influenza, which may worsen their morbidity and mortality as a result of immunosuppression caused by the chemotherapy itself [53], [54]. Although there are no data examining PCa chemotherapy during the SARS-CoV-2 epidemic, a recent retrospective study by Yu et al [55] reports on 1524 cancer patients treated at a center in Wuhan during the Chinese SARS-CoV-2 epidemic. Less than 50% of the patient cohort was receiving active treatment, but all had regular hospital visits and their overall infection rate was significantly higher than that of the community (0.79% vs 0.37%; 95% confidence interval 0.3–1.2%). Policies aimed at reducing hospital visits and careful assessment of the risks of SARS-CoV-2 infection versus the benefits of upfront or palliative chemotherapy must be considered under the current circumstances, before a chemotherapy recommendation is made.
3.4.9. Immunotherapy
Besides the Food and Drug Administration–approved autologous, active cellular immunotherapy sipuleucel, and checkpoint inhibitors ipilumab and nivolumab, which are used to treat metastatic castration-resistant prostate cancer [56], most immunotherapies for different PCa patient subpopulations with advanced disease will be given as a part of a clinical trial and will be suspended during the SARS-CoV-2 pandemic.
3.4.10. Telemedicine
Telehealth and other technologies that facilitate remote patient interactions have previously been discussed as a means of providing healthcare in times of disaster [57]. The literature has focused on specific care for patients who are victims of the disaster (eg, those infected with SARS-CoV-2 during the viral pandemic), but there is much to be learnt for allied specialties in the current period of “lockdown.” In the USA, over 50 large healthcare institutions have the digital telemedicine infrastructure in place, and from a urological perspective, the priority is triage of patients who are suitable compared with those who are not [58]. For PCa clinic appointments, these can be screened and categorized as urgent or nonurgent (eg, a high suspicion of high-risk PCa or uncomplicated active surveillance follow-up, respectively). Nonurgent patients described as “well” can have telemedicine follow-up consultations as an alternative (Table 4). Any additional investigations such as PSA or imaging studies can be arranged at local facilities, and prescriptions be sent to local pharmacies electronically (Table 4). The SARS-CoV-2 pandemic offers an opportunity to gain widespread experience of telehealth and associated technologies, and although all research in the USA is currently on hold, retrospective data will emerge, allowing the urological community to assess the success of these triage systems for both outpatient and inpatient care. One small silver lining to COVID-19 is the opportunity to assess whether telemedicine has any real potential for providing PCa clinical services over the long term.
3.4.11. Pre- and postsurge management
COVID-19 as a pandemic has three phases: presurge, surge, and postsurge. Table 4 deals with recommendations for the surge phase, but as hospitalization numbers gradually come down, the pandemic will enter the postsurge phase. However, given the predictions for more waves of the virus, the lines between the pre- and postsurge phases may become blurred. During these phases, stocks of PPE should be refilled, and departments should ensure that staff are fully cognizant of COVID-19 guidelines. Where possible, telemedicine infrastructure should be developed to allow remote follow-up, and all patients on treatment waiting lists should be meticulously stratified according to NCCN risk guidelines and according to comorbidities and COVID risk stratification [44]. During pandemic surge, NCCN risk groups 5 and 6 (high and very high risk) may be offered surgery or alternatively ADT according to their choice and their assessed risk of COVID-19 infection due to comorbidities. After the surge, departments can work their way down through risk groups according to local capacity, preparing for subsequent pandemic waves as described above. Similarly, patients with BCR or metastatic disease can be assessed according to Table 4 and be referred for therapy as appropriate.
3.5. Future implications
These are extraordinary times, and there is much that is unknown. As just-in-time information is processed to improve understanding on how the pandemic should be managed at clinical, social, and economic levels, critical questions need to be asked so that lessons are learnt [3]. In affected countries, the aim has been to transfer PCa services from predominantly hospitals to community-based systems, and surgeries have been delayed or cancelled. The questions are how long this will last and how long urological services can be put on hold. Many countries have government-imposed targets for PCa investigation and treatment from the point of referral, including Italy and the UK [3]. At the best of times, urology departments find meeting these targets challenging, but clear guidance are now needed on how to manage delays in patients under diagnosis and treatment for PCa, how long patients can wait to be seen, what red flags during initial assessment should expedite management, and how to deal with patients (and their families) who have suffered negative outcomes as a result of these delays.
Other direct effects of SARS-CoV-2 also need to be considered. Service reductions and banning of group meetings necessarily have a deleterious effect on training and education. Research efforts, both basic science and clinical, have generally been put on hold (barring local guidelines), and ramifications of interrupting projects and withholding grants will have long-term economic effects on institutional research programs and funding mechanisms, notwithstanding a negative impact on patient care. Finally, although SARS-CoV-2 predictions point to that it will pass following containment, there is the potential for recurrence, and also emergence of mutant viruses with enhanced sensitivity. The question then arises whether formal guidelines be put in place for viral pandemics, and from the perspective of basic science, whether SARS-CoV-2 will have a more long-lasting influence on PCa tumor biology, influencing etiology, and clinical management. Urological societies and institutional bodies have responded quickly and responsibly to the problem so far. Based on this response, and as the pandemic unfolds with tremendous consequences on global health, this review will be a critical guide for urologists across the globe on how best to approach management of the millions of patients with PCa with updated evidence-based advice.
4. Conclusions
PCa patients may have an increased risk of SARS-CoV-2 infection as well as morbidity and mortality if infected. Once appropriately triaged, and to reduce viral spread, PCa patients can have surveillance by telemedicine, and institute lifestyle changes and social quarantining measures. If risk stratification suggests that treatment should be planned, ADT can be started, or potentially surgery or RT is possible on a case-by-case basis.
Author contributions: Ash Tewari had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dovey, Mohamed, Gharib, Hammouda, Nair, Chakravarty, Lantz, Wiklund, Kyprianou, Tewari.
Acquisition of data: Dovey, Mohamed, Gharib, Hammouda, Ratnani, Stanislaw.
Analysis and interpretation of data: Dovey, Mohamed, Gharib, Hammouda, Ratnani, Stanislaw.
Drafting of the manuscript: Dovey, Mohamed, Gharib, Hammouda, Nair, Chakravarty, Lantz, Wiklund, Kyprianou, Tewari.
Critical revision of the manuscript for important intellectual content: Dovey, Mohamed, Lantz, Wiklund, Kyprianou, Tewari.
Statistical analysis: Ratnani, Stanislaw.
Obtaining funding: None.
Administrative, technical, or material support: Ratnani, Stanislaw, Nair.
Supervision: Dovey, Wiklund, Kyprianou, Tewari.
Other: None.
Financial disclosures: Ash Tewari certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Guillaume Ploussard
References
- 1.Wenham C., Smith J., Morgan R. Gender and COVID-19 Working Group. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naspro R., Da Pozzo L.F. Urology in the time of corona. Nat Rev Urol. 2020;17:251–253. doi: 10.1038/s41585-020-0312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stensland KD, Morgan TM, Moinzadeh A, et al. Considerations in the triage of urologic surgeries during the COVID-19 pandemic. Eur Urol. In press. 10.1016/j.eururo.2020.03.027. [DOI] [PMC free article] [PubMed]
- 5.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He F, Deng Y, Li W. Coronavirus disease 2019 (COVID-19): what we know? J Med Virol. In press. 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed]
- 8.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Doremalen N., Bushmaker T., Morris D.H. Aerosl and surface stability of HCov-19 (SARS-CoV-2) compared to SARS-CoV-2. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. C.D.C. resource on SARS-CoV-2. https://www.cdc.gov/sars/about/faq.html.
- 16.Zhao S, Chen H. Modeling the epidemic dynamics and control of COVID-19 outbreak in China. Quant Biol. In press. 10.1007/s40484-020-0199-0. [DOI] [PMC free article] [PubMed]
- 17.CBS News. www.cbsnews.com/news/coronavirus-update-china-lift-lockdown-wuhan-april-8-epicenter-quarantine/.
- 18.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Guan W., Ni Z., Liang W. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X., Yu Y.X., Jiqian S. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naicker S., Yang C.W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Luo R, Wang K, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. medRxiv. In press. 10.1101/2020.02.18.20023242. [DOI]
- 28.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foerster B., Pozo C., Abufaraj M. Association of smoking status with recurrence, metastasis, and mortality among patients with localized prostate cancer undergoing prostatectomy or radiotherapy: a systematic review and meta-analysis. JAMA Oncol. 2018;4:953–961. doi: 10.1001/jamaoncol.2018.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutcliffe S., Nevin R.L., Pakpahan R. Infectious mononucleosis, other infections and prostate-specific antigen concentration as a marker of prostate involvement during infection. Int J Cancer. 2016;138:2221–2230. doi: 10.1002/ijc.29966. [DOI] [PubMed] [Google Scholar]
- 34.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158 doi: 10.1053/j.gastro.2020.02.055. 1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling Y., Xu S.-B., Lin Y.-X. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fossati N., Rossi M.S., Cucchiara V. Evaluating the effect of time from prostate cancer diagnosis to radical prostatectomy on cancer control: can surgery be postponed safely? Urol Oncol. 2017;35 doi: 10.1016/j.urolonc.2016.11.010. 150.e9-15. [DOI] [PubMed] [Google Scholar]
- 37.Loeb S., Folkvaljon Y., Robinson D. Immediate versus 131 delayed prostatectomy: nationwide population-based study. Scand J Urol. 2016;50:246–254. doi: 10.3109/21681805.2016.1166153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park B., Choo S.H., Jeon H.G. Interval from prostate biopsy 127 to radical prostatectomy does not affect immediate operative outcomes for open or minimally 128 invasive approach. J Korean Med Sci. 2014;29:1688–1693. doi: 10.3346/jkms.2014.29.12.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta N., Bivalacqua T.J., Han M. Evaluating the impact of length of time from diagnosis to surgery in patients with unfavourable intermediate-risk to very-high-risk clinically localised prostate cancer. BJU Int. 2019;124:268–274. doi: 10.1111/bju.14659. [DOI] [PubMed] [Google Scholar]
- 40.ERUS Guidelines for COVID 19. www.uroweb.org/wp-content/uploads/ERUS-guidelines-for-COVID-def.pdf.
- 41.Kumar DS, O’Neill SB, Johnston JC, et al. SARS-CoV-2 infection in a 76-year-old man with negative results for nasopharyngeal swabs and possible nosocomial transmission. CMAJ. In press. 10.1503/cmaj.200641. [DOI] [PMC free article] [PubMed]
- 42.Thomas-Rüddel D, Winning J, Dickmann P, et al. Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020. Anaesthesist. In press. 10.1007/s00101-020-00760-3. [DOI] [PubMed]
- 43.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WRSE . 2020. COVID-19 Panel. April 28. https://4healthtv.play.livearena.com/ [Google Scholar]
- 45.Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. In press. 10.1097/SLA.0000000000003924. [DOI] [PMC free article] [PubMed]
- 46.Kimmig R., Verheijen R.H.M., Rudnicki M., for SERGS Council Robot assisted surgery during the COVID-19 pandemic, especially for gynecological cancer: a statement of the Society of European Robotic Gynaecological Surgery (SERGS) J Gynecol Oncol. 2020;31:e59. doi: 10.3802/jgo.2020.31.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novara G, Giannarini G, De Nunzio C, Porpiglia F, Ficarra V. Risk of SARS-CoV-2 diffusion when performing minimally invasive surgery during the COVID-19 pandemic. Eur Urol. In press. 10.1016/j.eururo.2020.04.015. [DOI] [PMC free article] [PubMed]
- 48.Sanguineti G., Giannarelli D., Petrongari M.G. Leukotoxicity after moderately hypofractionated radiotherapy versus conventionally fractionated dose escalated radiotherapy for localized prostate cancer: a secondary analysis from a randomized study. Radiat Oncol. 2019;14:23. doi: 10.1186/s13014-019-1223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaorsky NG, Yu JB, McBride SM, et al. Prostate cancer radiotherapy recommendations in response to COVID-19. Adv Radiat Oncol. In press. 10.1016/j.adro.2020.03.010. [DOI] [PMC free article] [PubMed]
- 50.Prostate Cancer Foundation, COVID Resource. www.curetoday.com/advocacy/prostate-cancer-foundation/prostate-cancer-foundations-covid19-resources.
- 51.Mohile S.G., Mustian K., Bylow K., Hall W., Dale W. Management of complications of androgen deprivation therapy in the older man. Crit Rev Oncol Hematol. 2009;70:235–255. doi: 10.1016/j.critrevonc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farooqi A.A., Hou M.F., Chen C.C., Wang C.L., Chang H.W. Androgen receptor and gene network: micromechanics reassemble the signaling machinery of TMPRSS2-ERG positive prostate cancer cells. Cancer Cell Int. 2014;14:34. doi: 10.1186/1475-2867-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ring A., Marx G., Steer C. Influenza vaccination and chemotherapy: a shot in the dark? Support Care Cancer. 2002;10:462–465. doi: 10.1007/s00520-001-0337-9. [DOI] [PubMed] [Google Scholar]
- 54.Boehmer L.M., Waqar S.N., Govindan R. Influenza vaccination in patients with cancer: an overview. Oncology (Williston Park). 2010;24:1167–1170. Nov 15. [PubMed] [Google Scholar]
- 55.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. In press. 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed]
- 56.Schepisi G., Farolfi A., Conteduca V. Immunotherapy for prostate cancer: where we are headed. Int J Mol Sci. 2017;18:2627. doi: 10.3390/ijms18122627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lurie N., Carr B.G. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;178:745–746. doi: 10.1001/jamainternmed.2018.1314. [DOI] [PubMed] [Google Scholar]
- 58.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 59.Wikipedia. 2020 Coronavirus pandemic in South Korea. March 29, 2020. https://en.wikipedia.org/wiki/2020_coronavirus_pandemic_in_South_Korea.
- 60.Coronavirus: why did infections shoot up in South Korea? February 25, 2020. https://www.bbc.com/news/world-asia-51609840.
- 61.Elflien J. 2020. Coronavirus COVID-19 exaggerated threat U.S opinion by gender. March 20, https://www.statista.com/statistics/1104711/coronavirus-exaggerated-threat-us-opinion-gender/ [Google Scholar]
- 62.abcNEWS. https://abcnews.go.com/Health/learn-americans-died-coronavirus/story?id=69588942.
- 63.Thomala L.L. 2020. China: gender distribution of novel coronavirus patients. March 12. https://www.statista.com/statistics/1095039/china-gender-distribution-of-wuhan-coronavirus-covid-19-patients/ [Google Scholar]
- 64.Statista Research Department . 2020. Italy: coronavirus cases distribution by gender. March 23,2020. https://www.statista.com/statistics/1103031/coronavirus-cases-distribution-by-gender-italy/ [Google Scholar]
- 65.Begley S., Archibald G., Sabriyya, Godfrey T.J. 2020. Who is getting sick? A look at coronavirus risk by age, gender, and more. March 10, https://www.statnews.com/2020/03/03/who-is-getting-sick-and-how-sick-a-breakdown-of-coronavirus-risk-by-demographic-factors/ [Google Scholar]
- 66.So W. 2020. South Korea: coronavirus cases by gender. March 23, https://www.statista.com/statistics/1102722/south-korea-coronavirus-cases-by-gender/ [Google Scholar]
- 67.Roser M., Ritchie H., Ortiz-Ospina E. 2020. Coronavirus disease (COVID-19)—statistics and research. March 4, https://ourworldindata.org/coronavirus. [Google Scholar]
- 68.Bendix A. Men represent the majority of coronavirus cases so far. Researchers think smoking could play a role. 2020 February 25, https://www.businessinsider.com/coronavirus-cases-why-more-men-than-women-2020-2. [Google Scholar]
- 69.KFF . 2020. Number of deaths per 100,00 population by gender. March 4, https://www.kff.org/other/state-indicator/death-rate-by-gender/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. [Google Scholar]
- 70.NYC Health. https://www1.nyc.gov/site/doh/covid/covid-19-data.page.
- 71.Medscape. Risk factors for death from COVID-19 Identified. March 9, 2020. https://www.medscape.com/viewarticle/926504.
- 72.Onder G. 2020. COVID-19 case fatality rate and characteristics of patients dying in Italy. March 23, 2020. https://jamanetwork.com/journals/jama/fullarticle/2763667. [DOI] [PubMed] [Google Scholar]
- 73.CEBM. Global COVID-19 case fatality rates. https://www.cebm.net/global-covid-19-case-fatality-rates/.
- 74.healthline.com. People with diabetes may have high risk. https://www.healthline.com/health-news/people-with-diabetes-risk-healthcare-covid19.
- 75.Cunningham A. 2020. Why some heart patients may be especially vulnerable to COVID-19. March 21, https://www.sciencenews.org/article/coronavirus-covid-19-why-some-heart-patients-especially-vulnerable. [Google Scholar]
- 76.Healio . 2020. COVID-19 and cancer: page 2. March 16, https://www.healio.com/hematology-oncology/practice-management/news/online/%7Bdbf76fca-5777-4c45-b5ff-0f09f6cc0540%7D/covid-19-and-cancer?page=2. [Google Scholar]
- 77.The ASCO Post. COVID-19 infection in patients with cancer in China. https://ascopost.com/news/march-2020/covid-19-infection-in-patients-with-cancer-in-china/.
- 78.Howard J. 2020. Cardiac injury among COVID-19 patients tied to higher risk of death. March 27, https://www.cnn.com/2020/03/26/health/cardiac-injury-coronavirus-study/index.html. [Google Scholar]
- 79.Pass W. MDedge News, March 31, 2020. COVID-19: extra caution needed for patients with diabetes. https://www.the-hospitalist.org/hospitalist/article/219144/diabetes/covid-19-extra-caution-needed-patients-diabetes.
- 80.Benyon B. Are patients with cancer at higher risk of COVID-19? https://www.oncnursingnews.com/web-exclusives/are-patients-with-cancer-at-higher-risk-of-covid-19.
- 81.CDC COVID-19 Response Team . 2020. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)— United States. February 12–March 16, 2020. March 26, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm?s_cid=mm6912e2_w#F1_down. [Google Scholar]
- 82.McCarthy N. 2020. How the coronavirus is impacting different U.S. age groups [Infographic]. March. March 19, 2020. https://www.forbes.com/sites/niallmccarthy/2020/03/19/how-the-coronavirus-is-impacting-different-us-age-groups-infographic/#7a5b9dc71fd4. [Google Scholar]
- 83.Markowski M.C., Chen Y., Feng Z. PSA doubling time and absolute PSA predict metastasis-free survival in men with biochemically recurrent prostate cancer after radical prostatectomy. Clin Genitourin Cancer. 2019;17 doi: 10.1016/j.clgc.2019.08.002. 470–475.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. In press. 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed]
- 85.Koo JR, Cook AR, Park M, et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect Dis. In press. 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed]
- 86.Dovey Z.S., Tewari A.K. Building a prostate cancer lifestyle medicine program. In: Mechanick J.I., Kushner R.F., editors. Building and implementing a lifestyle medicine program: from concept to clinical practice. Springer Nature; 2020. [Google Scholar]