Abstract
Aim:
The mathematical method used to calculate chest compression (CC) rate during cardiopulmonary resuscitation varies in the literature and across device manufacturers. The objective of this study was to determine the variability in calculated CC rates by applying four published methods to the same dataset.
Methods:
This study was a secondary investigation of the first 200 pediatric cardiac arrest events with invasive arterial line waveform data in the ICU-RESUScitation Project (NCT02837497). Instantaneous CC rates were calculated during periods of uninterrupted CCs. The defined minimum interruption length affects rate calculation (e.g., if an interruption is defined as a break in CCs ≥ 2 seconds, the lowest possible calculated rate is 30 CCs/min). Average rates were calculated by four methods: 1) rate with an interruption defined as ≥ 1 second; 2) interruption ≥ 2 seconds; 3) interruption ≥ 3 seconds; 4) method #3 excluding top and bottom quartiles of calculated rates. American Heart Association Guideline-compliant rate was defined as 100–120 CCs/min. A clinically important change was defined as ± 5 CCs/min. The percentage of events and epochs (30 second periods) that changed Guideline-compliant status was calculated.
Results:
Across calculation methods, mean CC rates (118.7 – 119.5/min) were similar. Comparing all methods, 14 events (7%) and 114 epochs (6%) changed Guideline-compliant status.
Conclusion:
Using four published methods for calculating CC rate, average rates were similar, but 7% of events changed Guideline-compliant status. These data suggest that a uniform calculation method (interruption ≥ 1 second) should be adopted to decrease variability in resuscitation science.
Keywords: Cardiopulmonary Resuscitation, Chest Compression Rate, American Heart Association Guideline, Utstein Style
INTRODUCTION:
Approximately 26,000 children and more than 500,000 adults are treated with cardiopulmonary resuscitation (CPR) for a cardiac arrest in the United States each year.1–3 Although survival rates have improved in recent years for both out-of-hospital and in-hospital cardiac arrest, most patients do not survive to hospital discharge.4–6 CPR quality is a principal determinant of survival;7–9 therefore monitoring CPR quality is a high priority.
Several adult studies have demonstrated that achieving established Guideline-based targets for chest compression rate,10 depth,11 and release velocity12 improves survival rates. Properly measuring these quantitative CPR variables is important for providing the rescuer with accurate feedback and for conducting research on CPR quality. While chest compression depth and release velocity can be calculated instantaneously per individual compression, chest compression rate is often based on a period of compressions. Multiple methods have been used to calculate chest compression rate in the literature and across manufacturers of CPR quality-recording defibrillators. This variability may lead to providers receiving different feedback depending on which method or manufacturer are used, and adds potential noise to scientific investigations designed to provide evidentiary support for CPR quality targets. Despite this concern, no study has determined if these methods result in substantially different chest compression rate calculations.
To that end, the primary objective of this study was to evaluate the variability of calculated chest compression rate across four existing published methods of chest compression rate calculation. To meet our objective, we leveraged the ICU-RESUScitation Project (NCT02837497, ICU-RESUS), currently being conducted in the Collaborative Pediatric Critical Care Research Network (CPCCRN). This study is prospectively enrolling pediatric in-hospital cardiac arrest events within 18 intensive care units (ICUs) in the United States.13 Data collection includes the capture and analysis of physiologic patient waveforms. Using data from the first 200 events, the objectives of this study were to 1) evaluate the differences in chest compression rate based on multiple calculation methods, and 2) determine the percentage of events that changed Guideline-compliant status or that had a clinically important change in the calculated rate across the methods evaluated.
METHODS:
Setting and Design
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, CPCCRN is a network of institutions that perform studies and investigations related to pediatric critical care practice.14 The clinical sites included in CPCCRN are supported by a comprehensive central data coordinating center (DCC) located at the University of Utah. In addition, to meet the enrollment goals of ICU-RESUS, the St. Louis Children’s Hospital (St. Louis, MO) and Nemours Alfred I. duPont Children’s Hospital (Wilmington, DE) were recruited to participate. The University of Utah serves as the central institutional review board (IRB) of ICU-RESUS. A waiver of informed consent was granted for the ICU-RESUS study. Please see our previous publication for more details on the ICU-RESUS study design.13
Enrollment for ICU-RESUS began in October 2016 and is scheduled to end in March 2021. The objective of the trial is to evaluate the effectiveness of a CPR quality improvement bundle (comprised of CPR quality-focused rolling refreshers and post-event cardiac arrest debriefings) concentrating on patient-specific physiology to improve survival outcomes from ICU pediatric cardiac arrests.15–17 To note, all of the sites have access to CPR quality monitoring defibrillators (Zoll Medical, Chelmsford, MA, USA). This investigation was a secondary methodological analysis of the multi-center interventional trial waveform data.
Patient Population
Children in an ICU who received external chest compressions and who had analyzable invasive arterial blood pressure monitoring prior to and during CPR were eligible for inclusion. Multiple events of the same subject were included and evaluated separately if there was ≥ 20 minutes of return of spontaneous circulation (ROSC) between CPR events. Events were excluded if at least 30 seconds of the arterial line data could not be used to determine chest compression start, chest compression stop, blood pressures, and interruptions. Subjects with an adjusted age < 37 weeks gestation or ≥ 19 years were excluded.
Data Analysis
Using BedMaster (Excel Medical, Jupiter, FL, USA) or hospital-based server clusters, all clinical sites obtained raw waveform data for all patients in the form of research-quality physiologic signals (respiratory plethysmography, central venous pressure, invasive arterial blood pressure, pulse oximetry, and electrocardiogram). These waveform data were digitally sampled, downloaded locally in comma-separated value (CSV) format, and then transmitted to the DCC de-identified. A member of the research team (WPL) then downloaded the files and reconstructed the waveforms using custom code (MATLAB; MathWorks, Inc., Natick, MA, USA) to allow for clinical review by one of the study investigators (RMS, RWM). During each clinical review, CC artifact on the above waveforms was utilized to identify periods of CPR and identify each individual compression. The following time stamps were annotated: 1) start of chest compressions; 2) start and stop of any interruptions in chest compressions (termed “pause”); 3) start and stop of periods of non-sustained ROSC (termed “any ROSC”); 4) start and stop of periods of unusable/non-interpretable arterial catheter data (termed “unusable data”); and 5) end of chest compressions.
Rate Calculation Methods
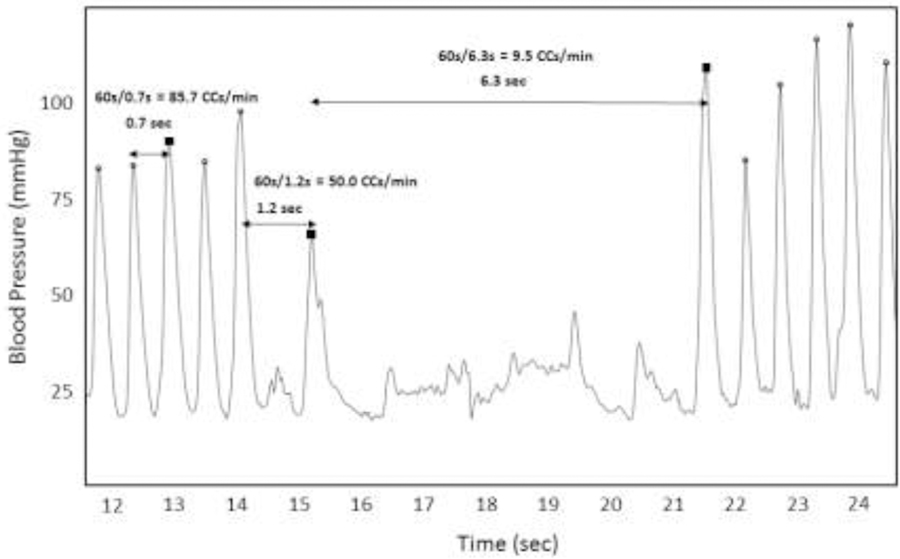
Time of compression and blood pressure are determined based on a systolic peak identification algorithm. Chest compression rate is calculated as the “instantaneous” rate per compression, with the units being chest compressions per minute (CCs/min). An instantaneous chest compression rate is calculated for each compression (i.e., systolic peak) during a period of uninterrupted chest compressions (Fig. 1). The first compression after an interruption (pause, any ROSC, unusable data) is assigned a null value and omitted from the calculation. The subsequent compressions are assigned an instantaneous rate value based on the following equation: [60 / (current compression time - previous compression time], where time is in seconds. As a specific example, a compression occurring 0.5 seconds after the previous compression would have an instantaneous chest compression rate of 120 CCs/min. See Fig. 1 for a graphical representation of the details regarding the calculation of instantaneous chest compression rate.
Figure 1: Calculation of chest compression rate.
This figure depicts the arterial blood pressure waveform during a representative period of CPR. Over this period, 12 compressions were identified (systolic peak marked by an open circle). Three compressions are highlighted (filled-in black square). The time to the previous chest compression and the resultant calculated instantaneous rate is noted for each of these highlighted compressions. Instantaneous Rate (RateI) is calculated as 60 divided by the time between compressions.
The average instantaneous rate over the course of a given time period yields the calculated chest compression rate for an event.
The definition of an interruption is an important component for calculating instantaneous chest compression rate.18 A significant interruption in chest compressions can either be defined as break in CPR, or as a slow rate. To illustrate, if the time from the current compression to the previous compression is 1.5 seconds, the current compression would have a calculated instantaneous rate of 40 CCs/min. If, however, this 1.5-second period between compressions is considered an “interruption” then the current compression would be assigned a null value in regard to the rate calculation, and therefore omitted from the calculation.
Instantaneous chest compression rates are averaged over epochs of 30 seconds in length for up to the first 10 minutes of an event. The chest compression rate associated with each epoch is averaged across an entire event for an event-level rate.
The following rate calculation methods were used in this analysis:
Instantaneous chest compression rate in which an interruption is defined as any break in compressions ≥ 1 second. The minimum possible calculated rate for any given compression is 60 CCs/min.19 (1-second method)
Instantaneous chest compression rate in which an interruption is defined as any break in compressions ≥ 2 seconds. The minimum possible calculated rate for any given compression is 30 CCs/min.20 (2-second method)
Instantaneous chest compression rate in which an interruption is defined as any break in compressions ≥ 3 seconds. The minimum possible calculated rate for any given compression is 20 CCs/min.21 (3-second method)
“Trimmed mean”: Instantaneous chest compression rate per compression calculated using the definition as in 3-second method. Within each 30-second data epoch, the top quartile and lower quartile of instantaneous rates are omitted from the calculation of the average chest compression rate.20 (trimmed mean method)
Statistical Analysis
Epoch-level chest compression rates within each CPR event were summarized by the mean of all epochs within each event, and by the median, minimum, and maximum epoch within each event. Averages of these event-level statistics for all CPR events were summarized for each of the four calculation methods. The frequency and percentage of epochs and events that were American Heart Association Guideline-compliant for chest compression rate (100 – 120 CCs/min)22 were summarized for all four rate calculation methods. Guideline-compliant status change was defined as the calculated rate being Guideline-compliant by one method, and Guideline-non-compliant by a different method. Events and epochs with Guideline-compliant status change between the reference method (1-second method) and the remaining three methods were summarized with the use of frequencies and percentages. Similarly, events and epochs with Guideline-compliant status changed between any combinations of two calculation methods were summarized. Despite Guideline-compliant status being the primary indicator of consistency between methods, clinically important differences between the methods were also summarized. These analyses were performed in order to evaluate the consistency of the calculation methods regarding chest compression rates that are on the border of being Guideline-compliant, well within compliance, or vastly non-compliant; whereas Guideline-compliant status may only evaluate chest compression rates on the border of being Guideline-compliant (chest compression rates near 100 and near 120 CCs/min). We used a change of at least ± 5 CCs/min to indicate a clinically important difference between two calculation methods, based on this degree of change representing a noticeable difference for clinical interpretation.
RESULTS:
Average event-level chest compression rate statistical summaries are contained in Table 1. Across calculation methods, mean, median, minimum, and maximum chest compression rates were similar. Table 2 contains the number and percentage of events (top row; n=200) and epochs (bottom row; n=1856) achieving the Guideline-compliant rate target. Again, the percentage of events and epochs achieving Guideline-compliant status was similar across the calculation methods.
Table 1:
Average event-level chest compression rate (cc/min) statistics by calculation method.
| Average event-level chest compression rate statistics | ||||
|---|---|---|---|---|
| Rate Calculation Method | ||||
| 1-Second Method | 2-Second Method | 3-Second Method | Trimmed Mean Method | |
| Statistic1 | ||||
| Mean | 119.5 | 118.9 | 118.7 | 118.8 |
| Median | 118.9 | 118.4 | 118.1 | 118.4 |
| Minimum | 107.9 | 107.0 | 106.9 | 106.9 |
| Maximum | 132.7 | 132.2 | 132.0 | 132.0 |
n = 200 events
Statistics (mean, median, minimum, maximum) are first calculated on an event level (e.g. for an event consisting of 8 30-second epochs, the Minimum Statistic is defined as the lowest of the 8 epoch-level chest compression rates). The statistics are then averaged across all events.
Table 2:
Guideline-compliant status across calculation methods.
| Guideline-compliant status across calculation methods | |||||
|---|---|---|---|---|---|
| Rate Calculation Method | |||||
| 1-Second Method | 2-Second method | 3-Second Method | Trimmed Mean Method | All Methods2 | |
| Events (n=200) | 74 (37.0%) | 69 (34.5%) | 72 (36.0%) | 73 (36.5%) | 67 (33.5%) |
| Epochs (n=1856) | 716 (38.6%) | 705 (38.0%) | 717 (38.6%) | 706 (38.0%) | 655 (35.3%) |
Guideline-compliant = 100–120 compressions per minute.
Indicates the number and percentage of events/epochs which were considered Guideline-compliant using all four methods.
The effect of rate calculation method on whether or not events and epochs changed Guideline-compliant status or whether or not the calculated rate changed by ± 5 CCs/min is detailed in Table 3. When compared to the reference method, number of events changing Guideline-compliant status ranged from seven (4%, 2-second method) to 10 (5%, 3-second method) and the number of events that had a clinically important difference ranged from three (2%, 2-second method) to 11 (6%, trimmed mean method). When comparing across all methods (i.e., a difference between any of the methods used), 14 events (7.0%) and 114 epochs (6.1%) changed Guideline-compliant status while 15 events (7.5%) and 162 epochs (8.7%) had a clinically important change in the calculated chest compression rate.
Table 3:
Effect of rate calculation method on CPR quality classification status.
| Effect of rate calculation method on CPR quality classification status | ||||
|---|---|---|---|---|
| Rate Calculation Method | ||||
| 2-Second method1 | 3-Second Method1 | Trimmed Mean Method1 | All Methods2 | |
| Events (n=200) | ||||
| Guideline-compliant status change | 7 (3.5%) | 10 (5.0%) | 9 (4.5%) | 14 (7.0%) |
| ≥ 5 bpm difference | 3 (1.5%) | 4 (2.0%) | 11 (5.5%) | 15 (7.5%) |
| Epochs (n=1856) | ||||
| Guideline-compliant status change | 31 (1.7%) | 49 (2.6%) | 80 (4.3%) | 114 (6.1%) |
| ≥ 5 bpm difference | 47 (2.5%) | 56 (3.0%) | 108 (5.8%) | 162 (8.7%) |
Reference method: Instantaneous rate with ≥ 1 sec pause (1-second method).
Denotes Guideline-compliant status change (top line) or average change ≥ 5 compressions per minute (bottom line) between any two of the calculation methods.
DISCUSSION:
In this study of 200 ICU pediatric cardiac arrest events, chest compression rates were similar across four known chest compression rate calculation methods. However, across methods, 7.0% of CPR events and 6.1% of data epochs changed Guideline-compliant status. In addition, 7.5% of events and 8.7% of epochs had a clinically important change in the calculated chest compression rate. As such, this study demonstrated that the clinical interpretation of CPR quality data can be influenced by the chest compression rate calculation method utilized. Lack of a uniform calculation method introduces potential noise into the resuscitation science literature, which supports evidence-based CPR targets for children and adults. This study quantified the magnitude of the variability across the currently utilized calculation methods, thereby highlighting the relevance of this issue.
Our decision to utilize a minimum interruption length of one second as the reference method deserves comment. In 2007, Kramer-Johansen, et al., published the results of an international consensus working group whose objective was to propose common definitions for reporting variables of CPR quality. In this report, a minimum interruption length of 1.5 seconds was proposed (i.e., a minimum compression rate of 40 CCs/min).18 This report was consistent with CPR quality publications from the early 2000s at which time compression rates < 60 CCs/min were not uncommon.23 In short, the definition matched the quality of the reporting period. However, after more than a decade of science and resuscitation quality improvement endeavors focused on improving CPR quality to rescue more patients from cardiac arrest, compression rates < 60 CCs/min are exceedingly rare, especially during pediatric in-hospital resuscitations.24,25 As such, we contend that interruptions of 1–2 seconds (corresponding to calculated rates of 30–60 CCs/min) are more likely to represent interruptions than actual chest compression rate. Moreover, there have been technological advancements that allow for rhythm analysis during active chest compressions, resulting in even less need to interrupt chest compressions.26 While one-second interruptions may not be common in all clinical scenarios, resuscitation science has established that it is feasible to check an electrocardiogram rhythm27 and change compressing providers28 in as little as one second.
Similarly, there is physiologic support for a minimum interruption length of one second. Early reports of CPR quality suggested a threshold in regard to the effect of chest compression rate on event survival. Specifically, a rate of ~80 CCs/min appeared necessary to achieve ROSC.23 As such, setting the trigger to receive feedback at rates of 40 CCs/min may permit detrimental effects on patient outcomes. Finally, a recent report published in Resuscitation established that that diastolic blood pressure decreases significantly in the first second of an interruption (consistent with historical work in animal studies).19,29 Considering this data, we advocate for establishing a higher minimum rate that is more consistent with contemporary CPR literature. In tandem, a shorter minimum interruption length would avoid the potential of missed opportunities for feedback when rates decline to levels associated with poor event outcomes.
There are strengths of this study worth noting. The use of invasive arterial catheter blood pressure data ensured accurate chest compression identification. In contrast, CPR recording defibrillators that use accelerometer-based technology to detect compression are limited by the lower detection limit for a compression to be registered. Given the smaller chest diameters of pediatric patients, it is not uncommon for these algorithms to miss compressions performed on young children. By completing our analysis using arterial line data, we have ensured detection of all delivered compressions. Additionally, the robust infrastructure of the CPCCRN network ensured the collection of all cardiac arrest events, thereby limiting selection bias in this dataset.
This study has limitations. First, although CPR quality recording defibrillators may have been deployed during these arrests, we were not able to determine what prospective feedback providers were receiving to be able to make any comparisons to our retrospectively calculated CC rates. Second, CPCCRN has a documented interest in monitoring and improving CPR quality. A previous study from this network showed that 62% of patients achieved and maintained blood pressure targets associated with improved survival.30 In this same previous dataset, only 6% of epochs (60-second averages) had a recorded rate of less than 100 CCs/min.24 In the data used for this analysis, the median number of pauses per event was 2 [IQR: 0, 5]. The near absence of “low” quality CPR (e.g., long interruptions) limited the variability in calculated chest compression rates that we could detect. Third, although the percentage of events and epochs changing Guideline-compliant categories or having a clinically important change exceeded 5% in almost all cases, to protect the integrity of the main trial, we were not permitted to evaluate whether the population of patients who changed categories were different in relation to other cardiac arrest variables associated with outcomes (e.g., age31, time of day32) or if there were actual outcome differences. Therefore, the effect that this variability has on studies designed to associate rate with outcomes remains unanswered. Finally, we acknowledge that our proposed minimal interruption length of one second may not seem clinically feasible in all settings (e.g., out-of-hospital cardiac arrest, extracorporeal membrane oxygenation CPR). However, given the detrimental effects on physiology and outcomes outlined above, as well as the technological and educational advancements that have eliminated longer pauses in several series, raising the CPR quality standard by establishing a minimum interruption length of one second for use in all clinical settings may save lives in the future.
CONCLUSION:
In this study of pediatric cardiac arrest patients, four different published methods for calculating chest compression rate resulted in similar mean chest compression rates. However, 7.0% of patients changed Guideline-compliant status across methods. As such, the use of a standard calculation method may reduce variability in the field of resuscitation science. We advocate for the 1-second method as the standard method for calculating CC rate. A minimum interruption length of one second is not only feasible due to technological and educational advancements, but also supported by physiologic and outcome studies across both pediatric and adult studies. This standard method is capable of reducing variability in the calculation of chest compression rate and creating more consistent results across the field of resuscitation science.
ACKNOWLEDGEMENTS:
The authors would like to thank Dr. Robert F. Tamburro and Tammara L. Jenkins for their leadership of the CPCCRN. In addition to the listed authors, the following ICU-RESUS investigators helped to support this project: Athena F. Zuppa, Martha Sisko, David L. Wessel, Elyse Tomanio, Mark W. Hall, Lisa Steele, Sabrina Heidemann, Ann Pawluszka, Todd Carpenter, Ruth Grosskreuz, Tina Day, Anne McKenzie, Ericka L. Fink, Leighann Koch, Theresa Kirkpatrick, Tanaya Deshmukh, Ramany John, Kylee Arbogast, Melissa Pederson, Russel Telford, and Whitney Coleman.
CONFLICT OF INTEREST STATEMENT:
Financial support was provided through the Department of Anesthesiology and Critical Care Medicine at The Children’s Hospital of Philadelphia, the ICU-RESUScitation Project, and CPCCRN.
Supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services: R01HL131544, UG1HD063108, and U01HD049934.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Young KD, Seidel JS. Pediatric Cardiopulmonary Resuscitation: A Collective Review. Annals of Emergency Medicine 1999;33(2):195–205. [DOI] [PubMed] [Google Scholar]
- 2.Slonim AD, Patel KM, Ruttimann UE, Pollack MM. Cardiopulmonary Resuscitation in Pediatric Intensive Care Units. Critical Care Medicine 1997;25(12):1951–1955. [DOI] [PubMed] [Google Scholar]
- 3.Girotra S, Chan PS, Bradley SM. Post-Resuscitation Care Following out-of-Hospital and in-Hospital Cardiac Arrest. Heart 2015;101(24):1943–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girotra S, Spertus JA, Li Y, et al. Survival Trends in Pediatric In-Hospital Cardiac Arrests. Circulation: Cardiovascular Quality and Outcomes 2013;6(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in Survival After In-Hospital Cardiac Arrest. Survey of Anesthesiology 2013;57(2): 73–74. [Google Scholar]
- 6.Neumar RW, Eigel B, Callaway CW, et al. American Heart Association Response to the 2015 Institute of Medicine Report on Strategies to Improve Cardiac Arrest Survival. Circulation 2015;132(11):1049–1070. [DOI] [PubMed] [Google Scholar]
- 7.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med 2016:44(4):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudson JD, Neish SR, Cabrera AG, et al. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: an analysis of the Kids’ Inpatient Database. Crit Care Med 2012:40(11):2940–4. [DOI] [PubMed] [Google Scholar]
- 9.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary Resuscitation quality, improving cardiac resuscitation outcomes inside and outside the hospital: a consensus statement from the American Heart Association. Circulation 2013;128:417–35. [DOI] [PubMed] [Google Scholar]
- 10.Idris AH, Guffey D, Pepe PE, et al. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med 2015;43(4):840–8. [DOI] [PubMed] [Google Scholar]
- 11.Stiell IG, Brown SP, Nichol G, et al. What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitations of adult patients? Circulation 2014;130(22):1962–70 [DOI] [PubMed] [Google Scholar]
- 12.Cheskes S, Common MR, Byers AP, et al. The association between chest compression release velocity and outcomes from out-of-hospital cardiac arrest. Resuscitation 2015;86:38–43. [DOI] [PubMed] [Google Scholar]
- 13.Reeder RW, Girling A, Wolfe H, et al. Improving Outcomes after Pediatric Cardiac Arrest – the ICU-Resuscitation Project: Study Protocol for a Randomized Controlled Trial. Trials 2018;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willson DF, Dean JM, Newth C, et al. Collaborative Pediatric Critical Care Research Network (CPCCRN)*. Pediatric Critical Care Medicine 2006;7(4):301–307. [DOI] [PubMed] [Google Scholar]
- 15.Niles D, Sutton RM, Donoghue A, et al. “Rolling Refreshers”: A Novel Approach to Maintain CPR Psychomotor Skill Competence. Resuscitation 2009;80(8):909–912. [DOI] [PubMed] [Google Scholar]
- 16.Sutton RM, Niles D, Meaney PA, et al. Low-Dose, High-Frequency CPR Training Improves Skill Retention of In-Hospital Pediatric Providers. Pediatrics 2011;128(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe H, Zebuhr C, Topjian AA, et al. Interdisciplinary ICU Cardiac Arrest Debriefing Improves Survival Outcomes. Critical Care Medicine 2014;42(7):1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer-Johansen J, Edelson DP, Losert H, Köhler K, Abella BS. Uniform Reporting of Measured Quality of Cardiopulmonary Resuscitation (CPR). Resuscitation 2007;74(3): 406–417. [DOI] [PubMed] [Google Scholar]
- 19.Morgan RW, Landis WP, Marquez A, et al. Hemodynamic Effects of Chest Compression Interruptions during Pediatric in-Hospital Cardiopulmonary Resuscitation. Resuscitation 2019;139:1–8. [DOI] [PubMed] [Google Scholar]
- 20.Idris AH, Guffey D, Pepe PE, et al. Chest Compression Rates and Survival Following Out-of-Hospital Cardiac Arrest. Critical Care Medicine 2015;43(4): 840–848. [DOI] [PubMed] [Google Scholar]
- 21.Brouwer TF, Walkler RG, Chapman FW, Koster RW. Association between Chest Compression Interruptions and Clinical Outcomes of Ventricular Fibrillation Out-of-Hospital Cardiac Arrest. Circulation 2015:32(11):1030–1037. [DOI] [PubMed] [Google Scholar]
- 22.Travers AH, Perkins GD, Berg RA, et al. “Part 3: Adult Basic Life Support and Automated External Defibrillation. Circulation 2015;132(16). [DOI] [PubMed] [Google Scholar]
- 23.Abella BS, Sandbo N, Vassilatos P, et al. Chest Compression Rates During Cardiopulmonary Resuscitation Are Suboptimal: a Prospective Study During in-hospital Cardiac Arrest. Circulation 2005;111(4):428–434. [DOI] [PubMed] [Google Scholar]
- 24.Sutton RM, Reeder RW, Landis WP, et al. Chest compression rates and pediatric in-hospital cardiac arrest survival outcomes. Resuscitation 2018;130:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton RM, French B, Nishisaki A, et al. American Heart Association cardiopulmonary resuscitation quality targets are associated with improved arterial blood pressure during pediatric cardiac arrest. Resuscitation 2013;84:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fumagalli F, Silver AE, Tan Q, Zaidi N, Ristagno G. Cardiac Rhythm Analysis during Ongoing Cardiopulmonary Resuscitation Using the Analysis During Compressions with Fast Reconfirmation Technology. Heart Rhythm 2018;15(2): 248–255. [DOI] [PubMed] [Google Scholar]
- 27.Partridge R, Tan Q, Silcer A, Riley M, Geheb F, Raymond R. Rhythm Analysis and Charging during Chest Compressions Reduces Compression Pause Time. Resuscitation 2015;90:133–137. [DOI] [PubMed] [Google Scholar]
- 28.Sutton RM, Maltese MR, Niles D, et al. Quantitative Analysis of Chest Compression Interruptions during in-Hospital Resuscitation of Older Children and Adolescents. Resuscitation 2009;80(11):1259–1263. [DOI] [PubMed] [Google Scholar]
- 29.Berg RA, Sanders AB, Kern KB, et al. Adverse Hemodynamic Effects of Interrupting Chest Compressions for Rescue Breathing During Cardiopulmonary Resuscitation for Ventricular Fibrillation Cardiac Arrest. Circulation 2001;104(20): 2465–2470. [DOI] [PubMed] [Google Scholar]
- 30.Berg RA, Sutton RM, Reeder RW, et al. Association between Diastolic Blood Pressure during Pediatric In-Hospital Cardiopulmonary Resuscitation and Survival. Circulation 2018;137(17): 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meaney PA, Nadkarni VM, Cook EF, et al. Higher Survival Rates among Younger Patients after Pediatric Intensive Care Unit Cardiac Arrests. Pediatrics 2006;118(6):2424–2433. [DOI] [PubMed] [Google Scholar]
- 32.Bhanji F, Topjian AA, Nadkarni VM, et al. Survival Rates Following Pediatric In-Hospital Cardiac Arrests During Nights and Weekends. JAMA Pediatrics 2017;171(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]