Abstract
Introduction:
Physical activity is one important factor in the nervous system of animals, it may affect the structure also the function of the brain. Regular aerobic exercises have a good effect on the whole body and thus improves the sense of well-being.
Aim:
The aim of this experiment was to evaluate the effect of aerobic physical activity in white laboratory rats for 21 days.
Methods:
The experimental animals used were twenty Wistar rats, divided into 2 groups of 10 animals they were subjected to forced swim test for 21 days for 60-90 min swim. The evaluation of levels of Adrenaline was performed on 1st, 7th, 14th day and on the last day of the experiment day 21 using ELISA kit protocol, also body mass was compared between groups.
Results:
Our results showed that inducing aerobic physical activity for 21 days on the rats affects their levels of adrenaline. Comparatively, the control group of rats had significantly higher levels of adrenalin compared with day 21 (p=0.435) but lower compared with day 7 and 14 (p=0.231). There was also a difference in body mass which demonstrates adaptability to the surroundings and better coping with physical stress.
Conclusion:
Regular aerobic activity for 21 days, for 60-90 min swim has a positive impact on adrenaline level also this aerobic exercise protocol could have a positive impact on reducing and maintaining body weight, thus preventing overweight.
Keywords: adrenaline, aerobic activity, physical stress, swim
1. INTRODUCTION
Regular physical activity and exercises have a very high impact on well being and very effective in the many-body system and this improves general fitness (1, 2) and overall health (3). There are many studies which have observed the effect of exercise on the immune and hormonal system, these studies have been conducted mainly on healthy people and the effects of exercise on these systems have been observed. The anatomical structures that mediate the stress response are found in both the central nervous system (4) and peripheral tissues. Except for the hypothalamic-pituitary-adrenal axis (HPA), several other structures play important roles in the regulation of adaptive responses to stress such as brain stem noradrenergic neurons, sympathetic adrenomedullary circuits, and parasympathetic systems (5).
Understanding the effect of stress hormones will give an idea of how stressors could influence homeostasis (6). To evaluate the stress hormones in experimental studies the most frequent used are rats because they are very sensitive to stress (7) also the degree of activation of the HPA is related to the intensity of stress (6).
Animal models of studies usually involve physical activities that simulate the human beings that are exposed, the most frequently used are treadmill running and swimming. Exercise intensity directly governs the quantity of adrenal medulla secretion. Exercise duration also influences catecholamine response as revealed by the direct relationship between plasma epinephrine and norepinephrine levels and mileage run (8). Adrenaline and noradrenaline like glucocorticoids may help the body to avoid or resist stress conditions, these hormones produce the body’s ”fight or flight” response to stress. It is well known that Adrenaline affects the cardiovascular system. If the level of Adrenaline is high this will produce a rapid powerful vasopressor effect, increasing heart rate, the force of contraction, respiratory rate and increasing blood flow towards the brain, heart and skeletal muscle and away from skin and kidneys (9-11).
The link between physical activity, exercise and health outcomes is well established (13). The effect of the exercises is well known for the positive effect on the central nervous system of animals (14), and many studies have reported the impact that the exercises have on morphological and behavioral aspects, exercise also induces antidepressant effects (12, 15) and enhances learning and memory (16).
2. AIM
The purpose of this experiment was to evaluate the effect of regular aerobic physical activity in adrenaline levels in white laboratory rats for 21 days.
3. MATERIAL AND METHODS
All experimental procedures were performed in accordance with the Manual for Care and Use of Laboratory Animals, approved by the Macedonian Center for Bioethics. The protocols were approved by the Ethics Committee for Animals at the University “St. Kiril and Metodij” Skopje, North Macedonia (nr. 2401-592, 2017) according to the recommendations for biomedical research involving animals, issued by the Council of International Organizations for Medical Sciences. The anesthetists were applied in accordance with the standards given in the EU Directive, Directive 86/609 / EEC. During the study, the animals were exposed to standard food and water regime available ad libitum and stayed in a room under a constant light regime of 12 hours (06:00 to 18:00) light and 12 hours dark, at a thermoneutral temperature of 26° C. There were used 20 female, white, laboratory rats from the Wistar strain, (n = 64) at the age of 4-5 months, with a body mass of 220 ± 20g. The experiment lasted for 22 days.
Experimental Procedures:
The experimental animal used were 20 Wistar rats, divided into 2 groups of 10 animals. The first group was the control group and the second group of rats that swam. The water temperature was measured before and after swimming. On the 1st, 7th and 14th day of the experiment, blood was taken from the tail of the rat, with its incision, in order to determine the level of adrenaline. The remaining 7 days to the end of the experiment, 5 rats from the examined group continued to swim. The last day (21-st day), the rats were sacrificed and blood from the abdominal aorta was collected. Serum obtained was used for analyzes of the amount of adrenaline, cortisol and serotonin. Physical stress was caused to the rats by being put in a water barrel with the water temperature ± 34 °. Previous studies have suggested that the depth of water affects the time of the immovability of the rats, a smaller amount of water causes increased immobility due to the ability of the rat to lean against the tail on the bottom of the barrel (17). In our research, a deeper barrel was used for rats to better deal with stress in swimming during the experiment. The rats swam every day for an hour to an hour and a half (60-90min). The serum analyzes were made with the ELISA technique.
Forced swimming test:
Porsolt and colleagues (18) are first authors that originally developed the Rat Forced Swimming test (FST), this is the most used test for animal models to assess antidepressant-like behavior (19), active or passive behavior of rats that are forced to swim and can’t escape from the swimming tank. Forced swimming test for rats later was modified for mice (20). The mouse version of the forced swim test is a relatively short and low-cost behavioral test that requires no training of the mice and can be conducted with minimal equipment. This is in contrast to the rat version of the test, which generally involves exposure to the water tank one day prior to the test day (21). There are two versions that are used, namely the traditional and modified FST which differ in their experimental setup, for both versions, a pretest of 10-15 min is included, as this accentuates the different behaviors in the 5-min swim test following drug treatment (19).
Rats were exposed to forced swim test the swimming program included two phases: adaptation and training. In the first week (adaptation), the rats swam 60-90 min per day for 6 days (1 week). The training period began from the beginning of the second-week swimming duration was progressively increased from 50 min to approximately 90 min per day. Rats were exposed to a swimming test between 08.30h till 12.00h AM. This intensity was maintained to the end of the training program which lasted 6 days per week for a total of three weeks (21 days), during weekdays Monday-Saturday, while Sunday was without activity for experimental rats (Picture 1).
Picture 1. The rats exposed to Forced Swim test.

Forced swimming stress was induced in rats by forcing them to swim in a cylindrical swim tank which was filled with tepid water (±34°C). All swimming protocol was recorded by a video camera and photo camera. The length of the cylindrical swim tank was approximately 80-90 cm which was filled with water till 50-60 cm of depth, this depth exceeded the length of the rat including tail (the length of the rats was approximately 40cm), the width of the tank was 60cm. During the experiment, it was measured body mass of the rats. Body mass was measured with a standard weighting scale of 1 kg, the measurement was taken every day at the same time in the morning approximately at 08.30 AM on day 2- 21.
Collection and Preparation of blood serum and plasma:
Blood collection was taken four times from the animals at day 1, 7, 14, and day 21, collection of blood procedure was performed always in the morning and before forcing them to swim. Blood was taken from the tail vein of the rats on days 1, 7, and 14, rats were awakened, and there was no need for anesthesia. Approximately 2-3 mm from the tip of the tail, it was done a small nick over the lateral tail vein using a sterile scalpel blade. When the nick was deep enough, the blood started welling up from the nick immediately. Blood was collected by allowing the blood to drop into a collection tube (Picture 2). The tail was gently stroked from the base of the tail toward the tail to encourage blood flow and the amount of the blood collected was 1.5 ml. The blood sample of 1.5 ml was allowed to clot for 30 minutes at room temperature. The serum was separated by centrifugation at 3000 rpm (rotations per minute) for 15 min. After centrifugation, the amount of serum collected was 0.75ml and subsequently stored at -20°C. The hormone was estimated by using the Epinefrine ELISA KIT protocol (ABNOVA, KA1882). While on day 21 all experimental rats were sacrificed and the blood was collected from the abdominal artery. Whole blood was collected into centrifuge tubes containing Potassium-EDTA as anticoagulant and centrifuged immediately for 3000 rpm for 15 min. After centrifugation, the amount of plasma collected was 5-6ml and subsequently stored at -20°C. The plasma samples were then subjected to the estimation of adrenaline by using Epinefrine ELISA KIT protocol (ABNOVA, KA1882).
Picture 2. Processing the analyzes on the ELISA plate.

Statistical analysis:
The data were analyzed using the statistical package SPSS 22.0 software for Windows (SPSS Inc., Chicago, IL, USA). Basic descriptive statistics were computed. Data normality distribution was determined using the skewness, kurtosis, and Kolmogorov-Smirnov method. Differences in variables were analyzed by One-Way Repeated-Measures ANOVA also LSD-least significant difference test was done.
4. RESULTS
On the basis of the bodyweight measurements obtained from day 2 and day 20 after the completion of the swimming the following results were obtained which are statistically processed. According to the results shown in the (Figure 1), it can be noticed that the bodyweight of the rats on day 8 during the physical stress showed the lowest values. While the total difference in body weight of rats before and after swimming for 20 days didn’t show any significant difference in body weight. From the figure itself, we can notice the difference between day 2, day 8, and day 20 of the bodyweight measured on the same day in the same rats. From the overview of (Table 1), it can be seen that the values of the skewness of adrenaline variable in control measurements, at day 7 and day 14 in rats who swam, are within the limits of the recommended values from -1 to +1, indicating that the distribution of the results are approximately symmetrical. Mild positive asymmetry (most of the results are in the higher zone), is observed in rats that swam on the 21 days of collection (Sk = 1.65) and in rats that rested on the 21st day of collection (Sk = 1, 07). From the values of the kurtosis (Table 1), it can be seen that the adrenaline variable at all times of collecting shows flatness.
Figure 1. A comparative overview of body weight in rats.
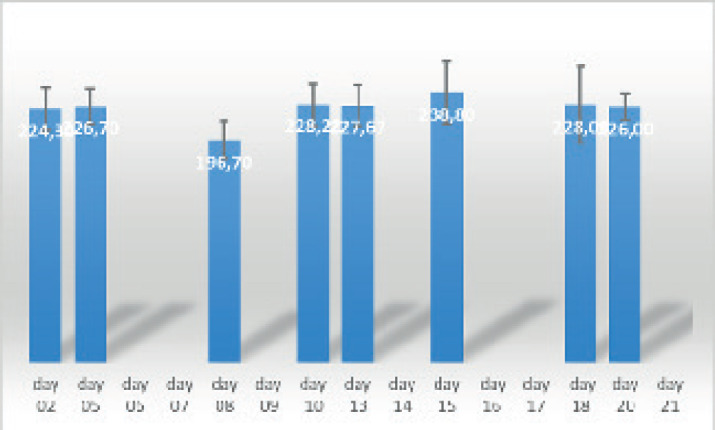
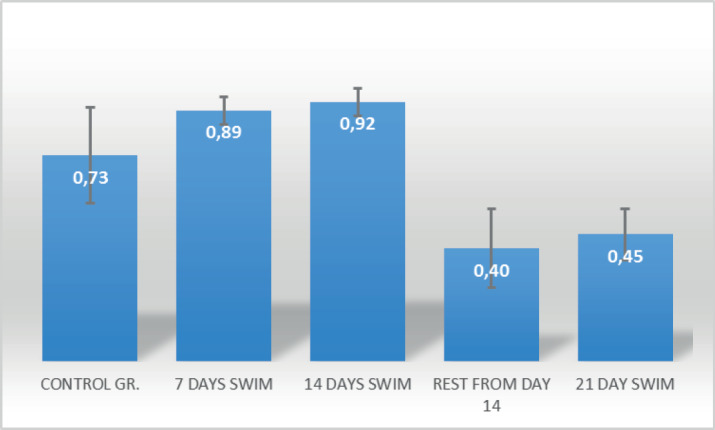
Table 1. Statistical data of Adrenaline levels among groups.
| ADRENALIN | Mean | Min | Max | SD | CV | s.e. | Skew | Kurtos |
|---|---|---|---|---|---|---|---|---|
| CONTROL | 0.73 | 0.55 | 1.01 | 0.17 | 23.64 | 0.06 | 0.47 | -1.70 |
| 7 DAYS SWIM | 0.89 | 0.81 | 0.97 | 0.05 | 5.75 | 0.02 | -0.61 | -0.45 |
| 14 DAYS SWIM | 0.92 | 0.81 | 1.01 | 0.05 | 5.82 | 0.02 | -0.75 | 2.14 |
| REST FROM DAY 14 | 0.40 | 0.29 | 0.70 | 0.14 | 35.35 | 0.05 | 1.65 | 1.98 |
| 21 DAY SWIM | 0.45 | 0.36 | 0.59 | 0.09 | 19.01 | 0.03 | 1.07 | -0.16 |
According to calculated coefficients of variability, it can be seen that homogeneity at all collecting points is at a satisfactory level. The lowest level of homogeneity is observed on the 7th day of swimming (CV = 5.75), while the highest level of dispersion of the results is observed in rats that were resting from 14th to 21st day (CV = 35.35). The value of the basic central and dispersive parameters of the applied variables in the interval of the minimal and maximal result contains about four or more standard deviations (SD), on the basis of which satisfactory susceptibility can be ascertained. Based on the values of the standard deviations (SD) and its ratio with arithmetic mean (Mean), it can be concluded that at all time points there is no statistically significant deviation of the results from the arithmetic mean. The numerical values of the standard error indicate a minimum dispersion, because, with proportional looking, there are not significant in relation to the corresponding value of the standard deviation. The results of Kolmogorov Smirnov’s procedure showed that, at all time points of collection, the results are normally distributed (Table 2). In order to determine in which of the examined groups there are statistically significant differences, the post hock (LSD-least significant difference test) tests are applied. The analysis of the tests is shown in Figure 3 and Table 3. From the overview of arithmetic means and the level of statistical significance, it can be seen that the control group shows lower values of adrenaline in the serum relative to the group that swam for 7 and 14 days, and lower values relative to the group that rested from day 14 up to day 21 and the group that swam up to 21 days. The group that swam for 7 days shows higher values compared to the group that was resting from day 14 up to 21st day and the group that swam for up to 21 days, while no statistically significant differences were found between the swimming group for 7 days and the group that swam for 14 days. The group that has been swimming for 14 days shows more adrenaline levels in the serum than the group that was resting from day 14 up to 21st day and the group that swam up to 21 days. There were no statistically significant differences (p=0.435) in the level of adrenaline in the serum between the group that was resting from day 14 and the group that swam up to 21 days.
Table 2. The results of Kolmogorov Smirnov's procedure for Adrenaline.
| Adrenaline | max D | K-S |
|---|---|---|
| CONTROL | 0.295 | p > .20 |
| 7 DAYS SWIM | 0.172 | p > .20 |
| 14 DAYS SWIM | 0.186 | p > .20 |
| REST FROM DAY 14 | 0.320 | p > .20 |
| 21 DAY SWIM | 0.256 | p > .20 |
Table 3. The comparison of Adrenalin serum among groups.
| Time | Mean Difference | Std. Error | p | |
|---|---|---|---|---|
| CONTROL | 7 DAYS SWIM | -,161* | ,064 | ,035 |
| 14 DAYS SWIM | -,190* | ,066 | ,020 | |
| REST FROM DAY 14 | ,334* | ,087 | ,005 | |
| 21 DAY SWIM | ,284* | ,036 | ,000 | |
| 7 DAYS SWIM | CONTROL | ,161* | ,064 | ,035 |
| 14 DAYS SWIM | -,029 | ,022 | ,231 | |
| REST FROM DAY 14 | ,496* | ,048 | ,000 | |
| 21 DAY SWIM | ,445* | ,039 | ,000 | |
| 14 DAYS SWIM | CONTROL | ,190* | ,066 | ,020 |
| 7 DAYS SWIM | ,029 | ,022 | ,231 | |
| REST FROM DAY 14 | ,525* | ,049 | ,000 | |
| 21 DAY SWIM | ,474* | ,041 | ,000 | |
| REST FROM DAY 14 | CONTROL | -,334* | ,087 | ,005 |
| 7 DAYS SWIM | -,496* | ,048 | ,000 | |
| 14 DAYS SWIM | -,525* | ,049 | ,000 | |
| 21 DAY SWIM | -,050 | ,061 | ,435 | |
| 21 DAY SWIM | CONTROL | -,284* | ,036 | ,000 |
| 7 DAYS SWIM | -,445* | ,039 | ,000 | |
| 14 DAYS SWIM | -,474* | ,041 | ,000 | |
| REST FROM DAY 14 | ,050 | ,061 | ,435 | |
5. DISCCUSION
The present study investigated the levels of Adrenaline in rats after forced swimming test for 21 days. It is well documented that any stress can elevate the level of adrenaline (22, 23, 24), regarding our results about levels of adrenaline after FST it can be seen that the control group showed lower values of adrenaline in the serum relative to the group that swam for 7 and 14 days, the average of adrenaline is slightly higher but there is no statistically significant differences found between these two groups, these data are in agreement with previous study (25), they concluded that in acute (7 days) and subacute (14 days) the levels of adrenaline after FST in rats didn’t show significant increases of adrenaline compared with control group, this study revealed the importance of physical activity regarding neurodegeneration they stated that ”14 days of FST stress didn’t cause neuronal damage”. Our results can indicate that acute stress-induced to rats was very stressful, Soria and colleagues reported the same data they confirmed high levels of adrenalin after acute physical stress (26). There were no statistically significant differences in the level of adrenaline in the serum between the group that was sleeping from day 14 of day 21 and the group that swam to 21 days, from these results we can conclude that the higher average of adrenaline level showed to be on day 14 of the experiment which are consistent with several studies (22, 25).
The day 14 showed to be the most stressful day of the experiment with highest level of adrenaline compared to control group and all other groups of the experiment, while the lowest levels of adrenaline during the experiment was day 14 of rats that were sleeping and the 21 day of the rats that swam, these results can be due to adaptation of rats with physical stress conducted from FST after day 14 of swimming. We can say that physical aerobic activity can activate adrenal catecholamine like adrenaline in adaptation to stressful conditions and these results confirm our hypothesis that regular aerobic exercise for 21 days can produce differences in adrenaline level among groups.
If we can connect this with sedentary life of human people, this fact should be considered because according to the evidence there is high number of persons with high levels of stress conditions like anxiety, depression, mood disorders and they should be aware that their sedentary life can have side effects in their stress hormone levels (27, 28) which can lead to several pathologies. Pain perception is another factor that has to be considered in people, many studies have been conducted about pain threshold. It is well known that exercises and stress have an influence on pain perception (29) according to another study, one group of rats were exposed to exhaustion exercises and the results showed that rats who were with higher aerobic capacity the pain threshold was higher (30). Physically trained individuals show lower levels of physiological and psychological stress responses not only in exercises but also in other types of stressful conditions (31). A recent study concluded that 21 days of exercise without rest will have effect on biochemical factors in the brain, also in memory function (32), exercise alters both bone modeling and energy balance (33). Another factor that should be considered during FST is also the immobility time during the test, according to one study the immobility time does not correlate with markers of depression and adrenaline levels (25). When we talk about the recuperation, there are many athletes that may be injured during the activity, some authors observed how e contused muscle of rats will react if they put them on wheel running for 21 days, they concluded that after 21 days of mobilization the healing process was better it was less inflammation and better nerve regeneration (34, 35). Recent data indicate that forced swimming stress for 21 days decreased the body weight and food intake but increased weights of liver, kidney, and adrenal gland, this reveals the effect of repeated forced swim stress can cause a wide range of adaptive changes in the central nervous system (36). According to recent study, the weight of the animals reported in point 7,14, and 21 after swimming test has been reduced gradually, also they reported decline in body weight of rats after exposed to exercise group (treadmill running for 1h) for 21 days (32), also after 8 weeks of FST for approximately 60 min per day in rats some authors reported reduced body weight gain (37), these data are consistent with our results also the type of exercise, timing and duration were approximately the same. A recent study used Wistar female rats in their experiment, they forced them to swim for 45 min for 21 days, and the results were consistent with our results regarding body weight (36).
6. CONCLUSION
Regular aerobic physical activity for 21 days, for 60-90 min swim have a positive impact to reduce stress hormone adrenaline, also aerobic exercise protocol could have a positive impact on reducing and maintaining body weight, thus preventing overweight. Limitations of this study are worthy of brief mention. Our study was gender-specific, including only female Wistar rats. Moreover, we had a small sample size. Taking into consideration these two limitations we can say that our results can’t be applicable in both genders.
Figure 2. The comparison of Adrenaline serum among groups.
Acknowledgements:
We would like to thank Faculty of Natural Sciences and Mathematics, Institute of Biology, Skopje, Macedonia for helping to conduct the experiment.
Authors contribution:
Ibrahimaj Gashi, Zivkovic, Gjorgovski and Gontarev gave substantial contributions to the conception or design of the work in acquisition, analysis, or interpretation of data for the work. Ibrahimaj Gashi, Zivkovic, Gontarev and Azemi had a part in article preparing for drafting or revising it critically for important intellectual content, and each author gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support and sponsorship:
None.
Conflicts of interest:
There are no conflicts of interest
REFERENCES
- 1.Pool AJ, Axford JS. The effect of exercise on the hormonal and imunde systems in rheumatoid arthritis. Rheumatology (Oxford) 2001;40(6):610–614. doi: 10.1093/rheumatology/40.6.610. [DOI] [PubMed] [Google Scholar]
- 2.Brown EJ, Vosloo A. The involvement of the hypothalamopituitary-adrenocortical axis in stress physiology and its significance in the assessment of animal welfare in cattle. Onderstepoort J Vet res. 2017;8(84):1–e9. doi: 10.3168/jds.2017-14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garber C E, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;3(7):334–339. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann NY Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal. The role of the hypothalamic-pituitary-adrenal. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gambarana C. Yehuda S, Mostofsky D, editors. Experimental protocols for the study of stress in animals and humans. Nutrients, stress and medical disorders. Human press Inc Torowa. 2005:21–30. doi: 10.1385/1-59259-952-4:021. [DOI] [Google Scholar]
- 8.Elizabetha A, Younga B, Abelsona J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Froniters in Neuroendocrinology. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Katch VL, McArdle WD, Katch FI. Essentials of Exercise Physiology. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2011. [Google Scholar]
- 10.Patton KT, Thibodeau GA. The human body in health and disease. Canada: Elsever Mosby; 2014. [Google Scholar]
- 11.Westfall TC, Westfall DP. Adrenergic agonists and antagonists. 12. New York: Goodman and Gilman’s THe pharmacological basis of therapeutics; 2011. [Google Scholar]
- 12.Goldstein DS. Clinical pharmacology of the autonomic nervous system. Amsterdam: Elsevier; 1999. [Google Scholar]
- 13.Hearing CM, Chang WC, Szuhany KL, Deckersbach T, Nierengerb AA, Sylvia LG. Physical Exercise for Treatment of Mood Disorders: A Critical Review. Curr behav neurosci. 2016;3(4):350–359. doi: 10.1007/s40473-016-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei M, Gibbson LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lin TW, Kuo YM. Exercises benefit brain function: the monoamine conection. Brain Sciences. 2013;3:39–53. doi: 10.3390/brainsci3010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson R, Robertson A, Jepsen R, Maxwell M. Walking for depression or depressive symptoms: a systematic revew and meta-analysis. mental health and Physical activity. 2012;5:66–75. doi: 10.1016/j.mhpa.2012.03.002. [DOI] [Google Scholar]
- 17.Kondo M, Shimada S. Serotonin and exercise-induced brain plasticity. Neurotransmitter. 2015;2:e793. doi: 10.14800/nt.793. [DOI] [Google Scholar]
- 18.Christianson JP, Drugan RC. Intermittent cold water swim stress increases immobility and interferes with escape performance inrat. Behav Brain Res. 2005;165:58–62. doi: 10.1016/j.pbb.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Porsolt RD, Deniel M, Jalfre M. Behavioural despair in rats and mice: strain differences and the effects of imipramine. European Journal of Pharmacology. 1978;51(3):291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- 20.Slatteryl DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature Protocols. 2012;7(6):1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 21.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 22.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. neurosciennce and Biobehavioral reviews. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez A, Toledo-Pinto EA, Menezes ML, Pereira OC. Changes in norepinephrine and epinephrine concentrations in adrenal gland of the rats submitted to acute immobilization stress. Pharmacological Research. 2003;49:607–613. doi: 10.1016/S1043-6618(03)00241-X. [DOI] [PubMed] [Google Scholar]
- 24.Scheurink AJ, Steffens AB, Bouritius H, Dretler GH, Bruntink R, Remie R, et al. Adrenal and sympathetic catecholamines in exercising rats. Am J Physiolo. 1989;256:155–160. doi: 10.1152/ajpregu.1989.256.1.R155. [DOI] [PubMed] [Google Scholar]
- 25.Sudo A. Accumulation of Adrenaline in sympathetic nerve endings in various organs of rats exopsed to swimming stress. Jpn J Pharmacol. 1985;38:367–374. doi: 10.1254/jjp.38.367. [DOI] [PubMed] [Google Scholar]
- 26.Abbas G, Nagyj S, Mehmood S, Kabir N, Dar A. Forced swimming stress does not affect monoamine levels and neurodegeneration in rats. Neurosci Bull. 2011;27(5):319–324. doi: 10.1007/s12264-011-1032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soria M, Gonzalez-Haro C, Anson M, Lopez-Colon JL. Plasamm leveles of trace elements and exercise induced stress homones in well-trained athletes. Journal of Trace elements in Medicine and Biology. 2015;31:113–119. doi: 10.1016/j.jtemb.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosomatic Medicine. 2005;67(1):S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 29.Jameel MK, Joshi AR, Dawane J, Padwal M, Joshi A, Pandit VA, et al. Effect of various physical stress model on serum cortisol level in wistar rats. Journal of clinical and diagnostic research. 2014;8(3):181–183. doi: 10.7860/JCDR/2014/7210.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzardo-Martins L, Martins DF, Marcon R, Santos UD, Speckhann B, Gadotti VM, et al. High-intensity extended swiming exercisereduces pain-related behaviour in mice: involvement of endogenous opioid and the serotonergic system. Journal of pain. 2010;11(12):1384–1393. doi: 10.1016/j.neuroscience.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 31.Geisser ME, Wang W, Smuck M, Koch LG, Britton SL, Lydic R. Nociception before and after exercise in rats bred for high and low aerobic capacity. Neuroscience letters. 2008;443:37–40. doi: 10.1016/j.neulet.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zschucke E, Renneberg B, Dimeo F, Wustenberg T, Strohle A. The stress-buffering effect of acute exercise: evidence for HPA axis negative feedback. Psychoneuroendocrinology. 2015;51:414–425. doi: 10.1016/j.psyneuen. [DOI] [PubMed] [Google Scholar]
- 33.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. Effects of different timing of stress on corticosterone, BDNF and memory in male rats. Physiol Behav. 2015;139:459–467. doi: 10.1016/j.physbeh.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Rosa BV, Firth EC, Vickers MH, Morel PC, Cockrem JF. Shortterm voluntary exercise in the rat causes bone modeling without initiating a physiological stress response. Am J Physiol regul Integr Comp Physiol. 2010;299(4):1037–1033. doi: 10.1152/ajpregu.00112.2010. [DOI] [PubMed] [Google Scholar]
- 35.Khattak MJ, Ahmad T, Rehman R, Umer M, Hasan SH, Ahmed M. Muscle healing and nerv regeneration in a muscle contusion model in rat. The Journal of bone and joint surgery. 2010;92(6):894–899. doi: 10.1302/0301-620X.92B6.22819. [DOI] [PubMed] [Google Scholar]
- 36.Kakihata CM, Malanotte JA, Karvat J, Brancalho RM, Ribeiro L C, Bertolini GR. The morphological and functional effects of exercise in the aquatic environment, performed before and/or after sciatic nerve compression in Wistar rats. Journal of exercise rehabilitation. 2016;12(5):393–400. doi: 10.12965/jer.1632670.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondam A, Kate NN, Lakshmi G, Chandrashekar MSM. Effect of forced swim test on wistar albino rats in various behavioral parameters. International Journale of medical research et health sciences. 2012;1(1):7–12. [Google Scholar]
- 38.Zhao Y, Liu LJ, Wang C, Li SX. Swiming exercises may not alleviate the depressive-like behavoiurs nad circadian alterations of neuroendocrine induced by chronic upredictable mild stress in rats. Neurology, Psychiatry and Brain Research. 2012;18(4):202–207. doi: 10.1016/j.npbr.2012.06.003. [DOI] [Google Scholar]


