Abstract
Radiation therapy (RT) is beneficial in Head and Neck Cancer (HNC) in both the definitive and adjuvant setting. Highly complex and conformal planning techniques are becoming standard practice in delivering increased doses in HNC. A sharp falloff in dose outside the high dose area is characteristic of highly complex techniques and geometric uncertainties must be minimised to prevent under dosage of the target volume and possible over dosage of surrounding critical structures. CTV-PTV margins are employed to account for geometric uncertainties such as set up errors and both interfraction and intrafraction motion. Robust immobilisation and Image Guided Radiation Therapy (IGRT) is also essential in this group of patients to minimise discrepancies in patient position during the treatment course. IGRT has evolved with increased 2-Dimensional (2D) and 3-Dimensional (3D) IGRT modalities available for geometric verification. 2D and 3D IGRT modalities are both beneficial in geometric verification while 3D imaging is a valuable tool in assessing volumetric changes that may have dosimetric consequences for this group of patients. IGRT if executed effectively and efficiently provides clinicians with confidence to reduce CTV-PTV margins thus limiting treatment related toxicities in patients. Accumulated exposure dose from IGRT vary considerably and may be incorporated into the treatment plan to avoid excess dose. However, there are considerable variations in the application of IGRT in RT practice. This paper aims to summarise the advances in IGRT in HNC treatment and provide clinics with recommendations for an IGRT strategy for HNC in the clinic.
Keywords: Image guided radiation therapy, Head and neck, Geometric verification, RTT, Best practice, Review
Introduction
The global prevalence of head and neck cancer (HNC) is 550,000 cases per year [1]. 90% of HNC are squamous cell carcinomas arising in the epithelial lining of the oral cavity, nasopharynx oropharynx, larynx and hypopharynx [2] Treatment approaches vary depending on tumour stage, location and patient characteristics with concurrent chemo-radiation an established standard of care in both early stage and locally advanced disease [3], [4]. Prognosis is favourable for early stage disease while survival rates for locally advanced disease have been rising steadily over the past decade [5].
A cumulative radiation therapy (RT) dose of 70 Gray (Gy) is frequently delivered with curative intent in 1.8–2.0 Gy daily fractions. Altered fractionation schedules such as hyper-fractionation, accelerated fractionation, hypo-fractionation and a combination of these are becoming common with the role of radiobiology in local control an important consideration for this group of patients [6].
In Europe 97% of patients are treated using highly complex and conformal planning techniques such as 3-Dimensional Conformal RT (3DCRT) and Intensity Modulated RT (IMRT) [7]. Unlike 3DCRT and conventional RT, a rapid fall off in dose is characteristic of IMRT planning – permitting delivery of a highly conformal dose to the Planning Target Volume (PTV) and improved sparing of surrounding Organs at Risk (OARs) [8], [9]. Robust immobilisation and Image Guided Radiation Therapy (IGRT) are essential to minimise geometric uncertainties and the risk of undertreating the target volume (TV) and over-treating adjacent OARs [10], [11], [12], [13].
The application of IGRT strategies in RT practice has grown exponentially in the past decade but internationally large variation has been reported in the availability and utilisation of IGRT in clinical practice [14], [15], [16]. Furthermore, IGRT modalities are both underused due to concerns regarding accumulated imaging dose [17] and potential time consuming nature of its execution [18].
This scoping review summarises the evidence for IGRT in HNC and provides recommendations for the implementation of an IGRT strategy for HNC cancer in the clinic.
Materials and methods
Study identification
EMBASE, MEDLINE, Web of Science and Cochrane electronic databases were searched for publications using the following terms in the title or abstract: (IGRT OR “image guided radiation therapy” OR “image guided radiotherapy”) AND (“Head and neck” OR Ear OR face OR head OR jaw OR lip OR mouth OR neck OR eye OR nose OR nasal OR pharynx OR tongue OR salivary OR sinus OR nasopharynx*) NEAR (cancer* OR tumo?r* OR carcinoma* OR neoplasm*)to retrieve all HNC studies reporting IGRT information published between 2009 and 2019. After duplicates were removed 727 papers were identified and screened for eligibility.
Study eligibility criteria
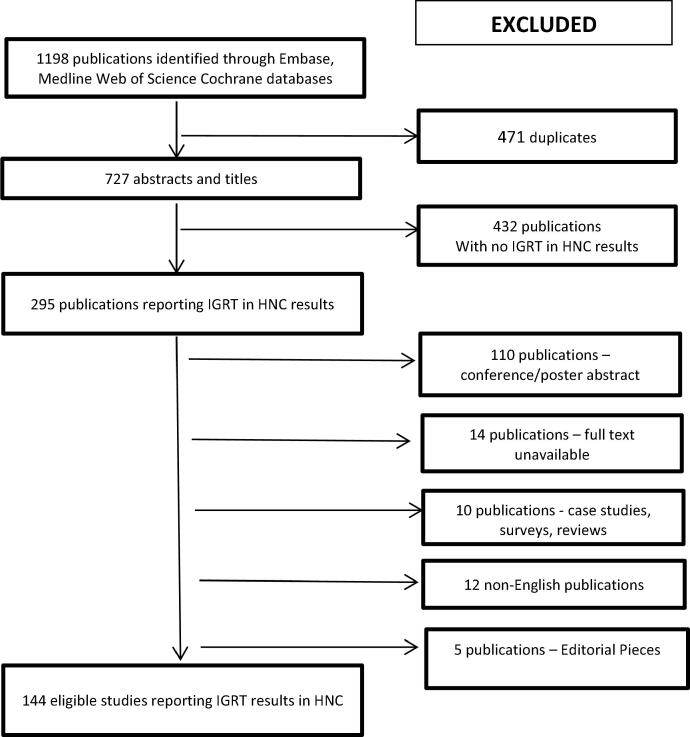
Eligible studies were identified using the Preferred Reporting Items for Systematic Reviews guidelines (Fig. 1) [PRISMA 2015]. Studies not reporting IGRT data for HNC were excluded. Reviews, case studies, non-human studies and surveys were also excluded. All studies reporting any data relating to IGRT in HNC were included. Following screening for eligibility there were 144 studies reporting IGRT data for HNC.
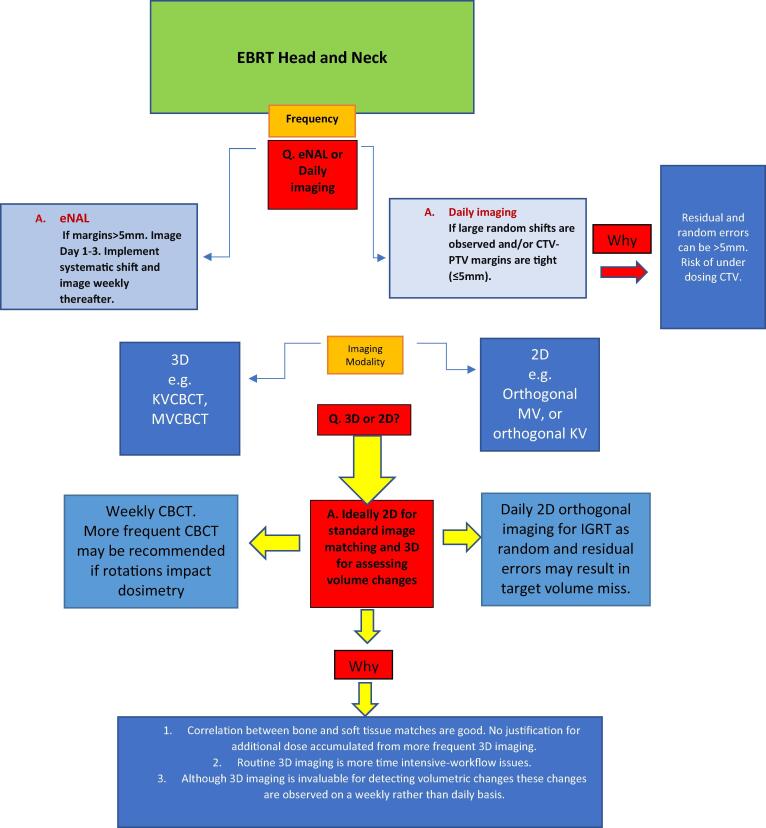
Fig. 1.
Flow chart of recommendations.
Data extraction
Each regime reported in these 144 studies were categorised according to IGRT modality – 2 dimensional (2D) / planar imaging, 3 dimensional (3D)/volumetric imaging e.g. Megavoltage cone beam CT (MVCBCT), Kilovoltage cone beam CT (KVCBCT) and CT on rails; imaging frequency (daily, weekly, extended No Action Level (eNAL), etc; and key messages from paper (e.g. set up errors and volumetric changes reported, correlation between 2D and 3D imaging, CTV-PTV margins used). The following variables were also noted: author, year, country of first author, type of study and number of participants. All key messages were analysed and categorised for discussion.
Results and discussion
Immobilisation
Thermoplastic masks provide rigid immobilisation minimising set up uncertainties with Head and Neck immobilisation studies consistently reporting average systematic interfraction motion of 2–5 mm with similar average values observed in mediolateral (ML), craniocaudal (CC) and anteroposterior (AP) directions [19], [20]. However, some immobilisation studies have reported translational shifts of up to 6 mm [19], [21]. Pitch, yaw and roll of < 10 have been consistently reported in studies investigating rotational displacements [12], [19].
Five-point fixation is recommended with fixation at head, neck and shoulders as standard to minimise sub regional variations in set up errors in the lower neck and shoulders [22], [23], [24]. Open face masks are growing in popularity as they can minimise distress in claustrophobic patients [25], [26]. The literature lacks set up data relating to interfraction motion associated with open face masks but mean intrafraction motion of 0.6–1.3 mm has been reported [25], [26], [27] – comparable to 0.5–1.8 mm observed with conventional masks [28], [29], [30].
CTV-PTV margins, set up errors & imaging frequency
ICRU 50 defines the Planning Target Volume (PTV) as ‘taking into consideration the net effect of all possible geometrical variations, in order to ensure that the prescribed dose is actually absorbed in the CTV’ [31]. The Gross Tumour Volume (GTV) is delineated based on demonstrable disease while the Clinical Target Volume(s) (CTV) encompass tissue at risk of containing subclinical disease both locally and within regional lymphatics [4]. A PTV margin is added to CTV volumes to compensate for possible daily geometric uncertainties such as set up discrepancies, bed sag and internal organ motion [32]. This CTV-PTV margin should avoid being too liberal – thus unnecessarily irradiating surrounding OARs, or too tight – increasing risk of the CTV falling outside the high dose area. Formulas including those reported by van Herk [33] and Stroom [34] have been used to calculate CTV-PTV margins based on systematic and random errors reported by individual institutions. Mzenda et al. [35] states the importance of using reported institutional set up errors to define CTV-PTV margins. Using a formula to expand margins has been questioned in some studies as set up errors are rarely consistent in their direction and magnitude over a course of head and neck IMRT [36], [37]. 5 mm CTV-PTV margins were standard in early IMRT planning [38], [39] and these margins have been reported as adequate to account for systematic and random errors in many HNC IGRT studies [40], [41], [42], [43], [44], [45], [46], [47].
3–5 mm margins have been reported as appropriate only where daily IGRT [45], [48], or alternating day IGRT [49], [43] is routine practice. Where daily IGRT is not practiced studies recommended CTV-PTV margins of greater than 5 mm due to potential sub region set up variabilities in mandible, nasal septum and neck [50], [51], [52], [53], [24], [54], [55] and unstable set ups in patients with a high Body Mass Index (BMI) [50]. Random errors of up to 2–5 mm have been reported which could result in geometric miss if not imaged and corrected online where standard CTV-PTV margins are used [13], [56], [57].
Where daily IGRT is routine clinical practice 40–80% reductions in margin sizes are possible [11], [13], [28], [29], [58], [59], [60]. Widespread use of IGRT has given clinicians confidence to reduce CTV-PTV margins to 3 mm reporting reduced patient toxicities with no reduction in local control [61], [62]. Van Kranen et al. reported total OAR doses reductions of approximately 1 Gy per mm reduction in CTV-PTV margin, with no compromise in CTV dose delivery where 3 mm CTV-PTV margins were used [62]. This was also supported in a recent study where a reduction in margins from 5 to 3 mm verified with daily CBCT showed reduced severity, frequency and duration of toxicity whilst maintaining disease-related outcomes [63]. Trans-Tasman Radiation Oncology Group (TROG) Guidelines extensively discuss the variability in CTV-PTV margins for head and neck cancers but typically suggest a 3–5 mm margin [64] and discourage a CTV-PTV below 2 mm regardless of IGRT practices [65]. This is to account for other possible sources of geometric uncertainty such as TV delineation [66] and intrafraction motion [30].
Where institutions wish to avoid daily imaging publications have recommended adopting an eNAL IGRT protocol – imaging day 1–3 and applying a systematic shift depending on departmental action level protocol (2–3 mm generally observed) [67]. However, use of an eNAL protocol may not always be adequate to account for random errors even when 5 mm margins are used [44], [48], [56].
Imaging frequency is a local, departmental decision but daily online imaging applying all translational shifts is recommended where 3–5 mm margins is clinical practice. Evaluation of setup accuracy at a department level and collaboration between clinicians, planning and treatment staff is strongly advised to validate margins that are utilised, or intend to be utilised, locally which will underpin the IGRT strategy in the clinic.
IGRT modalities and imaging frequency
2D imaging
IGRT modalities have evolved exponentially regarding in room availability and use. 2D and 3D images can be acquired using MV and KV exposures. An orthogonal set composed of a direct anterior/posterior and a direct lateral view should be acquired as a bare minimum to quantify shifts in ML, CC and AP directions [67]. Studies have reported user variability and a lack of consistency in shifts quantified where oblique images are acquired e.g. stereoscopic images [68], [69], [70]. KV imaging is the optimum choice for bone matching but in the absence of KV imaging facilities, orthogonal MV images produce enough bone detail for matching purposes [71].
3D imaging
3D Volumetric imaging such as KV CBCT and MV CBCT are extensively used for geometric verification – in conjunction with 2D images or as the solo imaging modality [17], [72]. Consensus on its frequency in IGRT strategies is lacking [73] with suggestions that it is often over used in IGRT [15]. HNC image registrations have reported good correlation in translational shifts between 2D and 3D image matches [74], [75], [76]. Shifts from 3D bony matched images have reported similar results to those from 2D images [47], [77] and standard planning margins provide adequate soft tissue coverage in HNC patients where IGRT matches are based on bone [78]. Bone based matches in HNC produce similar dosimetric outcomes to implanted fiducial matching [79].
Unlike 2D imaging, 3D imaging quantifies both translational and rotational displacements [75]. Robust immobilisation should produce rotations of less than 0.5 ° in pitch, yaw and roll planes [80], [19]. Lack of robotic couches in the clinic means rotational shifts were often quantified but can only be corrected by resetting up the patient [81]. Where significant rotations are observed global bony anatomy matching may still result in some treated sub-regions misaligned by >5 mm [82]. Improvements in the relative agreement of HNC anatomical sub regions (particularly the inferior neck) has been observed where rotational corrections were applied [83]. The dosimetric effect of rotations on PTV and OAR dose is multifaceted and depends on the size, shape and proximity of PTV and OARS, degree of dose grade steepness and margin sizes [84]. The reported dosimetric impact of HNC rotations is inconsistent with rotations of up to 3 degrees reporting no compromise to dose coverage [85] and rotations as small as one degree having a significant dosimetric impact [86].
In conclusion, as CBCT acquisition can increase the overall treatment session by three minutes [28] 3D imaging should be scheduled efficiently where the limitations of 2D imaging may compromise the integrity of the treatment plan. Departments that have real concerns regarding rotations and their potential impact on adjacent dose volume constraints (DVCs) should consider acquiring 3D imaging regularly to quantify and correct rotations.
3D IGRT, volume changes and Adaptive Radiation Therapy (ART)
Changes such as tumour shrinkage, weight loss and oedema during a course of RT leads to anatomical and positional variations that can be identified using 3D IGRT such as KVCBCT and MVCBCT [87], [88]. Average weight loss of 6–10% over a H&N RT course has been reported [89], [87], [90] while parotid glands can shrink by up to 30% [91], [92], [93], [62], [94]. Mean TV reductions reported vary – Liu reported a 1.5–1.8% reductions in CTV and GTV [93] while Berwouts et al. reported a 72% and 46.3% reduction in GTV and PTV volumes, respectively, over an entire treatment course [95]. PTV reduction of 50% was also reported by Bujold [66]. Due to the steep fall off associated with modern complex planning techniques, volumetric changes may require modification of original plan to avoid discrepancies in planned and delivered dose [96]. Weight loss can cause over dosage, under dosage and dose inhomogeneity in TVs [97].
Volumetric changes can result in a significantly increased dose to OARS if not corrected for – the parotid glands are especially susceptible to increased mean doses due to the medial migration of the shrinking parotids into high dose areas [98]. Castelli et al. reported a 1.1 Gy increase in mean dose delivered to ipsilateral parotid gland with changes in parotid glands in first 2 weeks indicative of an increased risk of acute xerostomia [98]. Worryingly the contralateral parotid gland which is considered the spared parotid gland reports significant increases (11.7–29%) in mean parotid dose [99], [100] exceeding DVC thresholds when volumetric changes are not addressed with a replan [101]. Total spinal cord doses can increase by 10 Gy [20] and in one study the maximum dose to the brain stem increased from 49.9 Gy to 52.6 Gy exceeding DVC for this organ [102].
Traditional RT practice accounted for anticipated volumetric changes by rescanning and replanning H&N patients at a fixed point in the treatment course or when triggered by significant weight loss.
21–65% of patients may benefit from plan revision as a result of dosimetric changes [94]. Adjuvant treated patients [103], HPV+ patients [104] and proton based plans [105] have been suggested as some categories of patients where a replan is likely to be beneficial during the treatment course. CBCT scans can produce safe and accurate dose distributions identifying where tumour shrinkage or other anatomical changes might compromise planned dose distributions [106]. This may be a more efficient provisional measure to assess the dosimetric consequences of observed volumetric changes and whether a replan is merited.
Adaptive Radiation Therapy is becoming more prevalent in RT practice, and is inherently reliant upon departmental IGRT protocols. A comprehensive discussion of ART is beyond the scope of this paper but has been well-covered by recent reviews [107], [108]. The growing body of ART literature does however provide an insight into when volumetric changes are likely to be detected that merit replanning during the treatment course. Significant volumetric changes have been reported in the second week of treatment [109] with fraction 10 suggested as an optimal ART intervention point by van Kranen et al. [62]. Other studies have suggested week 4–5 of a 7 week course as the most common period where significant volumetric changes are evident [110], [111]. Algorithms to identify patients (based on pre-treatment factors) who may benefit from ART have been developed but are still being validated [96].
While 2D and 3D imaging report similar levels of accuracy in daily IGRT, 3D facilities are valuable tools in detecting and calculating dosimetric consequences as a result of volume changes. As volumetric changes are a gradual process, weekly 3D imaging should be sufficient in a RT course to detect volumetric changes of dosimetric consequence.
In contrast to traditional ‘offline’ ART strategies that involve re-planning between fractions, ‘online’ ART enables plan adaption in response to daily volumetric changes while the patient remains on the treatment couch prior to treatment delivery [112]. Though still an emerging technology with limited clinical data available, online ART is commercially available with both MR- and CBCT-based treatment platforms [107]. Such systems represent a paradigm shift from linear workflow of conventional RT for RTTs at the treatment console, merging treatment planning with established practices in online IGRT [108], [107]. As such, the technological and clinical requirements for online ART are likely to supersede traditional considerations of IGRT protocol development calling for increased education and training and clear protocols in adaptive and on-line approaches and thresholds [113]. The introduction of an MRI based platform in RT requires an MRI compatible immobilisation solution and a comprehensive commissioning process to ensure geometric accuracy of the MRI data set [114] and provision of a reliable MR-IGRT option [115].
Imaging dose
While non-ionising radiation based IGRT systems such as MRI are likely to grow in the next decade this technology is in its infancy and radiation based IGRT systems largely remain the gold standard in routine clinical practice. In diagnostic imaging, Ionising Radiation (Medical Exposure) Regulations (IR(ME)R recommends establishing dose reference levels locally and monitoring these doses over time [116]. There are currently no similar recommendations for imaging dose in IGRT, likely due to the relatively low consequence of imaging dose when compared with prescribed treatment dose. The AAPM TG180 report provides broad guidance that imaging dose should not exceed 5% of the treated therapeutic dose [117].
Depending on IGRT modality and frequency of use, dose can vary considerably. Accumulated dose from daily orthogonal MV imaging over a standard 35 fraction H&N treatment can deliver approximately 1 Gy to the parotid gland and spinal cord [118] while daily MVCBCT can contribute the equivalent dose of one extra treatment fraction and increase toxicity based on NTCP modelling [119]. kVCBCT imaging typically contributes <0.5c Gy per session to structures within the head and neck region, though this can vary depending on the region measured and imaging system utilised [120], [121]. Accounting for KV dose in treatment plan is not standardly possible, but MV imaging doses can be modelled and incorporated into dose distribution at the planning stage [118], [122]. Variability in vendor specific customised exposure settings and imaging modality and frequency is an issue in establishing dose reference levels for IGRT protocols [123].
Incorporating MV imaging dose into the treatment plan and avoiding daily MVCBCT imaging is advised to reduce excess dose [124]. Other dose limiting recommendations include: use of a posterior CBCT acquisition angle (90–290 degrees) to reduce eye dose [125], utilising topogram feature to move field of view caudally [126] and using the lowest numbers of monitor units possible to acquire MV images [124].
At the commissioning and acceptance phase IGRT parameters could be optimised to minimise accumulated, incidental doses from IGRT. All RTTs should be educated on the potential risks associated with accumulated imaging dose and follow the principles of ALARA in IGRT practices.
Conclusion and recommendations
IGRT is now an integral part of RT delivery for HNC patients. Planning margins and techniques should be the strongest indicator when designing departmental IGRT strategies. Intradepartmental collaboration between RT disciplines is advised to ensure the IGRT strategy is appropriate to accurately deliver the treatment plan. As attention turns to the latent effects of RT treatment, RTTs should be mindful of ALARA principles in minimising excessive imaging dose in their daily practice and be prepared for an MR guided future in IGRT.
2D and 3D modalities both have their own unique advantages in HNC IGRT. RTTs must maximise available IGRT systems in an efficient manner by identifying optimum modality and frequency of 2D and 3D IGRT facilities in an HNC treatment course. All RTTs should be competent in image acquisition, registration and interpretation to maximise the benefits of available IGRT facilities with minimum impact on treatment session times providing high quality treatment to all HNC patients. This should provide a solid knowledge base to facilitate the growing implementation of ART into routine clinical practice where the role of RTTs in its safe and effective implementation cannot be underestimated.
Contributor Information
Maeve Kearney, Email: mkearne@tcd.ie.
Mary Coffey, Email: mcoffey@tcd.ie.
Aidan Leong, Email: aidan.leong@rad-onc.nz.
Appendix.
See Fig. 2.
Fig. 2.
The process of study identification for the review.
References
- 1.WHO. Latest global cancer data; 2018.
- 2.Vigneswaran N., Williams M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal M.S. Primary concurrent chemoradiation in head and neck cancers with weekly cisplatin chemotherapy: analysis of compliance, toxicity and survival. Int Arch Otorhinolaryngol. 2017;21(2):171–177. doi: 10.1055/s-0036-1594020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregoire V. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v184–v186. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 5.Gatta G. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE-5 population-based study. Eur J Cancer. 2015;51(15):2130–2143. doi: 10.1016/j.ejca.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 6.Mallick S. Altered fractionation radiotherapy in head and neck squamous cell carcinoma. J Egypt Natl Cancer Inst. 2016;28 doi: 10.1016/j.jnci.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Leech M. ESTRO ACROP guidelines for positioning, immobilisation and position verification of head and neck patients for radiation therapists. Tech Innovat Patient Support Radiat Oncol. 2017;1:1–7. doi: 10.1016/j.tipsro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gestel D. Intensity-modulated radiotherapy in patients with head and neck cancer: a European single-centre experience. Br J Radiol. 2011;84(1000):367–374. doi: 10.1259/bjr/67058055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roopashri G., Baig M. Current advances in radiotherapy of head and neck malignancies. J Int Oral Health. 2013;5(6):119–123. [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz M. IGRT versus non-IGRT for postoperative head-and-neck IMRT patients: dosimetric consequences arising from a PTV margin reduction. Radiat Oncol. 2012:133. doi: 10.1186/1748-717X-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiong A. Faculty of radiation oncology position paper on the use of image-guided radiation therapy. J Med Imag Radiat Oncol. 2016;60(6):772–780. doi: 10.1111/1754-9485.12463. [DOI] [PubMed] [Google Scholar]
- 12.Schubert L.K. A comprehensive assessment by tumor site of patient setup using daily MVCT imaging from more than 3,800 helical tomotherapy treatments. Int J Radiat Oncol Biol Phys. 2009;73(4):1260–1269. doi: 10.1016/j.ijrobp.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudat V. Impact of the frequency of online verifications on the patient set-up accuracy and set-up margins. Radiat Oncol. 2011;6 doi: 10.1186/1748-717X-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grau C. Radiotherapy equipment and departments in the European countries: final results from the ESTRO-HERO survey. Radiother Oncol. 2014;112(2):155–164. doi: 10.1016/j.radonc.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Nabavizadeh N. Image guided radiation therapy (IGRT) practice patterns and IGRT's impact on workflow and treatment planning: results from a national survey of american society for radiation oncology members. Int J Radiat Oncol Biol Phys. 2016;94(4):850–857. doi: 10.1016/j.ijrobp.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 16.Kron T. Medical physics aspects of cancer care in the Asia Pacific region: 2014 survey results. Australas Phys Eng Sci Med. 2015;38(3):493–501. doi: 10.1007/s13246-015-0373-2. [DOI] [PubMed] [Google Scholar]
- 17.Gupta T., Narayan C.A. Image-guided radiation therapy: Physician's perspectives. J Med Phys. 2012;37(4):174–182. doi: 10.4103/0971-6203.103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshpande S. Use of image guided radiation therapy techniques and imaging dose measurement at Indian hospitals: a survey. J Med Phys. 2015;40(4):220–225. doi: 10.4103/0971-6203.170788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Divneet M. Comparison of two thermoplastic immobilization mask systems in daily volumetric image guided radiation therapy for head and neck cancers. Biomed Phys Eng Express. 2018:4(5). [Google Scholar]
- 20.Castadot P. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol. 2010;20(2):84–93. doi: 10.1016/j.semradonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Li S. Clinical results of a pilot study on stereovision-guided stereotactic radiotherapy and intensity modulated radiotherapy. Technol Cancer Res Treat. 2010;9(6):603–617. doi: 10.1177/153303461000900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team NCA. National radiotherapy implementation group report image guided radiotherapy (IGRT) guidance for implementation and use; 2012.
- 23.Leech M. ESTRO ACROP guidelines for positioning, immobilisation and position verification of head and neck patients for radiation therapists. Tech Innovat Patient Support Radiat Oncol. 2016;1:1–7. doi: 10.1016/j.tipsro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoll M. Comparison of safety margin generation concepts in image guided radiotherapy to account for daily head and neck pose variations. PLoS One [Electron Resour] 2016;11(12):e0168916. doi: 10.1371/journal.pone.0168916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G. Migration from full-head mask to “open-face” mask for immobilization of patients with head and neck cancer. J Appl Clin Med Phys. 2013;14(5):243–254. doi: 10.1120/jacmp.v14i5.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiant D. A prospective evaluation of open face masks for head and neck radiation therapy. Pract Radiat Oncol. 2016;6(6):e259–e267. doi: 10.1016/j.prro.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Leitzen C. Quality of patient positioning during cerebral tomotherapy irradiation using different mask systems. Strahlenther Onkol. 2014;190(4):382–385. doi: 10.1007/s00066-013-0496-x. [DOI] [PubMed] [Google Scholar]
- 28.Den R.B. Daily image guidance with cone-beam computed tomography for head-and-neck cancer intensity-modulated radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 2010;76(5):1353–1359. doi: 10.1016/j.ijrobp.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Lu H. Interfractional and intrafractional errors assessed by daily cone-beam computed tomography in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy: a prospective study. J Radiat Res. 2012;53(6):954–960. doi: 10.1093/jrr/rrs041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang P.P.E. An assessment of the magnitude of intra-fraction movement of head-and-neck IMRT cases and its implication on the action-level of the imaging protocol. Radiother Oncol. 2014;112(3):437–441. doi: 10.1016/j.radonc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Jones D. ICRU report 50—prescribing, recording and reporting photon beam therapy. Int J Med Phys Res Pract. 1994;21(5):833–834. [Google Scholar]
- 32.Landberg T., Chavaudra J., Dobbs J., Gerard J.-P., Hanks G., Horiot J.-C., Johansson K.-A., Möller T., Purdy J., Suntharalingam N., Svensson H. ICRU report 62 prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50) J Int Comm Radiat Units Meas. 1999;os32(1) [Google Scholar]
- 33.Herk M.V. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14(1):52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Stroom J.C., Heijmen B.J. Geometrical uncertainties, radiotherapy planning margins, and the ICRU-62 report. Radiother Oncol. 2002;64:75–83. doi: 10.1016/s0167-8140(02)00140-8. [DOI] [PubMed] [Google Scholar]
- 35.Mzenda B. Determination of target volumes in radiotherapy and the implications of technological advances: a literature review. J Radiother Pract. 2009;8(1):41–51. [Google Scholar]
- 36.Yock A.D. Anisotropic margin expansions in 6 anatomic directions for oropharyngeal image guided radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87(3):596–601. doi: 10.1016/j.ijrobp.2013.06.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fangzheng W. Gemcitabine/cisplatin induction chemotherapy before concurrent chemotherapy and intensity-modulated radiotherapy improves outcomes for locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8(57):96798–96808. doi: 10.18632/oncotarget.18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mongioj Valeria. Set-up errors analyses in IMRT treatments fornasopharyngeal carcinoma to evaluate time trends, PTV and PRV margins. Acta Oncol. 2011;50(1):61–71. doi: 10.3109/0284186X.2010.509108. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y. Primary study of the threatening of unfixed planning of image guided radiotherapy to the volume margin of neck tumor. Shengwu Yixue Gongchengxue Zazhi/J Biomed Eng. 2013;30(3):503–507. [PubMed] [Google Scholar]
- 40.Baron C.A. Estimation of daily interfractional larynx residual setup error after isocentric alignment for head and neck radiotherapy: quality assurance implications for target volume and organs-at-risk margination using daily CT on- rails imaging. J Appl Clin Med Phys. 2014;16(1):5108. doi: 10.1120/jacmp.v16i1.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dionisi F. Set-up errors and planning target volume margins in head and neck cancer radiotherapy: a clinical study of image guidance with on-line cone-beam computed tomography. Int J Clin Oncol. 2013;18(3):418–427. doi: 10.1007/s10147-012-0395-7. [DOI] [PubMed] [Google Scholar]
- 42.Kataria T. Set-up uncertainties: online correction with X-ray volume imaging. J Cancer Res Ther. 2011;7(1):40–46. doi: 10.4103/0973-1482.80457. [DOI] [PubMed] [Google Scholar]
- 43.Nyarambi I., Chamunyonga C., Pearce A. CBCT image guidance in head and neck irradiation: the impact of daily and weekly imaging protocols. J Radiother Pract. 2015;14(4):362–369. [Google Scholar]
- 44.Saha A. Evaluating the need for daily image guidance in head and neck cancers treated with helical tomotherapy: a retrospective analysis of a large number of daily imaging-based corrections. Clin Oncol (Royal College Radiol) 2016;28(3):178–184. doi: 10.1016/j.clon.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Yin W.-J. Evaluation of inter-fraction and intra-fraction errors during volumetric modulated arc therapy in nasopharyngeal carcinoma patients. Radiat Oncol. 2013;8 doi: 10.1186/1748-717X-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Kranen S. Correction strategies to manage deformations in head-and-neck radiotherapy. Radiother Oncol. 2010;94(2):199–205. doi: 10.1016/j.radonc.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Tan W. Analysis of geometric variation of neck node levels during image-guided radiotherapy for nasopharyngeal carcinoma: recommended planning margins. Quant Imag Med Surg. 2018;8(7):637–647. doi: 10.21037/qims.2018.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houghton F. An assessment of action levels in imaging strategies in head and neck cancer using TomoTherapy. are our margins adequate in the absence of image guidance? Clin Oncol (Royal College Radiol) 2009;21(9):720–727. doi: 10.1016/j.clon.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Yu Y. Comparison of daily versus nondaily image-guided radiotherapy protocols for patients treated with intensity-modulated radiotherapy for head and neck cancer. Head Neck. 2014;36(7):992–997. doi: 10.1002/hed.23401. [DOI] [PubMed] [Google Scholar]
- 50.Lai Y.L. Impact of body-mass factors on setup displacement in patients with head and neck cancer treated with radiotherapy using daily on-line image guidance. Radiat Oncol. 2014;9:19. doi: 10.1186/1748-717X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheo T. Measuring radiotherapy setup errors at multiple neck levels in nasopharyngeal cancer (NPC): a case for differential PTV expansion. Radiother Oncol. 2015;117(3):419–424. doi: 10.1016/j.radonc.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 52.Goldsworthy S. A pilot study evaluating the effectiveness of dual-registration image-guided radiotherapy in patients with oropharyngeal cancer. J Med Imag Radiat Sci. 2017;48(4):377–384. doi: 10.1016/j.jmir.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Kapanen M. Effects of remedies made in patient setup process on residual setup errors and margins in head and neck cancer radiotherapy based on 2D image guidance. Rep Pract Oncol Radiother. 2015;20(4):292–298. doi: 10.1016/j.rpor.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leitzen C. Patient positioning in head and neck cancer: setup variations and safety margins in helical tomotherapy. Strahlenther Onkol. 2018;194(5):386–391. doi: 10.1007/s00066-018-1265-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhong R. Analysis of which local set-up errors can be covered by a 5-mm margin for cone beam CT-guided radiotherapy for nasopharyngeal carcinoma. Br J Radiol. 2018:91(1088). doi: 10.1259/bjr.20160849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zumsteg Z. Image guidance during head-and-neck cancer radiation therapy: analysis of alignment trends with in-room cone-beam computed tomography scans. Int J Radiat Oncol Biol Phys. 2012;83(2):712–719. doi: 10.1016/j.ijrobp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Srivastava S.P., Cheng C.W., Das I.J. Image guidance-based target volume margin expansion in IMRT of head and neck cancer. Technol Cancer Res Treat. 2016;15(1):107–113. doi: 10.1177/1533034614561162. [DOI] [PubMed] [Google Scholar]
- 58.Qi X.S. Evaluation of interfraction patient setup errors for image-guided prostate and head-and-neck radiotherapy using kilovoltage cone beam and megavoltage fan beam computed tomography. J Radiother Pract. 2014;12(4):334–343. [Google Scholar]
- 59.Piotrowski T. Impact of the spinal cord position uncertainty on the dose received during head and neck helical tomotherapy. J Med Imag Radiat Oncol. 2013;57(4):503–511. doi: 10.1111/1754-9485.12056. [DOI] [PubMed] [Google Scholar]
- 60.Oh Y.K. Assessment of setup uncertainties for various tumor sites when using daily CBCT for more than 2200 VMAT treatments. J Appl Clin Med Phys. 2014;15(2):4418. doi: 10.1120/jacmp.v15i2.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen A.M. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv Radiat Oncol. 2017;2(2):167–175. doi: 10.1016/j.adro.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Kranen S. Head and neck margin reduction with adaptive radiation therapy: robustness of treatment plans against anatomy changes. Int J Radiat Oncol Biol Phys. 2016;96(3):653–660. doi: 10.1016/j.ijrobp.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Navran A. The impact of margin reduction on outcome and toxicity in head and neck cancer patients treated with image-guided volumetric modulated arc therapy (VMAT) Radiother Oncol. 2019;130:25–31. doi: 10.1016/j.radonc.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 64.McKenzie A.L., van Herk M., Mijnheer B. The width of margins in radiotherapy treatment plans. Phys Med Biol. 2000;45(11):3331–3342. doi: 10.1088/0031-9155/45/11/315. [DOI] [PubMed] [Google Scholar]
- 65.Wang R.H., Zhang S.X., Lin S.Q. Recent advance in CT image-guided adaptive radiotherapy for nasopharyngeal carcinoma. Chinese J Cancer Prevent Treat. 2014;21(7):560–564. [Google Scholar]
- 66.Bujold A. Image-guided radiotherapy: has it influenced patient outcomes? Semin Radiat Oncol. 2012;22(1):50–61. doi: 10.1016/j.semradonc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Radiologists, RCo. On target: ensuring geometric accuracy in radiotherapy; 2006.
- 68.Clemente S. Is exactrac X-ray system an alternative to CBCT for positioning patients head and neck cancers? J Nucl Med Radiat Ther. 2013;4(4) doi: 10.1118/1.4824056. [DOI] [PubMed] [Google Scholar]
- 69.Tanyi J. Comparison of image-guidance modalities for cranial stereotactic radiosurgery (SRS) and radiotherapy (SRT) Int J Oncol Biol. 2011;81(2):826–827. [Google Scholar]
- 70.Fuller C.D. Method comparison of automated matching software-assisted cone-beam CT and stereoscopic kilovoltage x-ray positional verification image-guided radiation therapy for head and neck cancer: a prospective analysis. Phys Med Biol. 2009;54(24):7401–7415. doi: 10.1088/0031-9155/54/24/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devereux B. A comparison of kV and MV imaging in head and neck image guided radiotherapy. Radiography. 2010;16(1):8–13. [Google Scholar]
- 72.Nath S.K. Recent advances in image-guided radiotherapy for head and neck carcinoma. J Oncol. 2009;2009:752135. doi: 10.1155/2009/752135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nath S.K. Recent advances in image-guided radiotherapy for head and neck carcinoma. J Oncol Print. 2009;2009:752135. doi: 10.1155/2009/752135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang H. Accurate positioning for head and neck cancer patients using 2D and 3D image guidance. J Appl Clin Med Phy. 2011;12(1) doi: 10.1120/jacmp.v12i1.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciardo D. Set-up errors in head and neck cancer patients treated with intensity modulated radiation therapy: quantitative comparison between three-dimensional cone-beam CT and two-dimensional kilovoltage images. Physica Medica-Eur J Med Phys. 2015;31(8):1015–1021. doi: 10.1016/j.ejmp.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Dzierma Y. Set-up errors and planning margins in planar and CBCT image-guided radiotherapy using three different imaging systems: a clinical study for prostate and head-and-neck cancer. Physica Med. 2015;31(8):1055–1059. doi: 10.1016/j.ejmp.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Zhao L.R. The clinical feasibility and performance of an orthogonal X-ray imaging system for image-guided radiotherapy in nasopharyngeal cancer patients: comparison with cone-beam CT. Physica Med. 2016;32(1):266–271. doi: 10.1016/j.ejmp.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 78.Luisa B.M. Characterization of volume and shape modifications of PET-positive nodes during Tomotherapy for head and neck cancer as assessed by MVCTs. Radiother Oncol. 2015;115(1):50–55. doi: 10.1016/j.radonc.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 79.Zeidan O.A. A comparison of soft-tissue implanted markers and bony anatomy alignments for image-guided treatments of head-and-neck cancers. Int J Radiat Oncol Biol Phys. 2010;76(3):767–774. doi: 10.1016/j.ijrobp.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 80.Hansen C.R. Comparison of three immobilisation systems for radiation therapy in head and neck cancer. Acta Oncol. 2014;53(3):423–U156. doi: 10.3109/0284186X.2013.813966. [DOI] [PubMed] [Google Scholar]
- 81.Gevaert T. Clinical evaluation of a robotic 6-degree of freedom treatment couch for frameless radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(1):467–474. doi: 10.1016/j.ijrobp.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 82.van Kranen S., van Beek S., Rasch C., van Herk M., Sonke J.J. Setup uncertainties of anatomical sub-regions in head-and-neck cancer patients after offline CBCT guidance. Int J Radiat Oncol Biol Phys. 2009;75(5):1566–1573. doi: 10.1016/j.ijrobp.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 83.Kung J.S. Evaluation of the efficacy of rotational corrections for standard-fractionation head and neck image-guided radiotherapy. Technol Cancer Res Treat. 2018;18 doi: 10.1177/1533033819853824. 1533033819853824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu W. Dosimetric influences of rotational setup errors on head and neck carcinoma intensity-modulated radiation therapy treatments. Med Dosim. 2013;38(2):125–132. doi: 10.1016/j.meddos.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Peng Jean L. Dosimetric consequences of rotational setup errors with direct simulation in a treatment planning system for fractionated stereotactic radiotherapy. J Appl Clin Med Phys. 2011.:12. doi: 10.1120/jacmp.v12i3.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arumugam S. Impact of patient rotational errors on target and critical structure dose in IMRT: a 3D simulation study. J Phys Conf Ser. 2013:444. [Google Scholar]
- 87.Chen A.M., Daly M.E., Cui J., Mathai M., Benedict S., Purdy J.A. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck J Sci Special Head Neck. 2014;36(11):1541–1546. doi: 10.1002/hed.23477. [DOI] [PubMed] [Google Scholar]
- 88.Bhide S.A., Nutting C.M. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zia S.Y. The impact of weight loss on setup accuracy for head and neck cancer patients in the era of image guided radiation therapy. J Radiat Oncol. 2016;5(4):359–362. [Google Scholar]
- 90.Hou J. Response monitoring for concurrent chemo-radiation therapy of head and neck cancer: pre-treatment PET SUV and tumor regression. Int J Radiat Oncol Biol Phys. 2011;81(2):S800. [Google Scholar]
- 91.Schwartz D.L. Adaptive radiotherapy for head and neck cancer–dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106(1):80–84. doi: 10.1016/j.radonc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 92.Srivastava R. A hybrid approach for head and neck cancer using online image guidance and offline adaptive radiotherapy planning. J Radiother Pract. 2019;18(3):271–275. [Google Scholar]
- 93.Liu Q. Dosimetric evaluation of incorporating patient geometric variations into adaptive plan optimization through probabilistic treatment planning in head and neck cancers. Int J Radiat Oncol Biol Phys. 2018;101(4):985–997. doi: 10.1016/j.ijrobp.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 94.Ahn P.H. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys. 2011;80(3):677–685. doi: 10.1016/j.ijrobp.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 95.Berwouts D. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother Oncol. 2013;107(3):310–316. doi: 10.1016/j.radonc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 96.Surucu M. Adaptive radiotherapy for head and neck cancer: implications for clinical and dosimetry outcomes. Technol Cancer Res Treat. 2017;16(2):218–223. doi: 10.1177/1533034616662165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang C. Using an online adaptive replanning tool for offline adaptive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(2):S850. [Google Scholar]
- 98.Castelli J. Adaptive radiotherapy in head and neck cancer is required to avoid tumor underdose. Acta Oncol. 2018;57(9):1267–1270. doi: 10.1080/0284186X.2018.1468086. [DOI] [PubMed] [Google Scholar]
- 99.Jensen A.D. A clinical concept for interfractional adaptive radiation therapy in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):590–596. doi: 10.1016/j.ijrobp.2010.10.072. [DOI] [PubMed] [Google Scholar]
- 100.Loo H. Tumour shrinkage and contour change during radiotherapy increase the dose to organs at risk but not the target volumes for head and neck cancer patients treated on the TomoTherapy HiArt (TM) system. Clin Oncol. 2011;23(1):40–47. doi: 10.1016/j.clon.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Graff P. Head and neck adaptive radiotherapy. CancerRadiotherapie. 2013;17(5–6):513–522. doi: 10.1016/j.canrad.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 102.Chen X. Interfractional dose variations in parotid and submandibular glands in IG-IMRT for head and neck cancer. Med Phys. 2014;41(6):402. [Google Scholar]
- 103.Capelle L. Adaptive radiotherapy using helical tomotherapy for head and neck cancer in definitive and postoperative settings: initial results. Clin Oncol (Royal College Radiol) 2012;24(3):208–215. doi: 10.1016/j.clon.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Chen A.M. Differential response rates to irradiation among patients with human papillomavirus positive and negative oropharyngeal cancer. Laryngoscope. 2013;123(1):152–157. doi: 10.1002/lary.23570. [DOI] [PubMed] [Google Scholar]
- 105.Mueller B.S. Impact of interfractional changes in head and neck cancer patients on the delivered dose in intensity modulated radiotherapy with protons and photons. Physica Medica-Eur J Med Phys. 2015;31(3):266–272. doi: 10.1016/j.ejmp.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 106.Hvid C.A. Cone-beam computed tomography (CBCT) for adaptive image guided head and neck radiation therapy. Acta Oncol. 2018;57(4):552–556. doi: 10.1080/0284186X.2017.1398414. [DOI] [PubMed] [Google Scholar]
- 107.Heukelom J., Fuller C.D. Head and neck cancer adaptive radiation therapy (ART): conceptual considerations for the informed clinician. Semin Radiat Oncol. 2019;29(3):258–273. doi: 10.1016/j.semradonc.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sonke J.J., Aznar M., Rasch C. Adaptive radiotherapy for anatomical changes. Semin Radiat Oncol. 2019;29(3):245–257. doi: 10.1016/j.semradonc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 109.Bhide S.A., Nutting C.M. Advances in radiotherapy for head and neck cancer. Oral Oncol. 2010;46(6):439–441. doi: 10.1016/j.oraloncology.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 110.Ricchetti F. Feasibility of helical tomotherapy for radical dose retreatment in pelvic area: a report of 4 cases. Tumori. 2011;97(4):492–497. doi: 10.1177/030089161109700413. [DOI] [PubMed] [Google Scholar]
- 111.Barker J.L., Jr, Garden A.S., Ang K.K., O'Daniel J.C., Wang H., Court L.E., Morrison W.H., Rosenthal D.I., Chao K.S., Tucker S.L., Mohan R., Dong L. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59(4):960–970. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 112.Lim-Reinders S. Online adaptive radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(4):994–1003. doi: 10.1016/j.ijrobp.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 113.McNair H.A. Image guided radiotherapy: current status of soft tissue imaging. Radiography. 2014;20(2):158–161. [Google Scholar]
- 114.Pollard J.M. The future of image-guided radiotherapy will be MR guided. British J Radiol. 2017;90(1073):20160667. doi: 10.1259/bjr.20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Michael Gach H. MRI quality control for low-field MR-IGRT systems: lessons learned. J Appl Clin Med Phys. 2019;20(10):53–66. doi: 10.1002/acm2.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.British Institute of Radiology SaCoRatRCoR. A guide to understanding the implications of ionising radiation (medical exposure) regulations in diagnostic and interventional radiology; 2015.
- 117.Ding G.X., Alaei P., Curran B., Flynn R., Gossman M., Mackie T.R. Image guidance doses delivered during radiotherapy: quantification, management, and reduction: report of the AAPM Therapy Physics Committee Task Group 180. Med Phys. 2018;45(5):e84–e99. doi: 10.1002/mp.12824. [DOI] [PubMed] [Google Scholar]
- 118.Zabel-du Bois A., Nill S., Ulrich S., Oelfke U., Rhein B., Haering P., Milker-Zabel S., Schwahofer A. Dosimetric integration of daily mega-voltage cone-beam CT for image-guided intensity-modulated radiotherapy. Strahlentherapie und Onkologie. 2012;188(2):120–126. doi: 10.1007/s00066-011-0021-z. [DOI] [PubMed] [Google Scholar]
- 119.Bell K. Image guidance and positioning accuracy in clinical practice: influence of positioning errors and imaging dose on the real dose distribution for head and neck cancer treatment. Radiat Oncol. 2018;13(1):190. doi: 10.1186/s13014-018-1141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ding G.X., Malcolm A.W. An optically stimulated luminescence dosimeter for measuring patient exposure from imaging guidance procedures. Phys Med Biol. 2013;58:5885–5897. doi: 10.1088/0031-9155/58/17/5885. [DOI] [PubMed] [Google Scholar]
- 121.Spezi E. Patient-specific three-dimensional concomitant dose from cone beam computed tomography exposure in image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):419–426. doi: 10.1016/j.ijrobp.2011.06.1972. [DOI] [PubMed] [Google Scholar]
- 122.Korreman S. The European society of therapeutic radiology and oncology-european institute of radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother Oncol. 2010;94(2):129–144. doi: 10.1016/j.radonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 123.Goyal S., Kataria T. Image guidance in radiation therapy: techniques and applications. Radiol Res Pract. 2014;2014 doi: 10.1155/2014/705604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jia M.X. Peripheral dose from megavoltage cone-beam CT imaging for nasopharyngeal carcinoma image-guided radiation therapy. J Appl Clin Med Phys. 2012;13(5):3–11. doi: 10.1120/jacmp.v13i5.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ding G.X., Coffey C.W. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int J Radiat Oncol Biol Phys. 2009;73(2):610–617. doi: 10.1016/j.ijrobp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 126.Khamfongkhruea C. Posterior kV-CBCT scanning of the head and neck region minimizes doses to critical organs with sustained image quality. Physica Medica-Eur J Med Phys. 2015;31(5):524–528. doi: 10.1016/j.ejmp.2015.03.016. [DOI] [PubMed] [Google Scholar]