Abstract
The most common childhood malignancy is acute leukemia. Approximately 15- 20% of it, is Acute myeloid leukemia (AML). The general symptoms of this malignancy include fatigue, weakness, fever, paleness and bleeding disorders. There are two methods of classifying for AML: The French-American-British (FAB) and the World Health Organization (WHO) classification.The M4 eos subtype, also called myelomonocytic leukemia, is one subtype of AML with eosinophilia. The most common cytogenetic variations in this leukemia include inv (16) (p13q22) or the variant t (16; 16) (p13; q22). In this report, we present the first AML-M4 eos case with a new translocation that has not yet been reported.
Keywords: AML M4, Eosinophilia; Acute myelomonocytic leukemia; ETV6-JAK2; Chromosomal translocation
1. Introduction
Acute myeloid leukemia (AML) is a malignancy of the hematopoietic system in which the myeloid category is involved, it is diagnosed and divided based on the appearance of the cells and chromosomal abnormalities[1]. Classifying AML into its subtypes according to karyotype, before treatment, is a widely accepted approach. Cytogenetics is the most important prognostic factor in AML[2]. Each of the AML subtypes is associated with specific cytogenetic and chromosomal changes according to French-American-British (FAB) criteria[3]. A group of AML that contains inv (16) (p13q22) or t (16;16) (p13; q22), is associated with the subtype M4Eo[4]. AML M4 with inv (16) is commonly associated with eosinophilia; the abnormal eosinophils are part of the leukemic clone, as shown by fluorescence in situ hybridization (FISH)[5]. Translocations with breakpoints at chromosome band 12p13 frequently result in rearrangements of the Ets variant gene 6 (ETV6). It has been shown that ETV6 fused to several different partners due to different leukemia-related translocations[6]. In this report, we present the determination of janus kinase 2 (JAK2) as a fusion partner of ETV6 in t(9;12)(p24;p13) found in the leukemic cells of a child with AML M4eos.
2. Case presentation
A 20-month old girl was delivered by cesarean section at 37 weeks of gestation, in 2014. She was examined for a history of FUO, anorexia, weight loss and ill appearance. She had hepatosplenomegaly on examination. The CBC showed anemia and eosinophilia.
[WBC: 16.7 × 103/μL (seg:24%, L:18%, M:10%, EO: 22%, Blast: 26%), Hb:8.8 g/dl, Plt:165 × 103/μL]
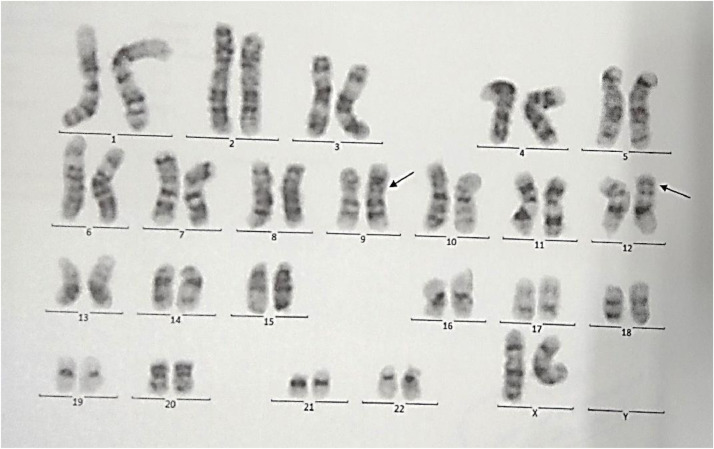
She was diagnosed with AML M4 eos, by bone marrow aspiration and immunophenotyping. The G-banded karyotype was 46 XX, t (9;12) (p24; p13) [20] (Fig 1). The cerebrospinal fluid was normal. She was treated for AML M4 eos with chemotherapy. She responded well to standard AML chemotherapy and is currently in remission. The flowcytometry and cytogenetic evidence of the patient are as follows (Fig 1 and Table 1):
Fig. 1.
Cytogenetic analysis.
Table 1.
. Flowcytometry.
| Test | Results | Reference value |
|---|---|---|
| CD8 | 8% | 19–37 |
| CD5 | 17% | 65–79 |
| CD4 | 6% | 35–55 |
| CD3 | 26% | 68–82 |
| CD7 | 27% | 75 |
| CD10 | 9% | <2 |
| CD13 | 17% | – |
| CD14 | 32% | – |
| CD15 | 43% | – |
| CD19 | 12% | 5–15 |
| CD20 | 8% | 5–15 |
| CD22 | 10% | – |
| CD33 | 64% | – |
| CD34 | 36% | – |
| CD45 | 82% | – |
| HLA-DR./PE | 30% | – |
Four slides were scanned using METAFER automated metaphase finder. Twenty random metaphase spreads were studied on the basis of GTG technique at 350–400 band resolution, revealing 46 chromosomes with translocation of chromosomes 9 and 12 with exchange of segments distal to 9p24 and 12p13 in all spreads.
Conclusion: 46,XX,t(9;12)(p24;p13) [20].
Compatible with translocation of chromosomes 9 and 12.
3. Discussion
Although acute myeloid leukemia is more common in adulthood, it is the second most common childhood leukemia, and it has a high mortality and morbidity. Acute myelomonocytic leukemia is the M4 subtype of AML. The cytogenetic study is the most important diagnostic and prognostic tool. Chromosomal alterations have been observed in a wide range of cancers. This information is not only used for diagnosis, but also leads to the development of molecular tests to predict cancer prognosis and develop therapeutic approaches. So far, we do not yet have enough information about the completely possible genetic changes associated with this disease. By cytogenetic studies, most of the chromosomal abnormalities are detectable.
The common chromosomal abnormalities in the AMLM4 include monosomy 5 or del(5q), monosomy 7 or del (7q), trisomy 8, t(6;9) (p23;q34), and rearrangements involving the MLL gene mapped at 11q23 [del(11)(q23); t(9;11)(p22;q23), t(11;19)(q23;p13)], and Core Binding Factor B (CBFβ) mapped at 16q22 [del(16)(q22), inv(16) (p13q22), t(16;16)(p13;q22)][3]. The common chromosomal abnormalities in AMLM4 eos include del(16)(q22), inv(16)(p13;q22), t(10;16)(p13;q22). Patients with inv(16) and t(16;16) have a favorable prognosis and good response to intensive chemotherapy[7].
Prognostic factors can be divided into two categories[8,9]:
-
1)
Patient-related factors, prognosis worsens with age.
-
2)
AML-related factors include white blood count (WBC), the existence of prior MDS, previous cytotoxic therapy for another disorder and cytogenetic, molecular genetic changes in the leukemic cells at diagnosis and MRD (Table 2).
Table 2.
. Favorable and unfavorable –risk molecular studies cytogenetics[9].
| Favorable-risk molecular studies: CEBP a NPM1 GATA1s |
Unfavorable-risk molecular studies: FLT3/ITD C-KIT |
| Favorable-risk cytogenetics: RUNX1-ETO T(8;21)(q22;q22) Inv(16) Inv(16)(p13.1q22),t(16;16)(13.1;q22) Del(16)(q22) PML-RARA T(15;17)(q22;q12) MLL T(1;11)(q21;q23) |
Unfavorable-risk cytogenetics: Preexisting MDS, particularly affecting chromosomes 5 and 7 Monosomy 5 Monosomy 7 Del(5q) Del(7q) MLL T(6;11)(q27;q23), t(10;11)(p12;q23) MISC T(7;12)(q36;p13), t(6;9)(p23;q34), t(5;11)(q35;p15.5) |
Among the cytogenetic abnormalities seen in AML, translocation (9;12) (p24; p13) is a rare gene and poorly known. In this translocation JAK2 gene (on chromosome 9p24) encoding for another protein tyrosine kinase resulting in, fusions to the ETS-family member ETV6 (also known as TEL)[10]. The Janus kinase 2 gene (JAK2) encodes a receptor tyrosine kinase that signals through the JAK-STAT pathway. JAK2 mutations are a common molecular mechanism underlying polycythemia vera and essential thrombocythaemia and primary myelofibrosis[11]. Some cases of myeloid and lymphoid neoplasms with the rearrangement of JAK2 have been reported. A study about this was done in 2014 by Bain et al. [11], according to this study, the t(9;12)(p24;p13) and its variants leading to ETV6-JAK2 has been reported in a small number of cases of ALL. In this study, 8 patients with myeloid and lymphoid neoplasms associated with t(9; 12)(p24;p13) were summarized. Two of them were children and both were male. Few cases have been described in detail, but bone marrow eosinophilia has been reported in only one patient.
Lacronique et al., had a study about childhood T-cell ALL and they detected a t (9;12) (p24; p13) from blasts of a 4-year-old male in relapse leading to a fusion of ETV6 exon 5 to JAK2 exon 19 containing the JH1 tyrosine kinase domain[12]. The survival of this patient was not available.
In a study by Peeters et al., t (9;12) (p24; p13) was reported which seen in a patient with early pre-B cell acute lymphoblastic leukemia. The patient was a 19-month-old infant with a diagnosis of pre-B cell ALL that is undergoing intensive chemotherapy regimen according to the European organisation for Radiotherapy and Chemotherapy (EORTC) protocol 58,881. The patient achieved complete remission (CR) at the end of induction therapy but after 7 months of consolidation courses, the patient developed bone marrow and testicular relapse. Salvage therapy was started for him and CR was obtained. Also, they identified a t (9;15;12) (p24; q15; p13) in a patient with atypical CML in an alteration that fused exon 5 of ETV6 to exon 12 of JAK2 (ETV6-JAK2, 5–12)[6].
In 2018 a multicenter study was done by Tang et al., on hematopoietic neoplasms with 9p24/JAK2 rearrangement. From 7 large medical centers, they identified 10 patients with t(8;9)(p22;p24) /PCM1-JAK2 and 3 with t(9p24;v)/JAK2 at diagnosis. Most of the cases showed myeloproliferative neoplasm. They show that hematopoietic neoplasms with 9p24/JAK2 rearrangement are extremely rare, and a large proportion of the cases are t(8;9)(p22;p24.1)/PCM1-JAK2. They did not obtain accurate information about patients with t(9;12)(p24.1;p13)/ETV6-JAK2 due to their rarity and the small number of cases[13].
In summary, t(9;12) (p24; p13) is a rare mutation that has been observed in some hematologic malignancies. It is less common in leukemic malignancies. So far, two cases of child patients with this translocation have been reported, one being a 19-month-old infant and the other a four-year-old child, both were male and with acute lymphoblastic leukemia.
According to our research, no additional cases of childhood leukemia have been reported with this mutation. We present the first female, AML M4 eos patient with translocation (9;12) (p24; p13).
Because of the rarity of this mutation, little is known about the prognosis and survival of these patients. To better understand the molecular and chromosomal events in leukemia, it is helpful to produce them in vivo models. Sufficient models of ETV6-JAK2 are needed for studying JAK2 oncogenic signaling at the molecular level. Further research will be helpful for future testing of molecule inhibitors and finding new therapies. We need to advance our knowledge and use most of the available molecular biology methods to understand the complex events that lead to leukemia.
Declaration of Competing Interests
None
References
- 1.Ubnitz J.E. Childhood acute myeloid leukemia. Curr. Treat. Options Oncol. 2008;9:95–105. doi: 10.1007/s11864-008-0059-z. [DOI] [PubMed] [Google Scholar]
- 2.Yrd J.C., Mrozek K., Dodge R.K. Cancer and Leukemia Group B (CALGB8461): pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 3.Al-Achkar W., Aljapawe A., Othman M.A., Wafa A. A de novo acute myeloid leukemia (AML-M4) case with a complex karyotype and yet unreported breakpoints. Mol. Cytogenet. 2013;6(1):18. doi: 10.1186/1755-8166-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson R.A., Williams S.F., Le Beau M.M. Acute myelomonocytic leukemia with abnormal eosinophils and inv (16) or t (16;16) has a favorable prognosis. Blood. 1986;68:1242–1249. [PubMed] [Google Scholar]
- 5.Haferlach T., Winkemann M., Loffler H. The abnormal eosinophils are part of the leukemic cell population in acute myelomonocytic leukemia with abnormal eosinophils (AML M4Eo) and carry the pericentric inversion 16: a combination of May-Grünwald-Giemsa staining and fluorescence in situ hybridization. Blood. 1996;87:2459–2463. [PubMed] [Google Scholar]
- 6.Peeters P., Raynaud S.D., Cools J. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t (9;12) in a lymphoid and t (9;15;12) in a myeloid leukemia. Blood. 1997;90(7):2535–2540. Oct 1. [PubMed] [Google Scholar]
- 7.Chen Z., Sandberg A.A. Molecular cytogenetic aspects of hematological malignacies: clinical implications. Am. J. Med. Genet. 2002;115:130–141. doi: 10.1002/ajmg.10689. [DOI] [PubMed] [Google Scholar]
- 8.Döhner H., Estey E.H., Amadori S. European LeukemiaNet: diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 9.Lanzkowsky P.H., Lipton JM,Fish J.D. Lanzkowsky's Manual of Pediatric Hematology and Oncology. 6th edition. Academic Press is an imprint of Elsevier; London: 2016. . [Google Scholar]
- 10.Schwaller J. Modeling ETV6-JAK2-induced leukemia: insights from the zebrafish. Haematologica. 2012;97(12):1783–1785. doi: 10.3324/haematol.2012.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bain B.J., Ahmad S. Should myeloid and lymphoid neoplasms with PCM1-JAK2 and other rearrangements of JAK2 be recognized as specific entities? Br. J. Haematol. 2014;166(6):809–817. doi: 10.1111/bjh.12963. [DOI] [PubMed] [Google Scholar]
- 12.Lacronique V., Boureux A., Valle V.D. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278(5341):1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 13.Tang G., Sydney Sir Philip J.K. Hematopoietic neoplasms with 9p24/JAK2 rearrangement: a multicenter study. Mod. Pathol. 2019;32(4):490–498. doi: 10.1038/s41379-018-0165-9. [DOI] [PubMed] [Google Scholar]