Highlights
-
•
CNS relapse in multiple myeloma after ASCT without medullary relapse is uncommon.
-
•
Isolated CNS relapse is extremely rare with only 7 cases reported in literature.
-
•
Prognosis is poor with very short median survival after detection of CNS relapse.
-
•
Management is based on IMiD's and intrathecal therapy with radiation.
Keywords: Immunomodulatory drugs, Plasma cells in CSF, Extramedullary relapse
1. Introduction
Multiple Myeloma (MM) is the most common plasma cell dyscrasia predominantly affecting the elderly. Management consists of induction chemotherapy with proteasome inhibitors, immunomodulatory drugs and steroids followed by autologous stem cell transplant (ASCT). Although majority of patients relapse, duration of response varies and prognosis depends on risk stratification. Majority of relapses post-transplant are medullary with central nervous system (CNS) involvement reported in only 1% of patients. Isolated central nervous system relapse is even rarer with only few case reports and portends a very poor prognosis. We report one such case of a young male with isolated CNS relapse of multiple myeloma post ASCT with an unfavorable outcome despite aggressive therapy.
2. Case presentation
A 29-year-old male, with no prior comorbidities presented with one-year history of bilateral chest pain and a three-month history of swelling in the scalp and inability to walk with bowel bladder incontinence. On examination, there was presence of multiple soft tissue lesions in bilateral chest wall, left temporal region and left half of mandible with flaccid paraparesis below L4 spinal level. Initial investigations revealed anemia, hypercalcemia, renal dysfunction (creatnine-3.2 g/dl) and multiple skeletal lytic lesions. Serum electrophoresis showed a M-spike of 5.6 g/dl (IgG kappa), with 40% plasma cells in the marrow. Fluorescence in-situ hybridization (FISH) was not available in house at that time. Magnetic Resonance Imaging of spine showed a large extradural soft tissue mass at L4-S1 with multiple vertebral lytic lesions. A diagnosis of multiple myeloma IgG K, International Staging System (ISS) III and Durie Salmon Staging system (DSS) IIIB was made. He was treated with was four cycles of bortezomib, cyclophosphamide and dexamethasone (VCD) and palliative radiation with which he had a complete response with disappearance of M spike, normalization of serum free light chain ratio and bone marrow showing 1-2% plasma cells. He had major neurological recovery with regain of bowel bladder function and was able to walk with 4/5 power in both lower limbs. He had incomplete renal recovery with creatinine of 2 g/dl at treatment completion but with normal urine output and no metabolic complications. He was taken up for ASCT with melphalan conditioning at dose of 140 mg/m2 in view of renal dysfunction which he tolerated well. He was asymptomatic for six months with monthly SPEP and SFLC normal till 4 months after transplant. After a treatment free period of six months, he presented with low backache, weakness and diminished sensation in bilateral lower limbs, inability to see towards left side, voice change with swallowing difficulty and bowel bladder incontinence. On examination, he had features of right sixth and left ninth and tenth nerve lower motor neuron palsy with flaccid paraparesis. Disease evaluation showed dense M band (4.5 g/dl) with normal hemogram, renal functions and bone marrow. MRI brain and whole spine was suggestive of multiple intraparenchymal deposits with multiple extradural spinal lesions with multiple level cord compression. Cerebrospinal fluid (CSF) was positive for plasma cells (Fig. 1) with CSF flow cytometry showing 80% plasma cells CD38, CD138, CD45, CD56 positive. Whole body fluorodeoxyglucose (FDG) PET-CT did not show any other site of disease involvement. He was counselled for Daratumumab based therapy but was not affordable for the same. He was started on cyclophosphamide, thalidomide, adriamycin and dexamethasone (CTAD) protocol with whole brain radiotherapy (WBRT) with IT-MTX (intrathecal methotrexate) with no significant improvement. He was shifted to DCEP (dexamethasone, cisplatin, etoposide, cyclophosphamide) protocol in view of refractory disease On day 23 he developed worsening shortness of breath with type one respiratory failure requiring mechanical ventilation. Imaging was suggestive of Pneumocystis pneumonia. He developed septic shock and expired the next day.
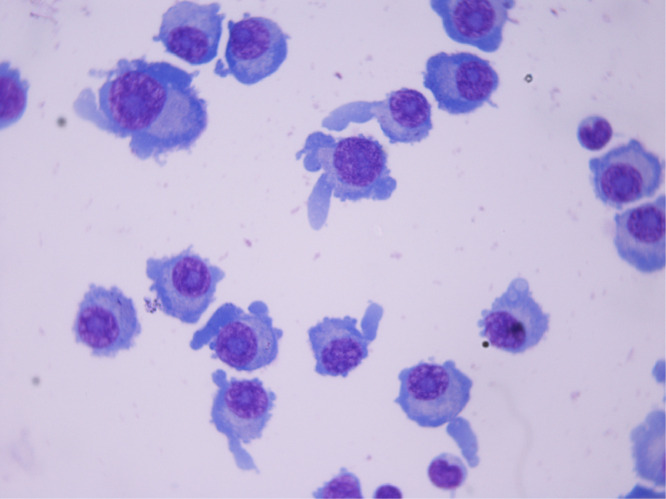
Fig. 1.
multiple plasma cells seen in CSF (giemsa stain, 100x).
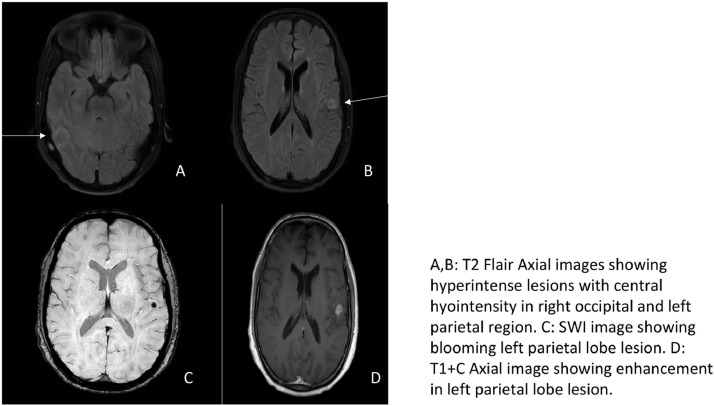
Fig. 2.
A,B: T2 Flair Axial images showing hyperintense lesions with central hyointensity in right occipital and left parietal region. C: SWI image showing blooming left parietal lobe lesion. D: T1+C Axial image showing enhancement in left parietal lobe lesion.
3. Discussion
Relapse after ASCT usually presents as medullary relapse with rising M spike and recurrence of plasma cells in the marrow. Central nervous system involvement in multiple myeloma is reported in about 1% of patients [1], involvement post ASCT is even rarer with only a few case reports.
CNS myeloma (CNS-MM) can present as isolated leptomeningeal involvement, leptomeningeal with intraparenchymal involvement or as isolated intraparenchymal lesions which is extremely rare [2]. Median survival in a cohort of CNS-MM patients was 4.6 months from time of diagnosis suggesting dismal outcomes [3]. In one series, high risk cytogenetics including 17p deletion were seen in 60% of patients [4].
CNS relapse after autologous stem cell transplant is rare and only seven cases have been reported in literature thus far. Their clinical characteristics are listed in Table 1 and comparison is done with our case presented above.
Table 1.
Published case reports of CNS relapse in multiple myeloma post ASCT.
| Patient | 1 [5] | 2 [6] | 3 [6] | 4 [7] | 5 [8] | 6 [9] | 7 [10] | 8 (current case) |
|---|---|---|---|---|---|---|---|---|
| Age | 39 | 55 | 50 | 32 | 58 | 61 | 54 | 29 |
| Sex | M | M | M | F | M | M | F | M |
| Type | IgA l | IgG k | IgD l | IgA k | IgA k | PCL (17p+) | IgG k | IgG k |
| Stage (DSS) | IIIB | IIIB | IIIA | IIIA | IIIB | IIIA | III (complex karyotype) | IIIB |
| Primary treatment | Pred, cy, VAD*3 | VAD*2, Mel | VAD*3, E/cy | VAD*3, Cy,spinal RT | MP*2, VAD*1, cy, MP*2 | 3*ROAD, 4*VAD | 3*VRD, 3*KRD, | 4*VCD |
| High dose therapy | Mel (200) | Mel (140) | Mel (140)/TBI | Mel (140)/TBI | Bu/Mel/ CY |
Mel (200) | Flu/By/Cy-Haplo | Mel (200) |
| Time to relapse | 3 m | 3 m | 3m | 10 weeks | 7.5 y | 3m | 3m | 6m |
| parenchymal | yes | no | no | no | yes | no | yes | yes |
| Treatment | IT | BCNU,CY MP, HDT |
IT | IT | Dexa, Cranial RT |
IT | RT | CTAD WBRT IT |
| Survival post CNS diagnosis | 9 days | 7months | 3months | 8days | 11months | 2months | – | 3months |
| Survival from initial diagnosis | 9months | 1year | 1year | 8.5year | 9months | – | 17months |
PCL- plasma cell leukemia, Mel- melphalan, TBI- total body irradiation, CY- cyclophosphamide, BU- busulfan, IT- intrathecal, RT-radiotherapy, CTAD- cyclophosphamide, thalidomide, Adriamycin, dexamethasone, WBRT- Whole brain radiotherapy, VAD- Bortezomib, Adriamycin, dexamethasone, VRD- Bortezomib, lenalidomide, dexamethasone, KRD- carfilzomib, lenalidomide, dexamethasone, MP- Melphalan prednisolone, HDT- High dose therapy, BCNU-carmustine, Flu- Fludarabine, Bu- Busulfan, ROAD- ranimustine, vincristine, melphalan,dexamethsaone, Haplo- Haploidentical stem cell transplant
The one noteworthy difference between our patient and others reported in literature was presence of IgG Kappa subtype whereas IgA and light chain subtype has classically been more associated with such aggressive disease biology and poor outcomes. FISH for 17p and other cytogenetic abnormalities was not performed in our case, presence of such risk cytogenetic abnormalities might explain aggressive behavior.
The mechanism leading to CNS relapse post ASCT is not well defined. It is hypothesized that high dose melphalan for ASCT might predispose to selection of extramedullary chemoresistant clone leading to relapse without bone marrow involvement [5]. Our patient had extensive extramedullary disease at baseline which in itself is a poor prognostic factor and underwent upfront ASCT after induction therapy. Patients with plasma cell leukemia are at a higher risk because of possibility of hematogenous spread [6]. Expression of CD11 and CD56 adhesion molecules on myeloma cells may also aid proliferation of these cells in CNS contributing to relapse [6]. Role of CD56 expression on plasma cells in this scenario has been debated. In a previous paper by Chang et al. 7/8 cases of CNS-MM had CD56 negative cells. However, 5/8 patients in their report had CD56 negative cells in bone marrow which was unexpected. Since then multiple case reports have reported presence of CD56 positive plasma cells in CNS owing to role of CD56 in cellular adhesion [11,12]
Management of CNS myeloma is uncertain because of rarity of this presentation and relatively poor CNS penetration of conventional myeloma active drugs. Intrathecal methotrexate with radiation and steroids was most commonly used in above cases with some response, though outcomes were poor. Yamashita et al. reported one case in which CNS relapse after allogenic stem cell transplant in a patient with primary plasma cell leukemia was successfully treated with intrathecal methotrexate, cytarabine and prednisolone plus cranial RT (10gy) along with low dose pomalidomide and dexamethasone to prevent systemic relapse [13]. Available proteasome inhibitors have poor CNS penetration; newer drug marizomib has been shown to be effective in CNS myeloma based on efficacy in two cases [14]. One case series from Mexico reported good responses to carfilzomib in CNS-MM [15]. Efficacy of daratumumab was reported by Elhassadi et al. in a patient with relapsed CNS plasmacytoma [16]. Since daratumumab was not affordable in our case, we treated our patient with immunomodulator thalidomide based regimen with intrathecal RT with minimal response. In future, based on limited data available and drug pharmacokinetics, marizomib, daratumumab, immunomodulators, radiation and intrathecal therapy might be the best approach to managing these high risk cases.
4. Conclusions
CNS relapse after ASCT in multiple myeloma is extremely rare and can precede systemic relapse. Prognosis after CNS involvement is poor and no definite data exist on how to manage these patients. Based on case reports and mechanistic models, immunomodulatory drugs may have higher CNS penetration than other antimyeloma drugs and may form the mainstay of management of these patients in combination with radiation and intrathecal therapy. Further study is required to establish whether any particular cytogenetic or IgG subtype is associated with CNS relapse. Lack of FISH at baseline or relapse in this case is a limitation and could have helped in understanding disease biology better.
Declaration of Competing Interest
None.
Acknowledgments
Authors' contributions
All three physicians were involved in care of the patient. AM compiled the history and wrote the manuscript. AM, DP and LK read and approved the manuscript.
Funding
None.
Acknowledgements
None.
References
- 1.Fassas A.B., Muwalla F., Berryman T. Myeloma of the central nervous system: association with high-risk chromosomal abnormalities, plasmablastic morphology and extramedullary manifestations. Br. J. Haematol. 2002;117(Apr (1):103–108. doi: 10.1046/j.1365-2141.2002.03401.x. Review. [DOI] [PubMed] [Google Scholar]
- 2.Patriarca F., Zaja F., Silvestri F. Meningeal and cerebral involvement in multiple myeloma patients. Ann. Hematol. 2001;80:758–762. doi: 10.1007/s00277-001-0387-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen C.I., Masih-Khan E., Jiang H. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br. J. Haematol. 2013;162(Aug (4):483–488. doi: 10.1111/bjh.12414. [DOI] [PubMed] [Google Scholar]
- 4.Varga G., Mikala G., Gopcsa L. Multiple myeloma of the central nervous system: 13 cases and review of the literature. J. Oncol. 2018;(Apr 23) doi: 10.1155/2018/3970169. 2018:3970169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen S.L., Wagner A., Gimsing P. Cerebral and meningeal multiple myeloma after autologous stem cell transplantation. A case report and review of the literature. Am. J. Hematol. 1999;62(4):228–233. doi: 10.1002/(sici)1096-8652(199912)62:4<228::aid-ajh5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Veinstein A., Brizard A., Randriamalala E., Babin P., Preud'homme J.L., Guilhot F. Central nervous system relapses after autologous stem cell transplantation for myeloma. Report of two cases. Hematol. Cell Ther. 1997;39:327–330. doi: 10.1007/s00282-997-0327-6. [DOI] [PubMed] [Google Scholar]
- 7.Ulusakarya A., Youssef A., Bayle C. Plasma cell meningitis after an autograft in a patient with multiple myeloma. Leuk. Lymphoma. 1999;34:633–634. doi: 10.3109/10428199909058496. [DOI] [PubMed] [Google Scholar]
- 8.Seftel M.D., Maguire J., Voss N. Intra-cerebral relapse following prolonged remission after autologous stem cell transplantation for multiple myeloma. Leuk. Lymphoma. 2002;43(Dec (12):2399–2403. doi: 10.1080/1042819021000040125. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura F., Narimatsu H., Furukawa K. Central nervous system relapse after autologous peripheral blood stem cell transplantation in primary plasma cell leukemia. Jpn. Med. Assoc. J. 2006;49:324–326. [Google Scholar]
- 10.Pan J., Chen J., Filicko J. Relapsed multiple myeloma presenting as intracranial plasmacytoma and malignant pericardial effusion following recent allogeneic stem cell transplantation. Case Rep. Oncol. 2017;10(2):582–587. doi: 10.1159/000478001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang H., Bartlett E.S., Patterson B., Chen C.I., Yi Q.L. The absence of CD56 on malignant plasma cells in the cerebrospinal fluid is the hallmark of multiple myeloma involving central nervous system. Br. J. Haematol. 2005;129:539–541. doi: 10.1111/j.1365-2141.2005.05493.x. [DOI] [PubMed] [Google Scholar]
- 12.Bergantim R., Bastos J., Soares M.J. Aggressive central nervous system relapse after autologous stem cell transplant in multiple myeloma: case reports and literature review. Case Rep. Hematol. 2020;2020 doi: 10.1155/2020/8563098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita Y., Tamura S., Oiwa T. Successful intrathecal chemotherapy combined with radiotherapy followed by pomalidomide and low-dose dexamethasone maintenance therapy for a primary plasma cell leukemia patient. Hematol. Rep. 2017;9(1):6986. doi: 10.4081/hr.2017.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badros A., Singh Z., Dhakal B. Marizomib for central nervous system-multiple myeloma. Br. J. Haematol. 2017;177(2):221–225. doi: 10.1111/bjh.14498. [DOI] [PubMed] [Google Scholar]
- 16.Elhassadi E., Murphy M., Hacking D., Farrell M. Durable treatment response of relapsing CNS plasmacytoma using intrathecal chemotherapy, radiotherapy, and Daratumumab. Clin. Case Rep. 2018;6(4):723‐728. doi: 10.1002/ccr3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinoza R., Nolasco D.B., Alejandro S. Report of 5 cases of extramedullary myeloma with central nervous system involvement treated with a combination of carfilzomib/thalidomide/dexamethasone as a first line treatment at a single institution in Mexico. Blood. 2016;128:5704. 5704. [Google Scholar]