Abstract
Type 2 diabetes (T2D) is a very prevalent, multisystemic, chronic metabolic disorder closely related to atherosclerosis and cardiovascular diseases. It is characterised by mitochondrial dysfunction and the presence of oxidative stress. Metformin is one of the safest and most effective anti-hyperglycaemic agents currently employed as first-line oral therapy for T2D. It has demonstrated additional beneficial effects, unrelated to its hypoglycaemic action, on weight loss and several diseases, such as cancer, cardiovascular disorders and metabolic diseases, including thyroid diseases. Despite the vast clinical experience gained over several decades of use, the mechanism of action of metformin is still not fully understood. This review provides an overview of the existing literature concerning the beneficial mitochondrial and vascular effects of metformin, which it exerts by diminishing oxidative stress and reducing leukocyte-endothelium interactions. Specifically, we describe the molecular mechanisms involved in metformin's effect on gluconeogenesis, its capacity to interfere with major metabolic pathways (AMPK and mTORC1), its action on mitochondria and its antioxidant effects. We also discuss potential targets for therapeutic intervention based on these molecular actions.
Keywords: Type 2 diabetes, Metformin, Oxidative stress, Pathophysiology, Treatment, Atherosclerosis, Mitochondria
Abbreviations: Advanced glycation end product, (AGE); AMP-activated protein kinase, (AMPK); cardiovascular diseases, (CVD); cAMP response element-binding, (CREB); CREB-binding protein, (CBP); electron transport chain, (ETC); glucagon-like peptide 1, (GLP-1); glycated haemoglobin, (HbA1c); glycerol 3-phosphate dehydrogenase, (GPD); intercellular adhesion molecule-1, (ICAM-1); inner mitochondrial membrane, (IMM); insulin receptor substrate, (IRS); insulin resistance, (IR); nitric oxide synthase, (NOS); organic cation transporter, (OCT); oxidative phosphorylation, (OXPHOS); peroxisome proliferator-activated receptor gamma coactivator 1-alpha, (PGC-1α); polycystic ovary syndrome, (PCOS); reactive nitrogen species, (RNS); reactive oxygen species, (ROS); sirtuin, (SIRT); superoxide dismutase, (SOD); type 1 diabetes, (T1D); type 2 diabetes, (T2D); vascular cell adhesion molecule-1, (VCAM-1)
1. Introduction
Type 2 diabetes (T2D) and hyperglycaemia are related to the presence of oxidative stress, caused by increased production of reactive oxygen species (ROS), among other factors [1,2]. Mitochondria are the main source of ROS in the majority of cell types [3]; when levels of glucose are high, mitochondrial ROS production is enhanced, thus inducing oxidative stress, lipid peroxidation and tissue impairment. In addition, mitochondrial dysfunction is related to the appearance of insulin resistance (IR), reduced sensitivity of tissues to glucose and its uptake [4], and several related comorbidities, including cardiovascular diseases (CVD) [5]. Furthermore, energy status is modulated by mitochondrial homeostasis, while increased ROS production is related to hyperglycaemic conditions and the development of microvascular pathologies, including neuropathy, retinopathy and nephropathy, and macrovascular complications such as myocardial ischaemia and stroke [6]. These characteristics suggest that T2D and IR are closely linked to mitochondrial impairment. Mitochondrial homeostasis is strongly modulated by several mechanisms, including mitochondrial dynamics i.e. mitochondrial fusion and fission [7]. It is important to highlight that mitochondria are not static organellae, but rather are extremely dynamic and constantly change in shape, size and location within the cells, which confers them high plasticity and metabolic versatility. Another important mechanism related to cellular homeostasis is mitophagy, by which impaired mitochondria are engulfed by autophagosomes and subsequently degraded within lysosomes [8]. An “adaptive” increase in mitophagy may delay the progression to T2D by preserving β-cell function in subjects with prediabetes, a prolonged pathogenic phase that lasts for 7–10 years [9].
It is patent that diabetes in general, including T2D and type 1 diabetes (T1D), is a serious global health problem affecting more that 400 million people. Its prevalence is increasing due to the current increasingly sedentary life style and obesity, a major risk factor for diabetes. T1D is an autoimmune disease resulting in an impairment of β-cell function; its incidence has also increased in the last 30 years, suggesting that the environment plays a significant role [[10], [11]]. T2D is the most common type of diabetes, with around 90% of cases resulting in IR. T2D is increasing worldwide; for example, between 2001 and 2009, the prevalence of both T1D and T2D among children and adolescents increased around 21% in 5 areas of the USA [12]. In 2018, the prevalence of diagnosed diabetes among US adults was reported to be 0.5% for T1D and 8.5% for T2D, and among US adults with diagnosed diabetes, T1D and T2D accounted for 5.6% and 91.2%, respectively [13]. In addition, the risk of developing T2D is higher among women with gestational diabetes. Different clinical trials have shown that glycaemic control is key to reducing cardiovascular complications associated with diabetes (UKPDS study). In fact, several studies have demonstrated that metformin and other hypoglycaemic drugs protect the endothelium from the development of vascular impairment and atherosclerosis [14,15]. The hypoglycaemic effects of metformin or dimethylbiguanide in the treatment of T2D in humans were demonstrated more than half a century ago by the French medical doctor Jean Sterne [16]. Nowadays, metformin is the gold standard drug for the treatment of T2D and other conditions with IR such as polycystic ovary syndrome (PCOS) [17].
1.1. Type 2 diabetes and oxidative stress
Hyperglycaemia can induce oxidative stress by different mechanisms, including glucose autoxidation, advanced glycation end (AGE) product formation, and activation of different enzymes (e.g. NADPH oxidase), particularly through DAG-PKC-NADPH-oxidase signalling, the polyol pathway and activation of PKCβ1/2 kinase. Mitochondria are a major contributor to ROS generation, and their role will be discussed later. Enhanced free fatty acids, leptin and other circulating factors in T2D may also contribute to cause ROS overproduction [18]. AGEs are a result of the binding of ketone or aldehyde groups of glucose with the free amino groups of proteins, which leads to formation of Schiff-bases, Amadori products and finally AGEs [19]. Oxidative stress in the circulation of T2D patients is also induced by the polyol pathway, in which ROS are generated by two enzymes: (i) aldose reductase, which uses NADPH to convert glucose to sorbitol. While this is a minor reaction in normal physiological conditions, 30%–35% of glucose in T2D conditions can be metabolized in this way [20]. During sorbitol overproduction, NADPH availability is reduced, as is the regeneration of glutathione and nitric oxide synthase (NOS) activity, all of which leads to oxidative stress [20]; and (ii) sorbitol dehydrogenase, which oxidizes sorbitol to generate fructose concomitant with NADH overproduction, which is used by NADH oxidases to increase O2.- production.
In clinical settings, it is very difficult to evaluate changes in ROS and reactive nitrogen species (RNS) levels, because these molecules have a very short half-life within cells and tissues. Therefore, other techniques have been employed to assess redox status in patients that usually involve the evaluation of stable oxidative stress by-products in the blood [21]. Lipid peroxidation is a typical parameter with which to evaluate the redox state of T2D; it can be assessed by measuring malondialdehyde (MDA), thiobarbituric acid reactive substances (TBARS) or 8-isoprostane (8-Isp) [22]. 8-Isp can also be generated by other reactive molecules, such as 4-hydroxynonenal (4-HNE), an aldehyde which itself is a product of lipid peroxidation [23]. High levels of these markers have been described in blood and urine of T2D patients [24]. Furthermore, ROS-mediated protein modifications occur against the backdrop of lipid peroxidation, including protein glycation, carbonylation, nitrosylation and glutathionylation, among others. The most common ROS-modified protein biomarker is glycated haemoglobin (HbA1c), which is routinely used to monitor long-term (3 months) average levels of glycaemia and is firmly established as a key diagnostic marker of diabetes [25].
Hyperglycaemia and dyslipidaemia are typical for IR and T2D, and both are related to oxidative stress. Under these conditions, a decrease in insulin-stimulated glucose disposal and a drop in ATP levels have been described [26]. In this context, abnormal lipid metabolism can undermine insulin signalling and therefore increase IR in different tissues [27]. In line with this idea, Fayyaz et al. demonstrated that palmitate can be metabolized into sphingosine 1-phosphate (S1P) by hepatocytes, which impairs insulin signalling by attenuating insulin-dependent protein kinase B (Akt) phosphorylation [28].
In addition, IR is induced by multiple mechanisms, including increased levels of serine phosphorylation of insulin receptor substrate (IRS) [29], decreased activation of insulin downstream signalling molecules such as Akt or PKC [30], enhanced degradation of insulin receptor proteins [31], or elevated activity of phosphatases [32]. Phosphorylation of IRS at key target residues can reduce PI3K activation, thus playing an important role in the response to insulin levels [32], and a decrease in IRS tyrosine phosphorylation has been reported in different animal models of IR and humans [33]. Importantly, ROS production can modify signals that activate the serine kinases that phosphorylate IRS proteins [34].
However, in the context of diabetes, ROS should not only be regarded as harmful molecules, but rather as an essential element of certain biological responses, including insulin secretion by the pancreas, insulin sensing, and glucose uptake by peripheral tissues. For example, in rat islets an increase in intracellular H2O2 causes a rapid and transient elevation of intracellular Ca2+ accompanied by insulin release at non-stimulatory basal glucose concentrations [35]. Furthermore, H2O2 derived from glucose metabolism has been shown to be one of the metabolic signals of insulin secretion, whereas oxidative stress may disturb its signalling function [36]. Leloup et al. [37] reported that mitochondrial ROS are required for hypothalamic glucose sensing. They found that glucose significantly increased ROS production and that antioxidants such as catalase and Trolox significantly suppressed arcuate neuronal activity and hypothalamic insulin release in response to intracarotid glucose infusion. Thus, β-cells and hypothalamus might share ROS as a common element in glucose sensing. The skeletal muscle is a major consumer of glucose and its metabolism is tightly related to diabetes and IR. In contrast to potentially harmful excess mitochondrial ROS production, the generation of ROS from non-mitochondrial sources, including NADPH oxidase and xanthine oxidase, has been shown to be important for insulin signalling in the skeletal muscle [38]. The role of H2O2 in enhanced insulin signalling has been shown to be a result of the ability of these species to oxidatively modify and inactivate key protein tyrosine phosphatases (PTEN and PTP1B). Muscle contraction has been highlighted as an important generator of ROS production in the skeletal muscle; of note, skeletal muscle glucose uptake is normal during exercise in those who suffer from IR and T2D [39]. In this sense, a list of potential regulators has been drawn up, including Ca2+ (via CaMK and/or CaMKK), AMPK, ROS, and NO signalling during exercise un human subjects. In fact, there is a basal generation of ROS in skeletal muscle, and this generation increases substantially with contraction [40]. ROS are involved in the activation of glucose transport in isolated muscle preparations [41], although different reviews suggest this is not the case during light-to-moderate exercise, occurring instead during intense physical activity [42].
At this point, it is important to highlight the antioxidant properties of some drugs employed in diabetic patients (reviewed by Ref. [43]). Specifically, statins, widely employed lipid-lowering agents, have attracted interest, with both in vitro and in vivo studies providing evidence of their antioxidant properties. Atorvastatin decreases O2.- production in human endothelial cells exposed to high glucose, and also protects these cells from H2O2-mediated damage [44]. In human studies, statins have been shown to protect lymphocytic DNA from oxidative damage, decrease plasma concentrations of oxidized-LDL and protein-bound tyrosines, diminish urinary F2-isoprostanes, and increase concentrations of the antioxidant enzyme superoxide dismutase (SOD) in erythrocytes [45]. On the other hand, there are controversies regarding the effect of statins on plasma tocopherols (vitamin E). For example, a retrospective study found that metabolic syndrome patients on statin therapy (simvastatin or atorvastatin) for 6 months or more had significantly higher concentrations of plasma vitamin E and lower concentrations of plasma 8-OHdG compared to metabolic syndrome subjects not treated with statins [46]. The statin-treated group had similar plasma vitamin E and 8-OHdG concentrations to the healthy control group. In another study, 12-week treatment of hyper-cholesterolemic subjects on simvastatin had no effect on their serum ascorbic acid levels, but did decrease serum α-tocopherol by 16.2% and β-carotene by 19.5% [47].
1.2. Type 2 diabetes and mitochondrial dysfunction
A vast body of evidence accumulated over recent decades has shown that mitochondria play a critical role in the pathophysiology of T2D. ROS production is directly related to mitochondrial dysfunction and IR [5], but how and why mitochondria generate more ROS in diabetes is a fundamental question that still needs answering.
Hyperglycaemia and hyperlipidemia enhance glucose and lipid catabolism, leading to increased production of NADH and FADH2, which are used by the mitochondrial electron transport chain (ETC) to generate ATP [48]. This overproduction of NADH can cause higher proton gradient production in mitochondria, with the surplus electrons being transferred to O2 to produce O2.- [49]. Importantly, the NADH dehydrogenase of the complex I ubiquinone oxidoreductase and complex III cytochrome c reductase are the two main sites of O2.- production via the ETC [3]. Mitochondria are among the different sources of ROS that can contribute to ROS-induced phosphorylation of IRS-1 and impair insulin signalling [50]. In this sense, using uncouplers or inhibiting NADPH oxidase can enhance glucose metabolism and insulin signalling [51]. In fact, it has been shown that ROS - both total and mitochondrial - can promote damage in the ETC, mainly at complex I [52], and hyperglycaemia is known to induce metabolic changes in β-cells that markedly reduce mitochondrial metabolism and ATP synthesis [53].
Mitochondrial impairment, mitochondrial ROS production and oxidative stress are inter-related. Low levels of ROS can activate signalling pathways to initiate biological processes, while high levels of ROS can damage DNA, protein and lipids and aggravate the inflammatory process by enhancing IκKβ activation [54]. Furthermore, studies in humans and animal models have provided evidence of impaired oxidative phosphorylation (OXPHOS) in muscle mitochondria under conditions of IR. For example [55], studied mitochondria isolated from human muscle biopsies from T2D, obese, and lean individuals, demonstrating a reduction in both NADH oxidoreductase and citrate synthase activity in the mitochondria of diabetic and obese subjects compared to lean subjects [55]. Moreover, decreased mRNA expression of several genes associated with OXPHOS has been described in diabetic patients, including genes regulated by PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) and nuclear respiratory factors [56]. This has been observed not only in subjects with T2D, but also in their first-degree relatives.
Mitochondria are known to be susceptible to a variety of genetic and environmental insults; the accumulation of mtDNA mutations and mtDNA copy number depletion can help to understand the prevalence of mitochondria-related diseases such as T2D. In this sense, a recent review has revealed the implication of novel aspects of mitochondrial biology in the progression of disease, such as mtDNA heteroplasmy, actions of non-coding RNA (ncRNA), epigenetic modifications of the mitochondrial genome, and epitranscriptomic regulation of the mtDNA-encoded transcriptome [57]. Indeed, there is growing interest in identifying genes and processes that can trigger IR, beyond defects in the insulin signalling cascade itself. For example [58], identified 286 genes that are associated simultaneously with insulin signalling and mitochondrial genes, and which, therefore, may act as a molecular bridge between the two systems. Two strong candidates were highlighted - TRAF2 and NFKB1- and their connections to insulin genes and mitochondrial genes were verified based on published literature. TRAF2 is reported to be connected to the insulin genes MAP3K1 (MEKK1) and CAV1 (caveolin 1), and to the mitochondrial genes MAP3K5 (ASK1) and CASP8 (caspase-8). A possible connection to mTOR has also been proposed [58].
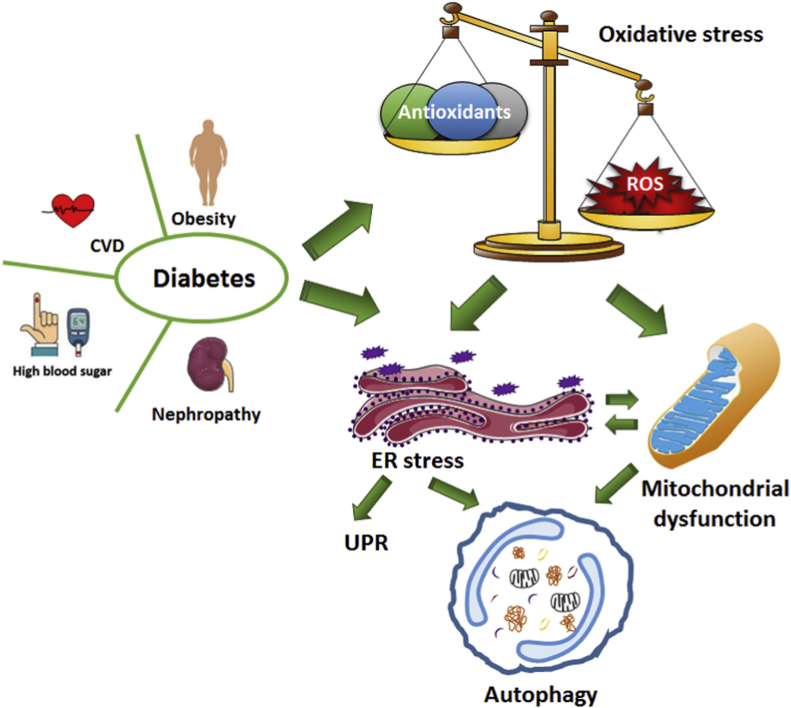
Under conditions which induce oxidative stress, different antioxidant systems, such as SOD and catalase, are activated to counteract ROS production and inhibit the formation of AGE products or the activation of one of the key transcription factors related to inflammation, nuclear factor kappa B (NF-κB) [59], thus combating the chronic proinflammatory state and oxidative stress, both which are hallmarks of diabetes. These effects can activate important cellular metabolic and energetic mediators such as sirtuins (SIRT1 and 3), PGC-1α or AMPK (AMP-activated protein kinase), therefore modulating and restoring mitochondrial function in cells (including β-cells) and tissues such as adipose tissue, liver and muscle, thereby preventing the development of CVD [60]. In summary, mitochondrial function depends on different factors, including OXPHOS and mitochondrial dynamics, and mitochondrial impairment plays a critical role in the development of T2D and IR-related conditions such as CVD (Fig. 1).
Fig. 1.
Pathophysiological mechanisms of Type 2 Diabetes Mellitus (T2D). The onset of T2D and its complications can lead to an imbalance in cellular homeostasis such as enhanced oxidative stress levels due to an increase in ROS production and a decrease in levels of antioxidant defenses. This can promote ER stress, which activates the UPR (unfolded protein response) on the one hand and can increase autophagy on the other hand. CVD, cardiovascular disease; ER, endoplasmic reticulum; ROS, reactive oxygen species.
1.3. Type 2 diabetes and cardiovascular disease
T2D is a complex and multi-systemic condition that impairs different organs through multiple mechanisms. In relation to diabetes-related oxidative/nitrosative stress, it is important to note that ROS and RNS have major functional and dysfunctional roles at the cellular level, particularly in tissues such as pancreatic islets, adipose, muscle and liver, which exacerbates T2D. In this way, T2D patients are also at risk of developing a variety of other diseases related to circulation and metabolism, including retinopathy, neuropathy, nephropathy, vascular diseases and cardiomyopathy [23].
In this sense, CVD is one of the main contributors to mortality and morbidity among T2D patients [61,62]. The endothelium is a glycolytic tissue, but under hyperglycaemic conditions, glucose can be shunted to other locations, leading to the generation of AGEs or activation of the polyol pathway [63]. Activation of PKCβ1/2 activation via diacylglycerol can alter different proteins in the altering hemodynamics, vascular permeability, vascular endothelium growth factor (VEGF) production and redox signaling in the vessels [64]. AGEs are related to the atherosclerotic process in T2D, and their levels are associated with the severity of the disease [65]. Furthermore, binding of AGEs to cell surface receptors that are specific for AGE can activate intracellular redox signaling. This subsequently triggers the expression of redox-sensitive transcription factors and inflammatory mediators; for example, the binding of AGEs to their endothelial receptor, which induces the expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in diabetic mice [66].
Moreover, it has been described that hyperglycaemia is not only related to mitochondrial ROS production and oxidative stress, but also affects autophagy and produces endoplasmic reticulum stress [67], both of which play an important role in the development of vascular disease and endothelial impairment [68,69,61] (Fig. 1). Alterations in endothelial function determined by an increase in the release of adhesion molecules such as sICAM-1 (soluble intercellular adhesion molecule-1) and sVCAM-1 are key indicators of the development of the atherosclerotic process and, eventually, CVD [70].
1.4. Metformin: pharmacological characteristics, clinical use and actions
Metformin is one of the most used medications in the world, and is currently prescribed to nearly 150 million people. It has been recommended as first-line oral therapy for T2D by both the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) since 2009, due to its ability to lower blood glucose in a safe and effective manner and to its affordable price (it is available as a generic since 2002). Despite its presence on the market for six decades now, this compound continues to intrigue researchers, especially due to mounting evidence of its effectiveness in other metabolic pathologies, such as obesity, PCOS [71], CVD [72], non-alcoholic fatty liver disease [73], premature puberty [74], kidney disease [75], T1D [[11], [76]], cancer [77,78] and neurodegenerative diseases [79]. There is a growing body of evidence showing that metformin can increase healthy lifespan in vivo in nematodes [80,81] and mice [82,83]. Metformin originates from galegine, an isoprenyl derivative of guanidine found in the perennial herb Galega officinalis, known as goat's rue, French lilac, or Italian fitch, used in popular medicine in Europe during the Middle Ages. Galegine was tested as a glucose-lowering agent in humans in the 1920s, but was found to be too toxic and limited by its short half-life and hypoglycaemic lethality at high doses [84,85]. A synthetic derivative of galegine – metformin – was then synthesized and shown to be effective, but it was not until 1957 that it was introduced as “Glucophage” (coined by Sterne) to treat diabetes [84,86]. The FDA approved it for the treatment of T2D in 1994, after 20 years of use in Europe. The bioavailability of oral immediate-release metformin in humans is approximately 70%, reaching plasma concentrations of 40–70 μM in the portal vein and 8–24 μM in the systemic circulation. For example, after a single 1.5 g dose, plasma concentration peaks at 18 μM at 3 h, and the mean plasma half-life is approximately 20 h [87]. Metformin is absorbed by enterocytes through the plasma monoamine transporter (PMAT) and organic cation transporter 3 (OCT3) on the apical membrane, and leaves the enterocytes to enter the portal vein via OCT1 on the basolateral membrane. It accumulates significantly in the intestines, liver, kidneys and bladder, is excreted unchanged in urine, and does not produce metabolites. Metformin's side effects are mainly gastrointestinal disturbances, including abdominal pain, nausea, diarrhoea and vomiting, which occur in 20%–30% of patients. Very rarely, metformin causes lactic acidosis, and the mortality risk is < 1.5/100,000 patients per year.
Despite vast clinical experience, the mechanism of action of metformin is still not fully understood. In comparison with other drugs, metformin is given at large doses, in the range of 2 g per day, suggesting that the effect may not be a result of the drug's interaction with a specific protein target, but rather due to multiple mechanisms. Abundant evidence obtained in clinical studies and animal models suggests that the primary function of metformin in the regulation of glucose homeostasis; specifically, the inhibition of liver glucose production through downregulation of hepatic gluconeogenesis and glycogenolysis [[88], [89]]. Several mechanisms have been proposed to explain this inhibitory action on hepatic gluconeogenesis, including changes in enzyme activity [90,91], such as the inhibition of gluconeogenic enzymes in mitochondria, a reduction in hepatic uptake of gluconeogenic substrates [92], and antagonism of the glucagon signalling pathway [93]. The preferential action of metformin on hepatocytes has been related to the predominantly hepatic expression of OCT1 and OCT3, the carriers that facilitate cellular uptake of metformin [94]. Consistent with this, more metformin is accumulated in the liver than in other tissues (2–5 times that of plasma) [95,96,97,94], reaching hundreds of μM in the periportal area. Metformin is also an insulin sensitizer, as it improves insulin sensitivity, thus enhancing peripheral glucose uptake, mainly in the skeletal muscle, and significantly reducing fasting insulin levels in plasma. The improvement in insulin sensitivity by metformin can be ascribed to its effects on insulin receptor expression and tyrosine kinase activity. In contrast to other antidiabetic drugs, metformin does not stimulate endogenous insulin production; therefore, it is not generally associated with a risk of hypoglycaemia [98] and, importantly, exerts a positive or neutral effect on body weight. Furthermore, an important contribution to antihyperglycaemic efficacy and other beneficial metabolic actions of metformin arise from its modulation of the incretin axis. In this regard, within the gut, metformin has been shown to increase the secretion of glucagon-like peptide 1 (GLP-1), a glucose-lowering gut incretin hormone that is secreted in response to food ingestion and normalizes postprandial glycaemia by stimulating glucose-dependent secretion of insulin and inhibiting glucagon release from the pancreas [99]. Related to this gut-mediated mechanism of metformin action, a gut–brain–liver crosstalk has been identified in rats linking intestinal metformin exposure to the suppression of hepatic glucose production via the nucleus tractus solitarius and nervus vagus efferents through AMPK and GLP-1 receptor activation [100]. Furthermore, the glucose-lowering effect of metformin has also been linked to its interaction with gut microbiota, leading to a rescue from dysbiosis associated with T2D, possibly through the regulation of metal homeostasis [101]. In line with this, a very recent landmark study on drug-nutrient-microbe interactions has revealed that gut microbes integrate cues from treatment with metformin and diet through the phosphotransferase signaling pathway that converges on the bacterial transcriptional regulators Crp and ArgR [102].
Metformin is widely regarded as a drug with pleiotropic effects. The number of effects and mechanisms involved in its hepatic and extra-hepatic response is constantly growing. Apart from its action on specific targets of the metabolism, it also increases antioxidant protection and regulates cell death processes [79,77]. Metformin favourably influences various metabolic and cellular processes, such as autophagy [103,104] and cellular senescence [105,106], which are closely linked to the development of age-related conditions. Metformin has also been shown to have direct effects on inflammation [107]; it acts on NF-κB signalling and differentiation of monocytes into macrophages [108] and is able to suppress several inflammatory cytokines in the plasma of non-diabetic individuals [109].
1.5. Molecular mechanisms involved in the actions of metformin
While it is clear that metformin's primary action is the suppression of hepatic glucose production, its secondary mechanisms are a subject of controversy, with various targets and signalling pathways having been proposed. The variety of actions and discrepancies described in the literature is probably due to the prolonged use of metformin at supratherapeutic doses/concentrations in animal and in vitro studies, which has somewhat obscured the physiological and clinical relevance of the reported findings. Moreover, cell and tissue responses are not only a product of dose, but also of treatment duration, and studies vary considerably in this respect. An additional factor is that many studies have been performed with phenformin, an analogue of metformin that was popular in the 1960s, but which was withdrawn in the early 1970s due to its capacity to cause lactic acidosis and increased cardiac mortality. Phenformin's actions are similar to those induced by metformin; however, it displays several characteristics that are not shared. For example, it shows greater lipid solubility than metformin, but is a better substrate of OCT, including OCTN1, which is located in the inner mitochondrial membrane (IMM). It is also a more effective inhibitor of complex I of the ETC, and thus about 20 times more likely to cause lactic acidosis in humans, which is the reason for its prohibition for human use in most countries.
1.5.1. Metformin and gluconeogenesis
A vast body of evidence supports the idea that the major mechanism of the hepatic effect of metformin occurs via or originates from its mitochondrial action (Fig. 2). Metformin-mediated ETC complex I inhibition (revised below) suppresses ATP production, which leads to an increase in cellular AMP and ADP levels and alters the AMP/ATP ratio. In fact, even modest decreases in ATP production can result in relative increases in AMP or ADP. An elevated AMP/ATP ratio triggers the activation of AMPK, the main bioenergetic sensor in eukaryotic cells, which promotes catabolic reactions that generate more ATP and inhibit energy-consuming anabolic pathways, including gluconeogenesis. Low intracellular energy levels can also suppress gluconeogenesis without lowering gluconeogenic enzyme expression; this can occur through the activation of the competing pathway of hepatic glycolysis, but whether this is the case for metformin is unclear. Alternatively, AMP can participate in the acute inhibition of gluconeogenesis via allosteric regulation of key enzymes in this pathway; e.g., AMP synergizes with fructose 2,6-bisphosphate to stimulate phosphofructokinase and inhibit fructose-1,6-bisphosphatase. The role of bioenergetic stress as the underlying mechanism of metformin's actions has also been challenged, as some studies have failed to detect any changes in the cellular ADP/ATP ratio after metformin treatment, while others have found that metformin suppresses glucose production in primary hepatocytes without altering either ATP levels or the AMP/ATP ratio [110]. Another signalling pathway that metformin may interfere with is the generation of cAMP, as increased AMP levels inhibit adenylyl cyclase [93]. However, the fact that this is achieved with high, probably suprapharmacological concentrations of metformin, together with metformin's ability to inhibit glucose production stimulated by dibutyryl-cAMP, a hydrolysis-resistant cAMP analog [110,111], challenges the cAMP hypothesis concerning the clinical action of metformin. On the other hand, lowering cAMP levels blocks the cAMP-PKA pathway. In a self-amplifying loop, this pathway may exert a negative effect on AMPK activation, as PKA phosphorylates the AMPKα subunit at S173, S485 and S497, reducing T172 phosphorylation and AMPK activity.
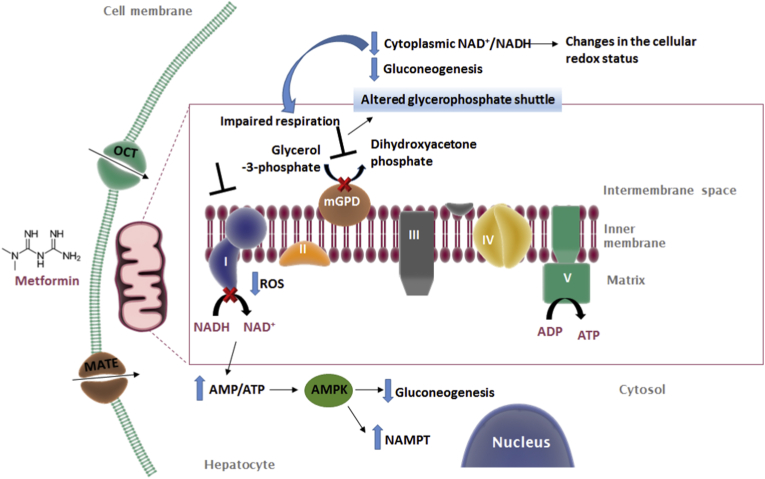
Fig. 2.
Molecular mechanisms of the mitochondrial action of metformin. Metformin exerts mild, transient and specific inhibition of complex I (NADH:ubiquinone oxidoreductase) of the respiratory chain, which leads to a change in both AMP/ATP ratio and NAD+/NADH ratio, effects that lead to downregulation of gluconeogenesis. Metformin also acts as a non-competitive inhibitor of mitochondrial glycerol 3-phosphate dehydrogenase (mGPD), and this inhibition of the glycerol-phosphate shuttle can result in impaired respiration, a reduced cytoplasmic NAD+/NADH ratio and undermined glucose production through gluconeogenesis. AMPK, AMP-activated protein kinase; NAMPT, nicotinamide phosphoribosyltransferase.
1.5.2. Activation of AMPK
Numerous studies have shown that metformin activates AMPK, but how exactly this occurs is still to be determined. Apart from triggering the activation of AMPK through the energetic dysbalance (increased AMP/ATP ratio), metformin has been reported to activate AMPK by increasing phosphorylation of the AMPK catalytic α subunit at Thr172 in primary hepatocytes [112]. At least a part of this response is mediated through the serine/threonine kinase LKB1, the upstream kinase for AMPKα phosphorylation at Thr172 [113]. As the activity of LKB1 is constitutive, it is possible that metformin mediates AMPK activation by promoting the binding of LKB to AMPK. AMPK activation results in inhibition of gluconeogenesis, an anabolic process mainly regulated through cAMP response element-binding (CREB) co-activator complex, a pivotal regulator of hepatic glucose output, by directing transcriptional activation of the gluconeogenic genes, especially phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase). The effect of metformin in this regard is extremely complex and occurs at multiple levels. AMPK activation results in phosphorylation of CREB-binding protein (CBP) at Ser436, which leads to disassembly of the CREB co-activator complex and the subsequent inhibition of gluconeogenic gene expression [114]. It also promotes the phosphorylation (Ser171) and nuclear exclusion (inactivation) of the transcriptional coactivator CREB-regulated transcription coactivator 2 (CRTC2), otherwise referred to as TORC2 [113]. An alternative mechanism for the inhibitory action of metformin on CRTC2-mediated gluconeogenesis involves deacetylation of CRTC2 by the NAD+-dependent protein deacetylase sirtuin 1 (SIRT1) through AMPK-mediated induction of nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme for NAD+ biosynthesis [115]. Metformin-activated AMPK has also been shown to mediate upregulation of the orphan nuclear receptor small heterodimer partner (SHP), which operates as a transcriptional repressor, as it inhibits CREB-dependent hepatic gluconeogenic gene expression via direct interaction with CREB and competition with CRTC2 binding in the CREB-CBP complex [116]. Despite the abundant bibliography that support the implication of AMPK, there is still controversy surrounding the absolute requirement of AMPK for metformin-induced suppression of glucose production [111], with the number of reports of the AMPK-independent actions of metformin increasing over the years [87].
1.5.3. Inhibition of mTORC1
The other major pathway affected by metformin is the mTORC1 pathway. The mTORC1 complex is the master regulator of protein synthesis through its main downstream target, S6 kinase, which initiates translation and protein synthesis. mTORC1 activation requires Rheb (Ras homolog enriched in the brain) which becomes active when bound to GTP and inactive when bound to GDP. Under starvation conditions, mTORC1 is negatively regulated by tuberous sclerosis complex 2 (TSC2), a GTP exchange factor for Rheb and a direct target of AMPK. There is evidence that metformin inhibits the mTORC1 complex in both AMPK-dependent and -independent manners [111,117]. AMPK-dependent mechanisms involve the activation of AMPK, which activates TSC2, the negative regulator of mTORC1. Additionally, AMPK can directly phosphorylate Raptor, a subunit of mTORC1, thus inhibiting mTORC1 activity. However, the AMPK-independent mechanism is less clear, and one of the actions to have been proposed involves localization of the mTORC1 complex in late endosomes/lysosomes. This is supported by the fact that metformin interacts with organelle Na+/H+ exchangers (eNHE) and the V type ATPase (VATPase) at the late endosome/lysosome [118].
1.6. Metformin and mitochondria
Many of the hepatic effects of metformin have been explained by its interference with mitochondria. As gluconeogenesis is an energy-intensive programme, these organelles are fundamental in providing the necessary ATP. The most intensively studied mitochondrial action of metformin is the mild, transient and specific inhibition of complex I (NADH:ubiquinone oxidoreductase) of the respiratory chain, which was first discovered in 2000 [119] and has since been demonstrated by numerous studies (for a detailed list see the comprehensive review by Fontaine [120]). Incubation of isolated mitochondria with mM concentrations of metformin leads to a fast (within a few minutes) inhibition of complex I. The major criticism of the authenticity of this mechanism has been the requirement of high extracellular concentrations (mM) for these rapid effects to be observed. Other consequences of the respiratory chain inhibition besides ATP production, such as changes in the NAD+/NADH ratio, may also contribute to the effects of metformin on gluconeogenesis [119]. When complex I is inhibited, electrons from NADH cannot be transferred to molecular oxygen (electron acceptor) to form water and regenerate NAD+. The mechanism by which metformin affects the activity of complex I is still largely debated. One notion is that it occurs differently to that of the reference complex I inhibitor rotenone. The inhibitory action of metformin is milder, and it significantly reduces mitochondrial ROS production by selective inhibition of the reverse electron flow through complex I, whereas rotenone triggers ROS production by increasing forward electron flow. Although the metformin binding site at complex I is not yet determined, it seems that it inhibits a rate-limiting step coupled to ubiquinone reduction. While some suggest that it does not competitively bind to the ubiquinone-binding site in complex I [121], others point to the 49 kDa subunit containing the ubiquinone binding site on the matrix side as the location of guanidine binding [122]. Some of the discrepancies regarding metformin's interference with complex I arise from the use of different models; i.e., intact cells, isolated mitochondria, isolated complex I, perfused organ or live animal. Despite the considerable amount of published evidence of metformin's interference with complex I, the extent to which this is physiologically relevant is still unclear. Some studies suggest that complex I is only affected by high, supratherapeutic concentrations of metformin [123].
Another aspect of debate is the capacity of metformin to enter and accumulate inside mitochondria, which is related to its peculiar physicochemical characteristics. As a biguanide, it is a hydrophilic compound charged positively at a physiological pH. Due to its hydrophilicity, its permeability through lipid membranes is limited, and transport of metformin in and out of cells (particularly liver, small intestine and kidney) is possible through several transporters, including OCTs and multidrug and toxin extrusion (MATE) transporters [124], whose genomic variations in humans affect pharmacokinetics and metformin concentration in tissues [125]. However, among the numerous mitochondrial carriers recognized, none has yet been identified specifically for metformin in the IMM. In addition, its apolar hydrocarbon side-chain is likely to promote its binding to hydrophobic structures, such as the constitutive phospholipids of mitochondrial membranes. Some studies have shown that metformin accumulates inside mitochondria, reaching concentrations up to 1,000-fold higher than in the extracellular medium; this is a result of metformin's positive charge, which enables the molecule to enter mitochondria using the IMM potential [121,126,127]. If metformin does accumulate within mitochondria, it is comprehensible that high (millimolar) concentrations of the drug are necessary to inhibit complex I in isolated mitochondria, while, when used at a therapeutic dose, the plasma metformin concentration remains in the micromolar range in both humans and animals [125,128,97]. However, metformin's accumulation in mitochondria has been challenged more than once. Firstly, drugs that are extensively sequestered in cells and, thus, in organellae have a very large apparent volume of distribution and a prolonged half-life in vivo, which is not the case of metformin, whose volume of distribution is 1.12 ± 0.08 L/kg [129], with a half-life of 1.74–7.3 h [129,97,94]. Secondly, the accumulation of numerous positive charges in the matrix, compensated by proton extrusion by the respiratory chain, should lead to a collapse of mitochondrial membrane potential; however, metformin does not depolarize isolated mitochondria [130]. Lastly, assuming that the total mitochondrial volume represents approximately 20% of the total cellular volume of hepatocytes, a 1,000-fold accumulation of metformin inside mitochondria would represent approximately a 200-fold accumulation in the liver (without accounting for its accumulation in the cytosol), which is 2 times higher than that reported by more than one study [96,97,94].
The mitochondrial interference of metformin seems to occur beyond ETC complex I. It has been suggested that direct binding of metformin to mitochondrial copper ions accounts for the metabolic effects of the drug [131]. Metformin also acts as a non-competitive inhibitor of mitochondrial glycerol 3-phosphate dehydrogenase (GPD2) (Fig. 2), as shown with the purified enzyme of different species. This inhibition of the glycerol-phosphate shuttle would result in impaired respiration, a reduced cytoplasmic NAD+/NADH ratio and undermined glucose production from both glycerol and lactate [123], which endorses the hypothesis that a change in cellular redox potential, rather than energy charge, underlies metformin's mechanism of action. GPD2 resides on the outer surface of the IMM, and, despite having no known transmembrane domain, it oxidizes glycerol 3-phosphate to produce dihydroxyacetone phosphate. Of note, GPD2′s location allows metformin to bind to it without having to pass through the IMM. However, several questions about this mechanism have been raised, which calls for a more profound analysis of its physiological relevance [132]. Firstly, the glycerophosphate shuttle is quantitatively much less important in hepatocytes than the malate-asparate shuttle, and contributes very little to ATP production (~0.5%). Secondly, lactate-driven gluconeogenesis may be unaffected, since the NADH produced by lactate-to-pyruvate conversion will be consumed by GAPDH in the gluconeogenic pathway. Also, if lactate and glycerol transformation to glucose in the liver are impaired, these metabolites should accumulate in the plasma; however, this does not seem to occur in metformin-treated T2D patients. Another argument against GPD2 as the primary target of metformin is the fact that GPD2 knockout mice have slightly lower fasting blood glucose levels than control animals [133].
1.7. Metformin as a promoter of antioxidant actions
Substantial and varied evidence shows that metformin exerts antioxidant effects. This has not been demonstrated purely in the field of diabetes, but also in various pathophysiological settings unrelated to diabetes, both in vivo and in vitro; e.g. liver damage induced by chemicals or bile duct ligation, global cerebral ischaemia or ischaemia/reperfusion, Parkinson's disease and sepsis-induced organ failure, among others [134,135,136]. Given that the antioxidant activity of metformin per se is minor, it is important to address which molecular mechanisms are responsible for its antioxidant actions. While multiple reports provide evidence that markers of oxidative stress unequivocally decrease in metformin-treated patients and animals, how exactly metformin promotes its antioxidants effects independently of its metabolic and hypoglycaemic actions remains a mystery. In one recent study that employed a rat model of arsenic-induced diabetes, metformin reversed oxidative stress in rat pancreas mitochondria via a Sirt3-dependent pathway [137]. Moreover, both in diabetic rats [138] and newly diagnosed diabetic patients [139] metformin has been shown to restore the activity of PON1 (paraoxonase 1), an antioxidant that circulates in association with HDL and hydrolyzes lipid peroxides within lipoproteins, mainly LDL. On a cellular level, it has been described that metformin reduces intracellular ROS levels by upregulating expression of the antioxidant protein thioredoxin via the AMPK-FOXO3 pathway, as observed in primary human aortic endothelial cells exposed to palmitic acid [140]. In rats exposed to the prooxidant rotenone, metformin co-treatment was found to block ROS production and maintain redox homeostasis by improving the function of antioxidant machinery and transporter proteins, thus reducing the osmotic fragility of erythrocytes [141]. Several studies employing very different experimental models have proven the capacity of metformin to downregulate NADPH oxidase, one of the major producers of cellular ROS [[142], [143],144,145]. Altogether, it seems that metformin exerts its role as an antioxidant through several mechanisms, including: 1) the direct trapping of hydroxyl radicals, though this is thought to be a minor and insignificant action [146]; 2) (more likely) by enhancing the endogenous antioxidant system, including the activity of antioxidant enzymes such as glutathione reductase, catalase and superoxide dismutase, or GSH content; and 3) by downregulating NADPH oxidase.
1.8. Metformin and leukocyte-endothelium interactions
Endothelial dysfunction and atherosclerosis have been related to T2D. In the early stages of atherosclerosis, activated leukocytes roll along the wall of these activated vessels, adhering to it and eventually transmigrating to the inflammatory focus. During this process, endothelial recruitment of leukocytes is mediated by adhesion molecules that are expressed on either leukocytes or endothelial cells. There are many adhesion molecules implicated in the atherogenic process, including VCAM-1, ICAM-1 and selectins [147].
As mentioned before, metformin has been widely used in the treatment of T2D due to its glucose-lowering properties. In addition, it exerts anti-inflammatory and antioxidant effects in T2D patients [148]. Its anti-inflammatory effects and protective actions in CVDs are mediated by multiple mechanisms (Fig. 3), including improvement of endothelial function in patients with IR - not only T2D patients, but also patients with metabolic syndrome [15]. In addition, multiple studies have highlighted the importance of hyperglycaemia and IR in exacerbated ROS production by leukocytes, decrease of antioxidant content, increase of proinflammatory cytokines, and a high incidence of leukocyte-endothelium interactions [149,150].
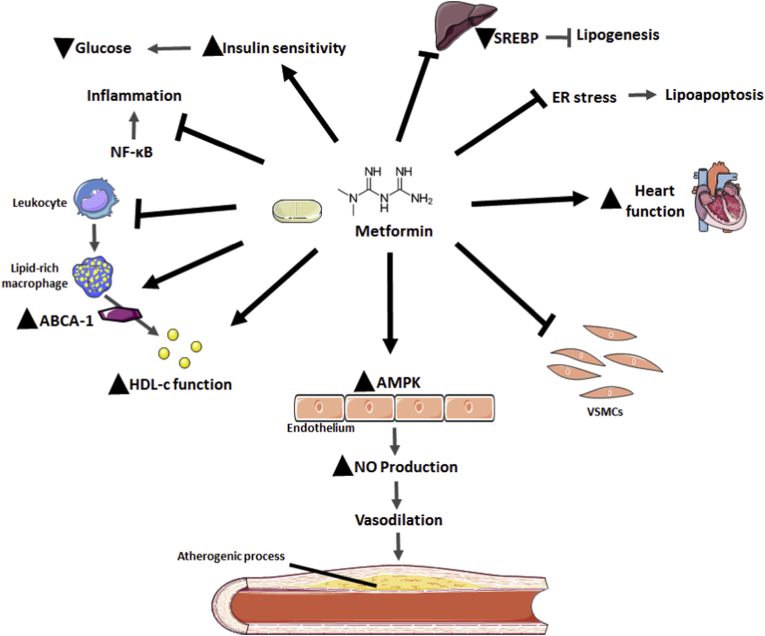
Fig. 3.
Beneficial effects of metformin on cardiovascular system. Metformin inhibits lipogenesis by downregulation of sterol regulatory element-binding protein (SREBP) expression and activity; it confers protection from lipoapoptosis by alleviating ER stress; it ameliorates cardiovascular system function by preventing abnormal vascular smooth muscle cell (VSMCs) proliferation and migration and by increasing AMPK signalling and nitric oxide (NO) production in endothelial vascular cells. Metformin ameliorates tissue insulin sensitivity, which leads to a decrease in glucose serum levels and inhibits NF-κB pathway signalling, thus diminishing inflammation; it prevents the conversion of monocytes into macrophages; it increases ATP cassette transporter type 1 (ABCA-1) activity, promoting the export of cholesterol from lipid-rich macrophages and ameliorating high-density lipoprotein cholesterol (HDL-c) function, thus reducing leukocyte-endothelium interactions and atherosclerotic risk.
Metformin has demonstrated beneficial effects in redox balance in different studies, such as that by Ref. [151]; which reported that metformin-treated T2D patients exhibited decreased mitochondrial ROS production, an increase in different antioxidant mRNA levels, including those of Gpx1 and SIRT3, and a decrease in leukocyte-endothelium interactions, as well as lower levels of ICAM-1 and P-selectin with respect to non-metformin-treated T2D patients. Metformin has also demonstrated beneficial effects on cholesterol metabolism in diabetic patients, such as HDL and LDL lipoprotein subfractions, by decreasing the risk of atherogenesis and reducing LDL levels [152]. It is widely accepted that oxidative stress and the atherosclerotic process are related to leukocyte recruitment to the arterial wall, processes that contribute to the development of vascular diseases. These results are in line with those of other studies showing that metformin exerts antioxidant effects by decreasing ROS from different sources, including mitochondria and NADPH oxidase in aortic endothelial cells [153]. Furthermore, metformin has beneficial effects by modulating ROS production by mitochondrial complex I, improving mitochondrial function and vascular homeostasis. Moreover, one study has demonstrated that metformin modulates the expression of the deacetylase SIRT3, whose activity promotes antioxidant effects in the cell and normalizes pulmonary hypertension associated with heart failure involving preserved ejection fraction [154]. In the study in question, injection of metformin reduced pulmonary pressure and vascular remodelling in a rat model of metabolic syndrome with a high activation of skeletal muscle SIRT3 and AMPK. SIRT3 is an NAD+-dependent deacetylase specifically located in the mitochondria that, when overexpressed, reduces ROS production in several tissues. Of note, metformin has been shown to enhance the mRNA expression of SIRT3, while increased SIRT3 activity leads to a reduction of mitochondrial ROS levels. In addition, it seems the drug increases the expression of Gpx1, thus protecting leukocytes against oxidative stress and hyperglycaemia through a reduction of hydroperoxides. Metformin has also been shown to protect the endothelium by improving endothelium-dependent vascular responses [15]. Mather et al. provided the first in vivo evidence that, compared to a placebo, 12-week metformin therapy significantly improved acetylcholine-stimulated, endothelium-dependent vasodilation in T2D patients. The authors proposed mechanisms for metformin's beneficial effects (other than glucose-lowering), including a reduction of IR, antioxidant effects, favorable effects on lipids and free fatty acids, and direct vasodilatory effects.
Metformin can also modulate the impact of hyperglycaemia on endothelial function in aortic tissue and microvascular endothelial cells by increasing phosphorylation of eNOS and Akt [155]. In line with this, one report demonstrated that metformin exerts its protective cardiovascular effect by reducing the activity of poly (ADP-ribose) polymerase 1 (PARP1) via the AMPK-PARP1 cascade in vascular endothelial cells, diabetic and hypertensive rodent models, and AMPKα2-knockout mice [156]. All of these features suggest that metformin has beneficial effects by preventing the atherosclerotic process and, consequently, CVD.
In leukocyte-endothelium interactions, a key role is played by soluble adhesion molecules, including ICAM-1, E-selectin and VCAM-1, which are expressed by endothelial cells and/or leukocytes in response to inflammation. These molecules are key markers of endothelial activation, as they play an important role in the recruitment of leukocytes to the site of inflammation. Moreover, it has been demonstrated that impaired endothelial activation is related to increased susceptibility to infection and, therefore, morbidity. Of note, several studies have described increased levels of adhesion molecules and proinflammatory cytokines in T2D patients [157,158], and it has been suggested that the beneficial effects of metformin on oxidative stress, mitochondrial function, endothelial function, and leukocyte-endothelium interactions may be key to preventing the vascular damage and development of an atherogenic process in T2D [159,160]. More specifically [160], demonstrated that metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission in streptozotocin (STZ)-induced diabetic ApoE-/- mice and in endothelial cells exposed to high levels of glucose. In summary, metformin is beneficial in the treatment of diabetes not only due to its hypoglycaemic capacity, but also because of its direct and indirect protective effects on leukocyte-endothelium.
2. Conclusions
Metformin has demonstrated positive results as a treatment for T2D with minimal adverse events. It provides beneficial effects as an insulin sensitizer and as an oral hypoglycaemic drug. There are numerous data generated by bench and clinical research highlighting the clinical effects of metformin on the endothelium, by which it provides protection against the development of hyperglycaemia-induced vascular disease. Metformin has been shown to exert beneficial effects in T2D by modulating oxidative stress, mitochondrial function, autophagy and endothelium-leukocyte interactions. Although the molecular mechanisms of its actions in skeletal muscle and liver and its vasoprotective effects are yet to be clarified, it seems that oxidative stress and mitochondrial function are secondary to an action that takes place at the ETC, specifically in complex I. Other actions include activation of the master bioenergetics regulator AMPK and related pathways, such as activation of eNOS and SIRT1.
Although there is abundant evidence to confirm that metformin activates AMPK, therefore acting as an insulin sensitizer while also inhibiting hepatic gluconeogenesis, there are data suggesting that it exerts important actions independent of AMPK. The wide range of concentrations of metformin used in the multiple studies carried out to date are compatible with this theory, as they would account for the discrepant findings regarding the compound's effect on the mTOR pathway and mitochondrial complex I. In this way, we still do not fully understand the molecular and cellular mechanisms which underlie the key therapeutic effects of metformin, despite its widespread use.
In conclusion, metformin has direct and indirect beneficial effects on mitochondrial function which involve actions on the vasculature in T2D. Future research in the form of in vivo and in vitro studies is needed to further explore the mechanisms responsible for the action and toxicity of this compound.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors give special thanks to Brian Normanly (University of Valencia-CIBERehd) for his editorial assistance. This work was funded by grants PI16/00090, PI19/00838, PI19/0437,PI19/01266 and CIBERehd CB06/04/0071 by Carlos III Health Institute and by the European Regional Development Fund (ERDF ‘‘A way to build Europe’‘); by PROMETEO/2019/027 by Ministry of Education of the Valencian Regional Government; by RTI2018-096748-B-100 (Spanish Ministry of Science, Innovation and Universities), by Andalusian Ministry of Economy, Innovation, Science and Employment (CTS-6264), Andalusian Ministry of Equality, Health and Social Policies (PI-0198-2016) and by an unrestricted grant from Menarini S.A. M.R and VM.V are recipients of contracts from the Ministry of Health of the Valencian Regional Government and Carlos III Health Institute (CPII16/00037 and CES10/030, respectively) while AG is supported by the Foundation “Juan Esplugues”.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.redox.2020.101517.
Contributor Information
Nadezda Apostolova, Email: nadezda.apostolova@uv.es.
Victor M. Victor, Email: victor.victor@uv.es.
Appendix A. Supplementary data
References
- 1.Folli F., Corradi D., Fanti P., Davalli A., Paez A., Giaccari A., Perego C., Muscogiuri G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011 Sep;7(5):313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 2.Iannantuoni F., Diaz-Morales N., Escribano-Lopez I., Sola E., Roldan-Torres I., Apostolova N., Bañuls C., Rovira-Llopis S., Rocha M., Victor V.M. Does glycemic control modulate the impairment of NLRP3 inflammasome activation in type 2 diabetes? Antioxidants Redox Signal. 2019;30(2):232–240. doi: 10.1089/ars.2018.7582. [DOI] [PubMed] [Google Scholar]
- 3.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen K.F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D.L., DiPietro L., Cline G.W., Shulman G.I. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rovira-Llopis S., Apostolova N., Bañuls C., Muntané J., Rocha M., Victor V.M. Mitochondria, the NLRP3 inflammasome, and sirtuins in type 2 diabetes: new therapeutic targets. Antioxidants Redox Signal. 2018;29(8):749–791. doi: 10.1089/ars.2017.7313. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.van der Bliek A.M., Shen Q., Kawajiri S. Vol. 5. pii; 2013. Mechanisms of mitochondrial fission and fusion; p. a011072. (Cold Spring Harb Perspect Biol). 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding W.X., Yin X.M. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012;393(7):547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhansali S., Bhansali A., Walia R., Saikia U.N., Dhawan V. Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and type 2 diabetes mellitus. Front. Endocrinol. 2017;8:347. doi: 10.3389/fendo.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egro F.M. Why is type 1 diabetes increasing? J. Mol. Endocrinol. 2013;51:R1–R13. doi: 10.1530/JME-13-0067. [DOI] [PubMed] [Google Scholar]
- 11.Ludvigsson J. The latest pharmacotherapy options for type 1 diabetes. Expet Opin. Pharmacother. 2014;15:37–49. doi: 10.1517/14656566.2014.855197. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D., Mayer-Davis E.J., Saydah S., Imperatore G., Linder B., Divers J., Bell R., Badaru A., Talton J.W., Crume T., Liese A.D., Merchant A.T., Lawrence J.M., Reynolds K., Dolan L., Liu L.L., Hamman R.F. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. J. Am. Med. Assoc. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G., Liu B., Sun Y., Du Y., Snetselaar L.G., Hu F.B., Bao W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. doi: 10.1136/bmj.k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arunachalam G., Samuel S.M., Marei I., Ding H., Triggle C.R. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br. J. Pharmacol. 2014;171:523–535. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mather K.J., Verma S., Anderson T.J. Improved endothelial function with metformin in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2001;37:1344–1350. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 16.Sterne J. Du nouveau dans les antidiabetiques. La NN dimethylamine guanyl guanide (N.N.D.G.) Maroc. Med. 1957;36:1295–1296. [Google Scholar]
- 17.Marshall J.C., Dunaif A. vol. 97. 2012 Jan. pp. 18–22. (All Women with PCOS Should Be Treated for Insulin Resistance Fertil Steril). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes. 2015;6(3):456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basta G., Schmidt A.M., De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Ramana K.V., Chandra D., Srivastava S., Bhatnagar A., Srivastava S.K. Nitric oxide regulates the polyol pathway of glucose metabolism in vascular smooth muscle cells. Faseb. J. 2003;17:417–425. doi: 10.1096/fj.02-0722com. [DOI] [PubMed] [Google Scholar]
- 21.Platat C., Habib H., Souka U., AlMaqbali F., Kamal H., Ibrahim W., Baynouna l., Ali H. Increased oxidative damage in the plasma of adult type 2 diabetics with poor glycemic control. Faseb. J. 2014;28 abstract 693.7. [Google Scholar]
- 22.Lodovici M., Bigagli E., Bardini G., Rotella C.M. Lipoperoxidation and antioxidant capacity in patients with poorly controlled type 2 diabetes. Toxicol. Ind. Health. 2009;25:337–341. doi: 10.1177/0748233709106464. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari B.K., Pandey K.B., Abidi A.B., Rizvi S.I. Markers of oxidative stress during diabetes mellitus. J. Biomark. 2013;8 doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Bandeira S.M., da Guedes G.S., da Fonseca L.J.S., Pires A.S., Gelain D.P., Moreira J.C.F., Rabelo A., Vasconcelos S.M.L., Goulart M.O.F. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxid. Med. Cell. Longev. 2012;13 doi: 10.1155/2012/819310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryden L., Grant P.J., Anker S.D., Berne C., Cosentino F., Danchin N. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur. Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 26.Han D.H., Hansen P.A., Host H.H., Holloszy J.O. Insulin resistance of muscle glucose transport in rats fed a highfat diet: a reevaluation. Diabetes. 1997;46(11):1761–1767. doi: 10.2337/diab.46.11.1761. [DOI] [PubMed] [Google Scholar]
- 27.Bloomgarden Z.T. Inflammation atherosclerosis and aspects of insulin action. Diabetes Care. 2005;28(9):2312–2319. doi: 10.2337/diacare.28.9.2312. [DOI] [PubMed] [Google Scholar]
- 28.Fayyaz S., Henkel J., Japtok L., Krämer S., Damm G., Seehofer D., Püschel G.P., Kleuser B. Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype. Diabetologia. 2014;57(2):373–382. doi: 10.1007/s00125-013-3123-6. [DOI] [PubMed] [Google Scholar]
- 29.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE. 2005;268:4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 30.Stratford S., Hoehn K.L., Liu F., Summers S.A. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 2004;279(2):36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 31.Zhande R., Mitchell J.J., Wu J., Sun X.J. Molecular mechanism of insulin-induced degradation of insulin receptor substrate 1. Mol. Cell Biol. 2002;22(4):1016–1026. doi: 10.1128/MCB.22.4.1016-1026.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinciguerra M., Foti M. PTEN and SHIP2 phosphoinositide phosphatases as negative regulators of insulin signaling. Arch. Physiol. Biochem. 2006;112:89–104. doi: 10.1080/13813450600711359. [DOI] [PubMed] [Google Scholar]
- 33.Cusi K., Maezono K., Osman A., Pendergrass M., Patti M.E., Pratipanawatr T., DeFronzo R.A., Kahn C.R., Mandarino L.J. Insulin resistance differentially affects the PI-3-kinase- and MAP kinase mediated signaling in human muscle. J. Clin. Invest. 2000;105(3):311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morino K., Petersen K.F., Dufour S., Befroy D., Frattini J., Shatzkes N., Neschen S., White M.F., Bilz S., Sono S., Pypaert M., Shulman G.I. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Invest. 2005;115(12):3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janjic D., Maechler P., Sekine N., Bartley C., Annen A.S., Wolheim C.B. Free radical modulation of insulin release in INS-1 cells exposed to alloxan. Biochem. Pharmacol. 1999;57:639–648. doi: 10.1016/s0006-2952(98)00346-3. [DOI] [PubMed] [Google Scholar]
- 36.Pi J., Zhang Q., Andersen M.E. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharmacol. 2010;244(1):77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leloup C., Magnan C., Benani A., Bonnet E., Alquier T., Offer G., Carriere A., Periquet A., Fernandez Y., Ktorza A., Casteilla L., Penicaud L. Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes. 2006;55:2084–2090. doi: 10.2337/db06-0086. [DOI] [PubMed] [Google Scholar]
- 38.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merry T.L., McConell G.K. Skeletal muscle glucose uptake during exercise: a focus on reactive oxygen species and nitric oxide signalling. IUBMB Life. 2009;61(5):479–484. doi: 10.1002/iub.179. [DOI] [PubMed] [Google Scholar]
- 40.Reid M.B. Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Katz A. Role of reactive oxygen species in regulation of glucose transport in skeletal muscle during exercise. J. Physiol. 2016;594(11):2787–2794. doi: 10.1113/JP271665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter E.A., Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Ver. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 43.Choi S.W., Ho C.K. Antioxidant properties of drugs used in Type 2 diabetes management: could they contribute to, confound or conceal effects of antioxidant therapy? Redox Rep. 2018;23(1):1–24. doi: 10.1080/13510002.2017.1324381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vecchione C., Gentile M.T., Aretini A., Marino G., Poulet R., Maffei A., Passarelli F., Landolfi A., Vasta A., Lemboe G. A novel mechanism of action for statins against diabetes-induced oxidative stress. Diabetologia. 2007;50(4):874–880. doi: 10.1007/s00125-007-0597-0. [DOI] [PubMed] [Google Scholar]
- 45.Kei A., Tellis C., Liberopoulos E., Tselepis A., Elisaf M. Effect of switch to the highest dose of rosuvastatin versus add-on-statin fenofibrate versus add-on-statin nicotinic acid/laropiprant on oxidative stress markers in patients with mixed dyslipidemia. Cardiovasc Ther. 2014;32(4):139–146. doi: 10.1111/1755-5922.12072. [DOI] [PubMed] [Google Scholar]
- 46.Cangemi R., Loffredo L., Carnevale R., Perri L., Patrizi M.P., Sanguigni V., Pignatelli P., Violie F. Early decrease of oxidative stress by atorvastatin in hypercholesterolaemic patients: effect on circulating vitamin E. Eur. Heart J. 2008;29(1):54–62. doi: 10.1093/eurheartj/ehm565. [DOI] [PubMed] [Google Scholar]
- 47.Jula A., Marniemi J., Huupponen R., Virtanen A., Rastas M., Rönnemaa T. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: a randomized controlled trial. J. Am. Med. Assoc. 2002;287(5):598–605. doi: 10.1001/jama.287.5.598. [DOI] [PubMed] [Google Scholar]
- 48.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Ceriello A., Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S.X., Khalyfa A., Wang Y., Carreras A., Hakim F., Neel B.A., Brady M.J., Qiao Z., Hirotsu C., Gozal D. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. 2014;38:619–624. doi: 10.1038/ijo.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J.A., Montagnani M., Koh K.K., Quon M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(5):1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez-Mijares A., Rocha M., Apostolova N., Borras C., Jover A., Bañuls C., Sola E., Victor V.M. Mitochondrial complex I impairment in leukocytes from type 2 diabetic patients. Free Radic. Biol. Med. 2011;50(10):1215–1221. doi: 10.1016/j.freeradbiomed.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Haythorne E., Rohm M., van de Bunt M., Brereton M.F., Tarasov A.I., Blacker T.S., Sachse G., Silva Dos Santos M., Terron R., Davis S., Baba O., Fischer R., Duchen M.R., Rorsman P., MacRae J.I., Ashcroft F.M. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells. Nat. Commun. 2019;10(1):2474. doi: 10.1038/s41467-019-10189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schieber M., Chandel N.S. ROS function in redox signalling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelley D.E., He J., Menshikova E.V., Ritov V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 56.Mootha V.K., Lindgren C.M., Eriksson, Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E., Houstis N., Daly M.J., Patterson N., Mesirov J.P., Golub T.R., Tamayo P., Spiegelman B., Lander E.S., Hirschhorn J.N., Altshuler D., Leif C. Groop PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 57.Pinti M.V., Fink G.K., Hathaway Q.A., Durr A.J., Kunovac A., Hollander J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am. J. Physiol. Endocrinol. Metab. 2019;316(2):E268–E285. doi: 10.1152/ajpendo.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercader J.M., Puiggros M., Segrè A.V., Planet E., Sorianello E., Sebastian D., Rodriguez-Cuenca S., Ribas V., Bonàs-Guarch S., Draghici S., Yang C., Mora S., Vidal-Puig A., Dupuis J., DIAGRAM Consortium. Florez J.C., MITIN Consortium. Zorzano A., Torrents D. Identification of novel type 2 diabetes candidate genes involved in the crosstalk between the mitochondrial and the insulin signaling systems. PLoS Genet. 2012;8(12) doi: 10.1371/journal.pgen.1003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiritoshi S., Nishikawa T., Sonoda K., Kukidome D., Senokuchi T., Matsuo T., Matsumura T., Tokunaga H., Brownlee M., Araki E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003;52(10):2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 60.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64(3):663–672. doi: 10.2337/db14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grundy S.M., Howard B., Smith S., Jr., Eckel R., Redberg R., Bonow R.O. Prevention Conference VI: diabetes and Cardiovascular Disease: executive summary: conference proceedings for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2003;105:2231–2239. doi: 10.1161/01.cir.0000013952.86046.dd. [DOI] [PubMed] [Google Scholar]
- 62.Rocha M., Apostolova N., Herance J.R., Rovira-Llopis S., Hernandez-Mijares A., Victor V.M. Perspectives and potential applications of mitochondria-targeted antioxidants in cardiometabolic diseases and type 2 diabetes. Med. Res. Rev. 2014;34(1):160–189. doi: 10.1002/med.21285. [DOI] [PubMed] [Google Scholar]
- 63.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 64.Shams N., Ianchulev T. Role of vascular endothelial growth factor in ocular angiogenesis. Ophthalmol Clin North Am. 2006;19:335–344. doi: 10.1016/j.ohc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura Y., Horii Y., Nishino T., Shiiki H., Sakaguchi Y., Kagoshima T., Dohi K., Makita Z., Vlassara H., Bucala R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am. J. Pathol. 1993;143:1649–1656. [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt A.M., Hori O., Chen J.X., Li J.F., Crandall J., Zhang J., Cao R., Yan S.D., Brett J., Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J. Clin. Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diaz-Morales N., Iannantuoni F., Escribano-Lopez I., Bañuls C., Rovira-Llopis S., Sola E., Rocha M., Hernandez-Mijares A., Victor V.M. Does metformin modulate endoplasmic reticulum stress and autophagy in type 2 diabetic peripheral blood mononuclear cells? Antioxidants Redox Signal. 2018;28(17):1562–1569. doi: 10.1089/ars.2017.7409. [DOI] [PubMed] [Google Scholar]
- 68.Escribano-Lopez I., Diaz-Morales N., Iannantuoni F., Lopez-Domenech S., de Marañon A.M., Abad-Jimenez Z., Bañuls C., Rovira-Llopis S., Herance J.R., Rocha M. Victor VM Sci Rep. 2018;8(1):15862. doi: 10.1038/s41598-018-34251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Escribano-Lopez I., Bañuls C., Diaz-Morales N., Iannantuoni F., Rovira-Llopis S., Gomis R., Rocha M., Hernandez-Mijares A., Murphy M.P., Victor V.M. The mitochondria-targeted antioxidant MitoQ modulates mitochondrial function and endoplasmic reticulum stress in pancreatic β cells exposed to hyperglycaemia. Cell. Physiol. Biochem. 2019;52(2):186–197. doi: 10.33594/000000013. [DOI] [PubMed] [Google Scholar]
- 70.Halcox J.P., Schenke W.H., Zalos G., Mincemoyer R., Prasad A., Waclawiw M.A., Nour K.R., Quyyumi A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 71.Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2010;1(3):117–128. doi: 10.1177/2042018810380215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nesti L., Natali A. Metformin effects on the heart and the cardiovascular system: a review of experimental and clinical data. Nutr. Metabol. Cardiovasc. Dis. 2017;27(8):657–669. doi: 10.1016/j.numecd.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Liu L., Wang B., Wang J., Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1(1):57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ibáñez L., Ong K., Valls C., Marcos M.V., Dunger D.B., de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J. Clin. Endocrinol. Metab. 2006;91(8):2888–2891. doi: 10.1210/jc.2006-0336. [DOI] [PubMed] [Google Scholar]
- 75.Corremans R., Vervaet B.A., D'Haese P.C., Neven E., Verhulst A. Metformin: a candidate drug for renal diseases. Int. J. Mol. Sci. 2018;20(1) doi: 10.3390/ijms20010042. pii: E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas I., Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatr. Diabetes. 2017;18(1):10–16. doi: 10.1111/pedi.12473. [DOI] [PubMed] [Google Scholar]
- 77.Yu X., Mao W., Zhai Y., Tong C., Liu M., Ma L., Yu X., Li S. Anti-tumor activity of metformin: from metabolic and epigenetic perspectives. Oncotarget. 2017;8(3):5619–5628. doi: 10.18632/oncotarget.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zi F., Zi H., Li Y., He J., Shi Q., Cai Z. Metformin and cancer: an existing drug for cancer prevention and therapy. Oncol Lett. 2018;15(1):683–690. doi: 10.3892/ol.2017.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rotermund C., Machetanz G., Fitzgerald J.C. The therapeutic potential of metformin in neurodegenerative diseases. Front. Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cabreiro F., Au C., Leung K.Y., Vergara-Irigaray N., Cochemé H.M., Noori T., Weinkove D., Schuster E., Greene N.D., Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Haes W., Frooninckx L., Van Assche R., Smolders A., Depuydt G., Billen J., Braeckman B.P., Schoofs L., Temmerman L. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc. Natl. Acad. Sci. U. S. A. 2014;111(24):E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anisimov V.N., Berstein L.M., Egormin P.A., Piskunova T.S., Popovich I.G., Zabezhinski M.A., Tyndyk M.L., Yurova M.V., Kovalenko I.G., Poroshina T.E., Semenchenko A.V. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7(17):2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 83.Anisimov V.N., Berstein L.M., Popovich I.G., Zabezhinski M.A., Egormin P.A., Piskunova T.S., Semenchenko A.V., Tyndyk M.L., Yurova M.N., Kovalenko I.G., Poroshina T.E. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (N Y) 2011;3(2):148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bailey C.J. Metformin: historical overview. Diabetologia. 2017;60(9):1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 85.Howlett H.C.S., Bailey C.J. Galegine and antidiabetic plants. In: Bailey C.J., Campbell I.W., Chan J.C.N., Davidson J.A., Howlett H.C.S., Ritz P., editors. Metformin—the Gold Standard. Wiley; Chichester: 2007. pp. 3–9. [Google Scholar]
- 86.Marshall S.M. 60 years of metformin use: a glance at the past and a look to the future. Diabetologia. 2017;60(9):1561–1565. doi: 10.1007/s00125-017-4343-y. [DOI] [PubMed] [Google Scholar]
- 87.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metabol. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 88.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viollet B., Guigas B., Sanz Garcia N., Leclerc J., Foretz M., Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin. Sci. (Lond.) 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Argaud D., Roth H., Wiernsperger N., Leverve X.M. Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur. J. Biochem. 1993;213(3):1341–1348. doi: 10.1111/j.1432-1033.1993.tb17886.x. [DOI] [PubMed] [Google Scholar]
- 91.Mithieux G., Guignot L., Bordet J.C., Wiernsperger N. Intrahepatic mechanisms underlying the effect of metformin in decreasing basal glucose production in rats fed a high-fat diet. Diabetes. 2002;51(1):139–143. doi: 10.2337/diabetes.51.1.139. [DOI] [PubMed] [Google Scholar]
- 92.Radziuk J., Zhang Z., Wiernsperger N., Pye S. Effects of metformin on lactate uptake and gluconeogenesis in the perfused rat liver. Diabetes. 1997;46(9):1406–1413. doi: 10.2337/diab.46.9.1406. [DOI] [PubMed] [Google Scholar]
- 93.Miller R.A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shu Y., Brown C., Castro R.A., Shi R.J., Lin E.T., Owen R.P., Sheardown S.A., Yue L., Burchard E.G., Brett C.M., Giacomini K.M. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin. Pharmacol. Ther. 2008;83(2):273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gormsen L.C., Sundelin E.I., Jensen J.B., Vendelbo M.H., Jakobsen S., Munk O.L., Hougaard Christensen M.M., Brøsen K., Frøkiær J., Jessen N. Vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution, and kinetic analyses. J. Nucl. Med. 2016;57(12):1920–1926. doi: 10.2967/jnumed.116.177774. [DOI] [PubMed] [Google Scholar]
- 96.Jensen J.B., Sundelin E.I., Jakobsen S., Gormsen L.C., Munk O.L., Frøkiær J., Jessen N. [11C]-Labeled metformin distribution in the liver and small intestine using dynamic positron emission tomography in mice demonstrates tissue-specific transporter dependency. Diabetes. 2016;65(6):1724–1730. doi: 10.2337/db16-0032. [DOI] [PubMed] [Google Scholar]
- 97.Scheen A.J. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 1996;30(5):359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 98.Panchapakesan U., Pollock C. Drug repurposing in kidney disease. Kidney Int. 2018;94(1):40–48. doi: 10.1016/j.kint.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 99.Bahne E., Hansen M., Brønden A., Sonne D.P., Vilsbøll T., Knop F.K. Involvement of glucagon-like peptide-1 in the glucose-lowering effect of metformin. Diabetes Obes. Metabol. 2016;18(10):955–961. doi: 10.1111/dom.12697. [DOI] [PubMed] [Google Scholar]
- 100.Duca F.A., Côté C.D., Rasmussen B.A., Zadeh-Tahmasebi M., Rutter G.A., Filippi B.M., Lam T.K. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015;21(5):506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Mannerås-Holm L., Ståhlman M., Olsson L.M., Serino M., Planas-Fèlix M., Xifra G., Mercader J.M., Torrents D., Burcelin R., Ricart W., Perkins R., Fernàndez-Real J.M., Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 102.Pryor R., Norvaisas P., Marinos G., Best L., Thingholm L.B., Quintaneiro L.M., De Haes W.5, Esser D., Waschina S., Lujan C., Smith R.L., Scott T.A., Martinez-Martinez D., Woodward O., Bryson K., Laudes M., Lieb W., Houtkooper R.H., Franke A., Temmerman L., Bjedov I., Cochemé H.M., Kaleta C., Cabreiro F. Host-microbe-drug-nutrient screen identifies bacterial effectors of metformin therapy. Cell. 2019;178(6):1299–1312. doi: 10.1016/j.cell.2019.08.003. e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song Y.M., Lee Y.H., Kim J.W., Ham D.S., Kang E.S., Cha B.S., Lee H.C., Lee B.W. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11(1):46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie Z., Lau K., Eby B., Lozano P., He C., Pennington B., Li H., Rathi S., Dong Y., Tian R., Kem D., Zou M.H. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60(6):1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jadhav K.S., Dungan C.M., Williamson D.L. Metformin limits ceramide-induced senescence in C2C12 myoblasts. Mech. Ageing Dev. 2013;134(11–12):548–559. doi: 10.1016/j.mad.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Moiseeva O., Deschênes-Simard X., St-Germain E., Igelmann S., Huot G., Cadar A.E., Bourdeau V., Pollak M.N., Ferbeyre G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12(3):489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- 107.Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr. Metab. Immune Disord. - Drug Targets. 2015;15(3):196–205. doi: 10.2174/1871530315666150316124019. [DOI] [PubMed] [Google Scholar]
- 108.Vasamsetti S.B., Karnewar S., Kanugula A.K., Thatipalli A.R., Kumar J.M., Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64(6):2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 109.Cameron A.R., Morrison V.L., Levin D., Mohan M., Forteath C., Beall C., McNeilly A.D., Balfour D.J., Savinko T., Wong A.K., Viollet B., Sakamoto K., Fagerholm S.C., Foretz M., Lang C.C., Rena G. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016;119(5):652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao J., Meng S., Chang E., Beckwith-Fickas K., Xiong L., Cole R.N., Radovick S., Wondisford F.E., He L. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK) J. Biol. Chem. 2014;289(30):20435–20446. doi: 10.1074/jbc.M114.567271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M.F., Goodyear L.J., Moller D.E. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shaw R.J., Lamia K.A., Vasquez D., Koo S.H., Bardeesy N., Depinho R.A., Montminy M., Cantley L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M.A., Radovick S., Wondisford F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137(4):635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Caton P.W., Nayuni N.K., Kieswich J., Khan N.Q., Yaqoob M.M., Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J. Endocrinol. 2010;205(1):97–106. doi: 10.1677/JOE-09-0345. [DOI] [PubMed] [Google Scholar]
- 116.Kim Y.D., Park K.G., Lee Y.S., Park Y.Y., Kim D.K., Nedumaran B., Jang W.G., Cho W.J., Ha J., Lee I.K., Lee C.H., Choi H.S. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 2008;57(2):306–314. doi: 10.2337/db07-0381. [DOI] [PubMed] [Google Scholar]
- 117.Kalender A., Selvaraj A., Kim S.Y., Gulati P., Brûlé S., Viollet B., Kemp B.E., Bardeesy N., Dennis P., Schlager J.J., Marette A., Kozma S.C., Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metabol. 2010;11(5):390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim J., Lee H.Y., Ahn J., Hyun M., Lee I., Min K.J., You Y.J. NHX-5, an endosomal Na+/H+ exchanger, is associated with metformin action. J. Biol. Chem. 2016;291(35):18591–18599. doi: 10.1074/jbc.C116.744037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El-Mir M.Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 120.Fontaine E. Metformin-induced mitochondrial complex I inhibition: facts, uncertainties, and consequences. Front. Endocrinol. 2018;9:753. doi: 10.3389/fendo.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bridges H.R., Jones A.J., Pollak M.N., Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014;462(3):475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murai M., Murakami S., Ito T., Miyoshi H. Amilorides bind to the quinone binding pocket of bovine mitochondrial complex I. Biochemistry. 2015;54(17):2739–2746. doi: 10.1021/acs.biochem.5b00187. [DOI] [PubMed] [Google Scholar]
- 123.Madiraju A.K., Erion D.M., Rahimi Y., Zhang X.M., Braddock D.T., Albright R.A., Prigaro B.J., Wood J.L., Bhanot S., MacDonald M.J., Jurczak M.J., Camporez J.P., Lee H.Y., Cline G.W., Samuel V.T., Kibbey R.G., Shulman G.I. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gong L., Goswami S., Giacomini K.M., Altman R.B., Klein T.E. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenetics Genom. 2012;22(11):820–827. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Christensen M.M., Brasch-Andersen C., Green H., Nielsen F., Damkier P., Beck-Nielsen H., Brosen K. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenetics Genom. 2011;21(12):837–850. doi: 10.1097/FPC.0b013e32834c0010. [DOI] [PubMed] [Google Scholar]
- 126.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 127.Wheaton W.W., Weinberg S.E., Hamanaka R.B., Soberanes S., Sullivan L.B., Anso E., Glasauer A., Dufour E., Mutlu G.M., Budigner G.S., Chandel N.S. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3 doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Higgins J.W., Bedwell D.W., Zamek-Gliszczynski M.J. Ablation of both organic cation transporter (OCT)1 and OCT2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab. Dispos. 2012;40(6):1170–1177. doi: 10.1124/dmd.112.044875. [DOI] [PubMed] [Google Scholar]
- 129.Pentikäinen P.J., Neuvonen P.J., Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur. J. Clin. Pharmacol. 1979;16(3):195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- 130.Carvalho C., Correia S., Santos M.S., Seiça R., Oliveira C.R., Moreira P.I. Metformin promotes isolated rat liver mitochondria impairment. Mol. Cell. Biochem. 2008;308(1–2):75–83. doi: 10.1007/s11010-007-9614-3. [DOI] [PubMed] [Google Scholar]
- 131.Logie L., Harthill J., Patel K., Bacon S., Hamilton D.L., Macrae K., McDougall G., Wang H.H., Xue L., Jiang H., Sakamoto K., Prescott A.R., Rena G. Cellular responses to the metal-binding properties of metformin. Diabetes. 2012;61(6):1423–1433. doi: 10.2337/db11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baur J.A., Birnbaum M.J. Control of gluconeogenesis by metformin: does redox trump energy charge? Cell Metabol. 2014;20(2):197–199. doi: 10.1016/j.cmet.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brown L.J., Koza R.A., Everett C., Reitman M.L., Marshall L., Fahien L.A., Kozak L.P., MacDonald M.J. Normal thyroid thermogenesis but reduced viability and adiposity in mice lacking the mitochondrial glycerol phosphate dehydrogenase. J. Biol. Chem. 2002;277(36):32892–32898. doi: 10.1074/jbc.M202408200. [DOI] [PubMed] [Google Scholar]
- 134.Dehkordi A.H., Abbaszadeh A., Mir S., Hasanvand A. Metformin and its anti-inflammatory and anti-oxidative effects; new concepts. Renal Inj Prev. 2019;8(1):54. [Google Scholar]
- 135.Ismail Hassan F., Didari T., Khan F., Niaz K., Mojtahedzadeh M., Abdollahi M. A review on the protective effects of metformin in sepsis-induced organ failure. Cell J. 2020;21(4):363–370. doi: 10.22074/cellj.2020.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paudel Y.N., Angelopoulou E., Piperi C., Shaikh M.F., Othman I. Emerging neuroprotective effect of metformin in Parkinson's disease: a molecular crosstalk. Pharmacol. Res. 2020;152:104593. doi: 10.1016/j.phrs.2019.104593. [DOI] [PubMed] [Google Scholar]
- 137.Javadipour M., Rezaei M., Keshtzar E., Khodayar M.J. Metformin in contrast to berberine reversed arsenic-induced oxidative stress in mitochondria from rat pancreas probably via Sirt3-dependent pathway. J. Biochem. Mol. Toxicol. 2019;33(9) doi: 10.1002/jbt.22368. [DOI] [PubMed] [Google Scholar]
- 138.Roxo D.F., Arcaro C.A., Gutierres V.O., Costa M.C., Oliveira J.O., Lima T.F.O., Assis R.P., Brunetti I.L., Baviera A.M. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetol. Metab. Syndrome. 2019;11:33. doi: 10.1186/s13098-019-0431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mirmiranpour H., Mousavizadeh M., Noshad S., Ghavami M., Ebadi M., Ghasemiesfe M., Nakhjavani M., Esteghamati A. Comparative effects of pioglitazone and metformin on oxidative stress markers in newly diagnosed type 2 diabetes patients: a randomized clinical trial. J. Diabet. Complicat. 2013;27:501–507. doi: 10.1016/j.jdiacomp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 140.Hou X., Song J., Li X.N., Zhang L., Wang X., Chen L., Shen Y.H. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem. Biophys. Res. Commun. 2010;396(2):199–205. doi: 10.1016/j.bbrc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 141.Tripathi S.S., Singh A.K., Akhtar F., Chaudhary A., Rizvi S.I. Metformin protects red blood cells against rotenone induced oxidative stress and cytotoxicity. Arch. Physiol. Biochem. 2019:1–10. doi: 10.1080/13813455.2019.1620288. [DOI] [PubMed] [Google Scholar]
- 142.An H., Wei R., Ke J., Yang J., Liu Y., Wang X., Wang G., Hong T. Metformin attenuates fluctuating glucose-induced endothelial dysfunction through enhancing GTPCH1-mediated eNOS recoupling and inhibiting NADPH oxidase. J. Diabet. Complicat. 2016;30(6):1017–1024. doi: 10.1016/j.jdiacomp.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 143.Bułdak Ł., Łabuzek K., Bułdak R.J., Machnik G., Bołdys A., Basiak M., Bogusław O. Metformin reduces the expression of NADPH oxidase and increases the expression of antioxidative enzymes in human monocytes/macrophages cultured in vitro. Exp Ther Med. 2017;13(2):794. doi: 10.3892/etm.2016.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng G., Lanza-Jacoby S. Metformin decreases growth of pancreatic cancer cells by decreasing reactive oxygen species: role of NOX4. Biochem. Biophys. Res. Commun. 2015;465(1):41–46. doi: 10.1016/j.bbrc.2015.07.118. [DOI] [PubMed] [Google Scholar]
- 145.Sato N., Takasaka N., Yoshida M., Tsubouchi K., Minagawa S., Araya J., Saito N., Fujita Y., Kurita Y., Kobayashi K., Ito S., Hara H., Kadota T., Yanagisawa H., Hashimoto M., Utsumi H., Wakui H., Kojima J.1, Numata T., Kaneko Y., Odaka M., Morikawa T., Nakayama K., Kohrogi H., Kuwano K. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir. Res. 2016 Aug 30;17(1):107. doi: 10.1186/s12931-016-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khouri H., Collin F., Bonnefont-Rousselot D., Legrand A., Jore D., Gardès-Albert M. Radical-induced oxidation of metformin. Eur. J. Biochem. 2004;271(23–24):4745–4752. doi: 10.1111/j.1432-1033.2004.04438.x. [DOI] [PubMed] [Google Scholar]
- 147.Galkina E., Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27(11):2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 148.D1 Pavlović, Kocić R., Kocić G., Jevtović T., Radenković S., Mikić D., Stojanović M., Djordjević P.B. Effect of four-week metformin treatment on plasma and erythrocyte antioxidative defense enzymes in newly diagnosed obese patients with type 2 diabetes. Diabetes Obes. Metabol. 2000;2(4):251–256. doi: 10.1046/j.1463-1326.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 149.Chait A., Bornfeldt K.E. Diabetes and atherosclerosis: is there a role for hyperglycemia? J. Lipid Res. 2009;50(Suppl):S335–S339. doi: 10.1194/jlr.R800059-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Diaz-Morales N., Rovira-Llopis S., Bañuls C., Escribano-Lopez I., de Marañon A.M., Lopez-Domenech S., Orden S., Roldan-Torres I., Alvarez A., Veses S., Jover A., Rocha M., Hernandez-Mijares A., Victor V.M. Are mitochondrial fusion and fission impaired in leukocytes of type 2 diabetic patients? Antioxidants Redox Signal. 2016;25(2):108–115. doi: 10.1089/ars.2016.6707. [DOI] [PubMed] [Google Scholar]
- 151.Diaz-Morales N., Rovira-Llopis S., Bañuls C., Lopez-Domenech S., Escribano-Lopez I., Veses S., Jover A., Rocha M., Hernandez-Mijares A., Victor V.M. Does metformin protect diabetic patients from oxidative stress and leukocyte-endothelium interactions? Antioxidants Redox Signal. 2017;27(17):1439–1445. doi: 10.1089/ars.2017.7122. [DOI] [PubMed] [Google Scholar]
- 152.Goldberg R., Temprosa M., Otvos J., Brunzell J., Marcovina S., Mather K., Arakaki R., Watson K., Horton E., Barrett-Connor E. Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the Diabetes Prevention Program. J. Clin. Endocrinol. Metab. 2013;98(10):3989–3998. doi: 10.1210/jc.2013-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ouslimani N., Peynet J., Bonnefont-Rousselot D., Thérond P., Legrand A., Beaudeux J.L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54(6):829–834. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 154.Lai Y.C., Tabima D.M., Dube J.J., Hughan K.S., Vanderpool R.R., Goncharov D.A., St Croix C.M., Garcia-Ocaña A., Goncharova E.A., Tofovic S.P., Mora A.L., Gladwin M.T. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133(8):717–731. doi: 10.1161/CIRCULATIONAHA.115.018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ghosh S., Lakshmanan A.P., Hwang M.J., Kubba H., Mushannen A., Triggle C.R., Ding H. Metformin improves endothelial function in aortic tissue and microvascular endothelial cells subjected to diabetic hyperglycaemic conditions. Biochem. Pharmacol. 2015;98(3):412–421. doi: 10.1016/j.bcp.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 156.Shang F., Zhang J., Li Z., Zhang J., Yin Y., Wang Y., Marin T.L., Gongol B., Xiao H., Zhang Y.Y., Chen Z., Shyy J.Y., Lei T. Cardiovascular protective effect of metformin and telmisartan: reduction of PARP1 activity via the AMPK-PARP1 cascade. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hernandez-Mijares A., Rocha M., Rovira-Llopis S., Bañuls C., Bellod L., de Pablo C., Alvarez A., Roldan-Torres I., Sola-Izquierdo E., Victor V.M. Human leukocyte/endothelial cell interactions and mitochondrial dysfunction in type 2 diabetic patients and their association with silent myocardial ischemia. Diabetes Care. 2013;36(6):1695–1702. doi: 10.2337/dc12-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rovira-Llopis S., Rocha M., Falcon R., de Pablo C., Alvarez A., Jover A., Hernandez-Mijares A., Victor V.M. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxidants Redox Signal. 2013;19(13):1452–1458. doi: 10.1089/ars.2013.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Forouzandeh F., Salazar G., Patrushev N., Xiong S., Hilenski L., Fei B., Alexander R.W. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. J Am Heart Assoc. 2014;3(6) doi: 10.1161/JAHA.114.001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Q., Zhang M., Torres G., Wu S., Ouyang C., Xie Z., Zou M.H. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of drp1-mediated mitochondrial fission. Diabetes. 2017;66(1):193–205. doi: 10.2337/db16-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.