Abstract
Introduction:
COVID-19 is a new viral illness that can affect the lungs and airways with lethal consequences leading to the death of the patients. The ACE2 receptors were widely disturbed among body tissues such as lung, kidney, small intestine, heart, and others in different percent and considered a target for the nCOVID-19 virus. S-protein of the virus was binding to ACE2 receptors caused downregulation of endogenous anti-viral mediators, upregulation of NF-κB pathway, ROS and pro-apoptotic protein. Nrf2 was a transcription factor that’s play a role in generation of anti-oxidant enzymes.
Aim:
To describe and establish role of Nrf2 activators for treatment COVID-19 positive patients.
Methods:
We used method of analysis of the published papers with described studies about COVID-19 connected with pharmacological issues and aspects which are included in global fighting against COVID-19 infection, and how using DMF (Nrf2 activator) in clinical trial for nCOVID-19 produce positive effects in patients for reduce lung alveolar cells damage.
Results:
we are found that Nrf2 activators an important medication that’s have a role in reduce viral pathogenesis via inhibit virus entry through induce SPLI gene expression as well as inhibit TRMPSS2, upregulation of ACE2 that’s make a competition with the virus on binding site, induce gene expression of anti-viral mediators such as RIG-1 and INFs, induce anti-oxidant enzymes, also they have a role in inhibit NF-κB pathway, inhibit both apoptosis proteins and gene expression of TLRs.
Conclusion:
We are concluded that use DMF (Nrf2 activator) in clinical trial for nCOVID-19 positive patients to reduce lung alveolar cells damage.
Keywords: nCOVID-19, Nrf2 activators, ACE2, RIG-1, NF-κB, ROS
1. INTRODUCTION
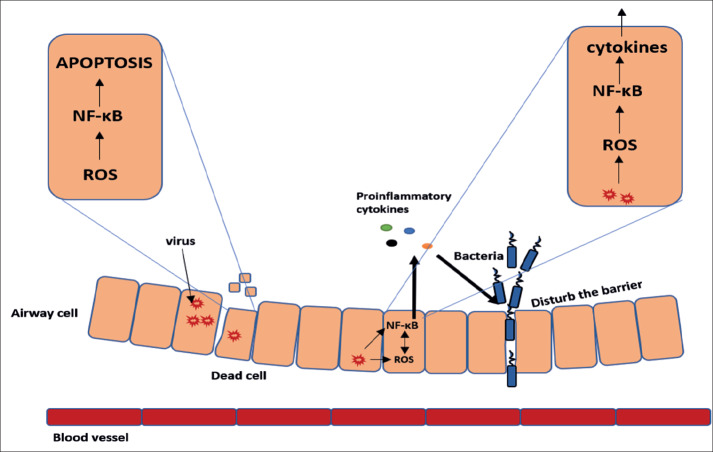
Corona virus diseases 19 (COVID 19) has become a public health problem and its prevalence varies from country to country but it is present in 195 countries of the world. The risk of death from COVID-19 varies from 1% in Germany to 11% in Italy indicating high fatality among subjects above 60 years (1). In humans, Transmembrane serine protease (TMPRSS2) is a protease under transmembrane serine protease family, TMPRSS2 was disturbed widely in the upper airways, bronchi, and lung. TMPRSS2 was cleave coronavirus fusion glycoproteins, the spike protein, such as severe acute respiratory syndrome-related coronavirus (SARS-CoV) as well as Middle East respiratory syndrome-related coronavirus (MERS-CoV) (2) facilitated entry into the host cell after binding to ACE2 receptor that’s make the virus entry to increased 2.6-fold in the presence of TMPRSS2, in the absence of TMPRSS2, the entry was decreased five-fold in absent it and this is accompanied by a nine-fold increase of the levels of the RNA and SARS-CoV in cells, expressing active TMPRSS2 compared to those cells with inactive TMPRSS2 (3). It is now known that ACE2 expression in the lung tissues of Asian population is much higher than white Caucasians and African-American (4). ACE2 is a carboxypeptidase which convert Ang I to angiotensin (1–9) and Ang II to angiotensin (1–7). Recent studies and analyses indicate SARS-coronavirus (SARS-CoV) and the novel coronavirus (2019-nCoV) have been target the angiotensin-converting enzyme 2 via spike proteins in the receptor-binding domain that maintains van der Waals forces and also showed that 2019-nCoV receptor-binding domain (RBD) stronger than SARS-CoV due to glutamine residue 479 can recognized lysine 31 on the human ACE2 receptor that 2019-nCoV to transmit from person to person (6, 7). After entering of the virus to the cells, begin the host defense mechanisms such as Retinoic acid-Inducible Gene I (RIG-I)-like receptors (RLRs) was directly recognizes and binds to virus, then ubiquitin by the E3 ligase leading to the phosphorylation and activation of NF-κB, In turn, this lead to the production of IFN-β which has antiviral activity (8). The N protein of SARS-CoV has been inhibits the synthesis of RIG-I ubiquitination and IFN-β (9, 10). ACE2 protein was downregulated during SARS-CoV infection that lead to lung injury due to ACE2 activity has negative regulatory effect on RAS in protection of the lung from injury (11) and ACE2 down regulation can also enhance NF–κB phosphorylation and further degrades a number of processes including IκBα protein, translocation, DNA binding, gene expression of pro-apoptotic proteins and cycline D1. As a result, pro-inflammatory cytokines such as IL-8 and IL-6 cytokines are secreted in large quantities. IL-8 and IL-6, in turn, stimulate monocytes, macrophages, NK, neutrophil and also monocyte migration to the site of the infection (12). Ang II has a proinflammatory properties via enhance NF-κB gene expression while ACE2 has an anti-inflammatory action by counteracting the actions of Ang II apoptosis and IL–1β and TNF–α secretion attenuated by ACE2 overexpression (13). One of the major pathogenic mechanisms for virus-induced inflammatory and tissue injury was generation of free radicals. Oxidative stress was induced after viruses entering the host cell to facilitate their replication (14, 15) On other hand increase ACE2 level may competitive with 2019-nCoV on binding (16). In a study by Li F et. al (2005) show that ACE2 was injection in a soluble form can slowdown entering of viral into host cells (7). Similarly, Zhang R et. al show that ACE2 has protect the lung from injury (17) (Figure 1).
Figure 1. Schematic diagram showing the pathogenesis of nCOVID-19 induce inflammation and cell damage via activation of NF-κB pathway and generation of ROS that lead to production of pro-inflammatory cytokines as well as cause secondary bacterial infection (17).
2. AIM
The aim of the study was to describe role of Nrf2 activators for treatment COVID-19 positive patients.
3. METHODS
We used method of analysis of the published papers with described studies about COVID-19 connected with pharmacological issues and aspects which are included in global fighting against COVID-19 infection, and how using DMF (Nrf2 activator) in clinical trial for nCOVID-19 produce positive effects in patients for reduce lung alveolar cells damage.
4. RESULTS
Antiviral activity of Nrf2 activators
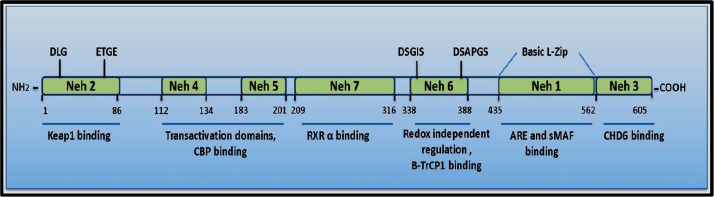
Nrf2 is a member of the cap‘n’collar (CNC) basic-leucine-zipper transcription-factor, Nrf2 is considered as a master regulator of oxidative stress through enhance expression of anti-oxidant genes of glutathione and thioredoxin-antioxidant systems and also Aldo-keto reductase, and NAD (P) H quinine-oxide-reductase-1, HO-1, NQO-1, SODs, GPx, GSTs, GCL, CAT, TRX Nrf2 system has anti-inflammatory, anti-apoptotic, and anti-oxidant through different pathways (18, 19). Nrf2 is a member of the CNC family which includes AP-1, NFE-2, Nrf-1, Nrf-2, Nrf-3, Bach-1 and -2, small-Mafs, and CREB/ATF and it can be divided into seven domains called Nrf2–ECH-homology (Neh) domains Neh-1 to Neh-7 (20) (Figure 2).
Figure 2. Diagram showing the structure of human Nrf-2 (20).
Inhibit virus penetration
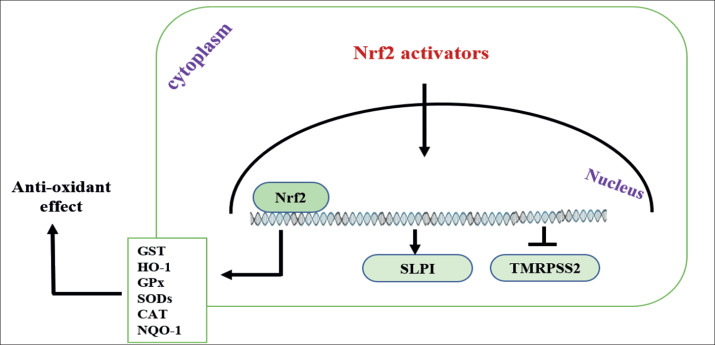
Nrf2 activators can increased anti-proteases secretion such as the secretory leukocyte protease inhibitor (SLPI) that inhibit serine protease activity, It can also protect target cell against viral infection by binding to its promoter and thus decreases TMPRSS2 expression (21), Hence, these activators can protect the cell against the entry of viruses into respiratory epithelial cells and thereby decreasing their replication (Figure 3). In a study by Iizuka et al. 2005, they found that SLPI not express in Nrf2-knockout mice that made the cells predisposed to inflammation by disturb protease/anti-protease balance, and they found SLPI gene expression was increase when activation of Nrf2 not only by the anti-oxidant effect, but by maintenance the protease/anti-protease balance (22). Similarly, Ling JX et al. 2012 showed that oral administration of EGCG (Nrf2 activator) improved the survival by decreased the entry and replication of viral pneumonia in the lungs in same equivalent to oral administration of oseltamivir.
Figure 3. Nrf2 activator wasinducing gene expression of anti-oxidant enzymes and SLPI antiprotease protein in addition to inhibit gene expression of TMRPSS2, therefore inhibit virus entry and replication (26).
Alveolar protection role
Alveolar epithelial type II cells were considered the more abundant cells in lung that express ACE2 which about 83% (23). Moreover, ACE2 protein is expressed in an abundant quantity on the apical airway epithelia and moreover, the expression ACE2 is more likely to be link with the epithelia differentiation. As such, well differentiated cells are more likely to be infected by SARS-CoV (24). Nrf2 was prevent the alteration in lung injury by increased level of ACE2 and expression of AT2R. On other hand, Nrf2 was decreased serum levels of Ang II and the expression of AT1R by suppressed oxidative stress, inflammation, and fibrosis induced by Ang II/AT1R axis (25) Nrf2 was play a role in induction of host defense mechanism through induction of interferon gene expression that have antiviral activity. In their study, Kesic et al. show that Nrf2 activators induced the expression of antiviral genes of RIG-I and IFN-β (26).
Anti-inflammatory and anti-apoptotic activities
Nrf2 pathway inhibits the NF-κB activity through the degradation of IKKβ by ubiquitination and induction gene transcription when NF-κB react with Nrf2 transcriptional-CBP. Activation of the Nrf2- dependent anti-oxidant genes suppress the production of inflammatory factors such as TNF-α, IL-6, MCP-1, MIP2 (27) in addition to downregulation of the selectins and VCAM-1, adhesion molecules (28). Nrf2 was play an important role in expression of TLRs through inhibit expression of these receptors (29). Li et al. observed that Nrf2 knockout mice increases the expression of the TLR4 as compared with the wild type mice (30). Yanzhe Wang et al. (2018) observed that Nrf2 activator treatment decreases number TLR-positive cells as compared with control when measured by immunohistochemistry (31).
The activation of Nrf2 can improve phagocytosis and clearance by alveolar macrophages through a mechanism that is independent of the intracellular-antioxidant glutathione (32). Nrf2 is the major regulatory system of GSH via controlling and modulatory release of γ-glutamyl cysteine ligase (33). Nrf2 induces production a number of anti-oxidant enzymes such as NQO1, HO-1, SODs, Grx1, and Trx1 (34) and reduced upregulated expression of HO-1, Bcl-2/Bax, decreased expression and activity of caspases 3, 9 (35).
Dimethyl fumarate (DMF)
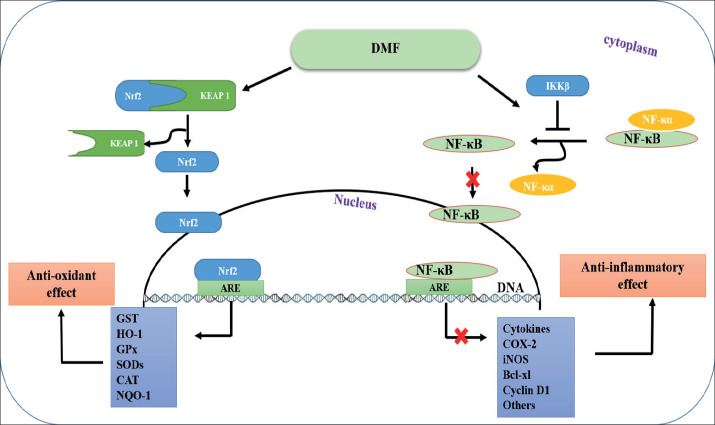
DMF a fumarate ester, is a recently FDA approved drug for the treatment of multiple sclerosis (MS) and psoriasis under market name Ticfedra (36). DMF is a potent anti-oxidant and anti-inflammatory agent via inhibits the NF-κB pathway through binding and activation of the IKKβ at Cys-179. This binding inhibits the release of NF-κB wfrom its complex (NF-κB-IκB) in the cytoplasm, thereby inhibiting downstream pro-inflammatory signaling pathways (37). In addition, it’s modulation of the glutathione system and enhancement of cellular response to oxidative stress (38). The molecular mechanism of action of the DMF was activation of Nrf2 and blocking the activity of NF-κB thereby plays an important role in the suppress of inflammation. In the absence of DMF, Nrf2 binds to Keap1 which makes it subjected to ubiquitination. DMF was disrupt Keap1-Nrf2 complex at its cysteine residues causes the release of Nrf2 and translocation of Nrf2 to the nucleus (37). DMF is a potent inhibitor of the NF-κB pathway through binding and activation of the IKKβ at Cys-179. This binding inhibits the release of NF-κB from its complex (NF-κB-IκB) in the cytoplasm, thereby inhibiting downstream pro-inflammatory signaling pathways (38) (Figure 4).
Figure 4. Schematic flow diagram showing the proposed mechanism (s) of action of DMF (38).
5. CONCLUSION
In conclusion and from the supporting literature review, it is tempting to suggest that DMF may be able to inhibit the entry of nCOVID-19 into the alveolar cells of the lungs via an increase in anti-protease (SPLI) and a concurrent decrease in the protease protein (TRMPSS2). Furthermore, DMF can decrease inflammation and ROS through the inhibition of NF-kB by inducing anti-oxidant enzymes thereby down regulating TLRs and increasing anti-viral mediators such as RIG-1 and INFs via the induction of gene expression, an increase ACE2 enzyme and a decrease in AT1R. As such, DMF should be recommended in clinical trials in patients with nCOVID-19.
Authors contribution:
Each author were included in all steps of preparation this article. Final proof reading was made by the first author.
Conflict of interest:
There is no conflict of interest
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Masic I, Naser N, Zildzic M. Public Health Aspects of COVID-19 Infection with Focus on Cardiovascular Diseases. Mater Sociomed. 2020 Mar;32(1):71–76. doi: 10.5455/msm.2020.32.71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. Journal of Virology. 2019:93. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen LW, Mao HJ, Wu YL, Tanaka Y, Zhang W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circulation research. 2000;87(5):e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 6.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 8.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annual review of immunology. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus research. 2008;133(1):101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Li W, Gao T, Cui Y, Jin Y, Li P, et al. The Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Inhibits Type I Interferon Production by Interfering with TRIM25-Mediated RIG-I Ubiquitination. J Virol. 2017;91(8):e02143–16. doi: 10.1128/JVI.02143-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature medicine. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dosch SF, Mahajan SD, Collins AR. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Research. 2009;142(1):19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehm M, Nabel EG. Angiotensin-converting enzyme 2—a new cardiac regulator. New England Journal of Medicine. 2002;347(22):1795–1797. doi: 10.1056/NEJMcibr022472. [DOI] [PubMed] [Google Scholar]
- 14.Paracha UZ, Fatima K, Alqahtani M, Chaudhary A, Abuzenadah A, Damanhouri G, et al. Oxidative stress and hepatitis C virus. Virology journal. 2013;10(1):251. doi: 10.1186/1743-422X-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salihefendic N, Zildzic M, Ahmetagic S. Acute Respiratory Distress Syndrome (ARDS) from Endemic Influenza A/H1N1: Prehospital Management. Med Arch. 2015;69(1):62–63. doi: 10.5455/medarh.2015.69.62-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Pan Y, Fanelli V, Wu S, Luo AA, Islam D, et al. Mechanical stress and the induction of lung fibrosis via the midkine signaling pathway. American journal of respiratory and critical care medicine. 2015;192(3):315–323. doi: 10.1164/rccm.201412-2326OC. [DOI] [PubMed] [Google Scholar]
- 18.Al-Mudhaffer R, Abbas Al-Huseini L, Hassan S, Hadi N. Bardoxolone Ameliorates Cerebral Ischemia/ Reperfusion Injury in Male Rats. Africa Health Research Organization. 2019;22:122–130. [Google Scholar]
- 19.Al-Huseini LMA, Al-Mudhaffer RH, Hassan SM, Hadi NR. DMF Ameliorating Cerebral Ischemia/ Reperfusion Injury in Male Rats. Systematic Reviews in Pharmacy. 2019;10(1):206–213. [Google Scholar]
- 20.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Schultz MA, Hagan SS, Datta A, Zhang Y, Freeman ML, Sikka SC, et al. Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells. PLoS One. 2014;9(1):e87204–e. doi: 10.1371/journal.pone.0087204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, et al. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes to cells: devoted to molecular & cellular mechanisms. 2005;10(12):1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. 2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. Journal of virology. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang I-A, Kim EN, Lim JH, Kim MY, Ban TH, Yoon HE, et al. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients. 2018;10(11):1741. doi: 10.3390/nu10111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med. 2011;51(2):444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochemical and Biophysical Research Communications. 2006;351(4):883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. The Journal of Immunology. 2004;172(6):3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mudhaffer RH, Al-Huseini LMA, Hassan SM, Hadi NR. Bardoxolone Ameliorates Cerebral Ischemia/Reperfusion Injury in Male Rats
- 30.Wang Y, Li L, Deng S, Liu F, He Z. Ursolic Acid Ameliorates Inflammation in Cerebral Ischemia and Reperfusion Injury Possibly via High Mobility Group Box 1/Toll-Like Receptor 4/NFkappaB Pathway. Frontiers in neurology. 2018;9:253. doi: 10.3389/fneur.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Li L, Deng S, Liu F, He Z. Ursolic Acid Ameliorates Inflammation in Cerebral Ischemia and Reperfusion Injury Possibly via High Mobility Group Box 1/Toll-Like Receptor 4/NFκB Pathway. Front Neurol. 2018;9:253. doi: 10.3389/fneur.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3(78):78ra32–78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correa F, Ljunggren E, Mallard C, Nilsson M, Weber SG, Sandberg M. The Nrf2-inducible antioxidant defense in astrocytes can be both up- and down-regulated by activated microglia: Involvement of p38 MAPK. Glia. 2011;59(5):785–799. doi: 10.1002/glia.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biology and Medicine. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan H, Wang H, Zhu L, Wang X, Cong Z, Sun K, et al. The involvement of Nrf2-ARE pathway in regulation of apoptosis in human glioblastoma cell U251. Neurological research. 2013;35(1):71–78. doi: 10.1179/1743132812Y.0000000094. [DOI] [PubMed] [Google Scholar]
- 36.Fox R. The New England Journal of Medicine publishes pivotal data demonstrating efficacy and safety of oral BG-12 (dimethyl fumarate) in multiple sclerosis. Can J Neurosci Nurs. 2012;34(3):7–11. [PubMed] [Google Scholar]
- 37.Gold R, Linker RA, Stangel M. Fumaric acid and its esters: an emerging treatment for multiple sclerosis with antioxidative mechanism of action. Clinical immunology (Orlando, Fla) 2012;142(1):44–48. doi: 10.1016/j.clim.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Dibbert S, Clement B, Skak-Nielsen T, Mrowietz U, Rostami-Yazdi M. Detection of fumarate-glutathione adducts in the portal vein blood of rats: evidence for rapid dimethylfumarate metabolism. Archives of dermatological research. 2013;305(5):447–451. doi: 10.1007/s00403-013-1332-y. [DOI] [PubMed] [Google Scholar]