Abstract
Objective
Clopidogrel is a commonly used P2Y12 inhibitor to treat and prevent arterial thrombotic events. Clopidogrel is a prodrug that requires bioactivation by cytochrome P450 (CYP) enzymes to exert anti-platelet activity. Diabetes mellitus is associated with an increased risk of ischemic events, and impaired ability to generate the active metabolite (AM) from clopidogrel. The objective of this study is to identify the mechanism of clopidogrel resistance in a murine model of diet-induced obesity (DIO).
Approach and results
C57BL/6J mice and IL-1R−/− mice were given high fat diet for 10 weeks to generate a murine model of diet-induced obesity. Platelet aggregation and carotid arterial thrombosis were assessed in response to clopidogrel treatment. Wild type DIO (WT DIO) mice exhibited resistance to anti-platelet and anti-thrombotic effects of clopidogrel that was associated with reduced hepatic expression of CYP genes and reduced generation of the AM. Interleukin-1 receptor deficient DIO (IL1R−/− DIO) mice showed no resistance to clopidogrel. Lack of resistance was accompanied by increased exposure of the clopidogrel AM. This resistance was also absent when WT DIO mice were treated with the conjugate of the clopidogrel active metabolite, DT-678.
Conclusions
These findings indicate that anti-platelet effects of clopidogrel may be impaired in the setting of diabetes due to reduced pro-drug bioactivation related to interleukin-1 receptor signaling. Therapeutic targeting of P2Y12 in patients with diabetes using the conjugate of clopidogrel active metabolite may lead to improved outcomes.
Keywords: Diabetes, Obesity, Anti-platelet, Cytochromes p450, Thrombosis, Pharmacology
Graphical Abstract
Introduction
Clopidogrel, a P2Y12 inhibitor, is widely used in combination with aspirin for treatment of acute coronary syndromes and following percutaneous coronary interventions.1–3 Clopidogrel is a prodrug requiring bioactivation by cytochrome P450 (CYP) enzymes via two oxidative steps to release the active metabolite (AM).4 Generation of the AM has been shown to be impaired in patients carrying CYP2C19 loss-of-function alleles and in patients with diabetes mellitus (DM).5,6 The effect of diabetes is particularly relevant since DM is associated with an increased risk of ischemic events.7–9 The excess vascular risk related to DM may be mediated by both heightened platelet reactivity and impaired response to antiplatelet agents such as clopidogrel. Some studies have indicated that DM is an independent risk factor for impaired clopidogrel responsiveness,10,11 which may be due to reduced generation of the AM.12–14
The liver plays an important role in the pathophysiology of type 2 diabetes. Hepatic insulin resistance is commonly associated with both hepatic lipid accumulation and inflammatory events.15 In vitro studies have shown that inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6, and tumor necrosis factor α can down-regulate CYP expression in isolated hepatocytes.16,17 Inflammatory cytokines in DM may lead to alterations of CYP enzymes and result in reduced efficacy of clopidogrel. By use of a murine model of interleukin-1 receptor (IL-1R) deficiency in the setting of diet-induced obesity (DIO), the effect of IL-1R pathways on clopidogrel responsiveness in DM can be tested.
DT-678 is a mixed disulfide conjugate of the clopidogrel AM with 3-nitropyridine-2-thiol, and it is readily converted to the active metabolite in the presence of glutathione (GSH) through a thiol disulfide exchange reaction.18 Since DT-678 does not require bioactivation by hepatic CYP enzymes, it serves as a useful tool to confirm the role of impaired CYP enzyme bioactivation of clopidogrel in DM and also as a potential therapeutic antithrombotic strategy in diabetic patients.
Material and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Chemicals
(S)- (+)-clopidogrel sulfate was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). DT-678 was synthesized by Beijing Shuanglu Pharmaceutical Company (Beijing, China).
Animals
Male C57BL/6J mice and Il1r1tm1Imx mice were purchased from The Jackson Laboratory. Mice were fed a standard laboratory rodent diet (SD; No. 5001, TestDiet, Richmond, IN, USA) or a high-fat, high-sucrose diet (D12451, Research Diets Inc, New Brunswick, NJ, USA) and tap water ad libitum in a temperature-controlled room with a 12:12-h light/dark cycle. DIO mice were given high fat diet for 10 weeks, beginning at age 10 weeks. Male mice were studied due to variable influence in response to high fat diet in female mice.19 All procedures complied with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Michigan Committee on Use and Care of Animals. Euthanasia was performed with combined pentobarbital overdose and induction of bilateral pneumothorax, a method consistent with the recommendations of the AVMA Guidelines on Euthanasia.
Measurement of metabolic factors
5-hour fasting blood glucose levels were measured using an Ascensia Contour Blood Glucose Meter and Ascensia Contour test strips (Bayer Healthcare LLC, NJ, USA). 5-hour fasting plasma insulin levels were measured by the University of Michigan Animal Phenotyping Core using Millipore Mouse/Rat insulin ELISA Kit. The following equation was used to calculate HOMA-IR values: Blood Glucose (mg/dL) x Serum Insulin (μU/mL)/405.20
Pharmacokinetics of clopidogrel and DT-678 in mice
Clopidogrel or DT-678 was dissolved in a 15:5:80 (v/v) mixture of N, N-dimethylacetamide, polyethylene glycol 400, and PBS. Mice were treated with a single oral dose of clopidogrel (5 mg/kg) or DT-678 (0.5 mg/kg) via oral gavage. Blood samples (50 μl each) were withdrawn from the retro-orbital venous plexus into heparinized polythene tubes at various time points. The blood samples were immediately treated with 5μl of 0.5 M 3’-methoxyphenacyl bromide to derivatize the AM. Plasma was separated by centrifugation at 7,000 g for 10 min and then kept frozen at −80 °C until analysis.
Aliquots (15 μl) of plasma were mixed with 15 μl acetonitrile, followed by the addition of 60 μl acetonitrile containing 1 mM trans-clopidogrel-MP (13C, D) as the internal standard. The mixture was then vortexed and centrifuged at 13,000 g for 10 minutes. A 5μl aliquot of the supernatant was injected for liquid chromatography–tandem mass spectrometry analysis as previously described.18
Pharmacokinetic parameters were calculated using PKSolver using non-compartmental analysis (extravascular model).21
Preparation of mouse liver microsomes
For hepatic microsome preparation, mice were euthanized and livers were removed and washed with ice-cold saline to remove excess blood and then cut into pieces on ice. Liver samples were then weighed and homogenized in 4 ml/gram of ice-cold homogenizing buffer (200 mM sucrose, 50 mM HEPES (PH 7.5), 50 mM MgCl2, 1 mM EDTA, 1 mM DTT and protease inhibitors). The resultant homogenates were transferred to clean centrifuge tubes followed by centrifuging at 10,000 g for 10 min at 4 °C. The supernatant was collected and further centrifuged at 140,000 g for 120 min at 4 °C using an ultracentrifuge. The resultant microsomal pellet was re-suspended at 20 mg/ml with ice-cold microsomal buffer (200 mM sucrose, 50 mM HEPES (PH 7.5), 1 mM DTT) and stored at −80 °C until use.
Kinetics of clopidogrel metabolism in vitro
Clopidogrel metabolism in vitro was carried out in 0.1 M potassium phosphate buffer (pH 7.4) containing 1.0 mg/ml microsomal protein (0.2 mg/ml microsomal protein for 2-oxo-clopidogrel as substrate), 0.1 M potassium fluoride, 1 mM GSH, 1 mM NADPH, and a specified concentration of clopidogrel (0–50μM) or 2-oxo-clopidogrel (0–100μM) as substrate, in a final volume of 100μl. The reaction mix was incubated at 30°C on shaker for 20 min. The metabolic reaction was terminated by adding 150μl acetonitrile containing the internal standard trans-clopidogrel-MP (13C, D) (0.3 μM) and the derivatizing reagent 3’-methoxyphenacyl bromide (10mM), followed by centrifugation at 13,000 g for 10 min. The supernatant was then analyzed by LC-MS/MS as previously described.18
RNA isolation and mRNA analysis
Total RNA was extracted from liver tissues using TRIzol reagent (Thermo Fisher, Carlsbad, CA, USA) and purified with the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Equal amounts of RNA (1 μg) were used for reverse transcription using the SuperScript™ III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). qPCR was performed with Fast SYBR™ Green Master Mix (Applied Biosystems, Vilnius, Lithuania) with 7500 Fast System. Primers (Sequence listed in Supplemental Materials Table I) were obtained from IDT (IA, USA). The relative amount of each studied mRNA was normalized to GAPDH mRNA levels as housekeeping gene, and the data were analyzed according to the 2−ΔΔCT method.22
Light transmission aggregometry
Platelet aggregation was performed 2 hours after drug administration as reported previously.23 Briefly, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (67 mg/kg), blood from the inferior vena cava was collected directly into 3.2% sodium citrate (9:1 blood/citrate ratio) and diluted with an equal volume of Tyrode’s buffer. Platelet-rich plasma was obtained by centrifugation at 50 g for 10 min. Then PRP was centrifuged at 1,200 g for 10 min, and the supernatant was collected as platelet-poor plasma. Platelet concentration was corrected to 3 × 108 platelets/ml with PPP. Adenosine diphosphate (ADP) (10 μM) stimulated platelet aggregation was performed using a Lumi-aggregometer (Chrono-Log Corp., Havertown, PA, USA) under stirring conditions (1,200rpm) at 37°C.
Carotid arterial thrombosis
To induce thrombosis, FeCl3 injury was performed on the carotid artery 2 hours after drug administration. This model has been shown to be sensitive to alterations in platelet activation and aggregation, as well as other coagulation factors.24 Briefly, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (67 mg/kg) and secured in the supine position under a dissecting microscope (Nikon SMZ-2T, Mager Scientific, Inc., Dexter, MI, USA). The right common carotid artery was isolated, dried, and treated with ferric chloride (7.5 % on 1.0 × 2.0 mm filter paper) for 3 min. The artery was then rinsed with warm saline and blood flow was continuously monitored by a Doppler flow probe (Transonic, Ithaca, NY, USA). The time to vessel occlusion was defined as the time between removal of FeCl3 and cessation of blood flow for 1 minute.
Liver histology
Livers were collected, fixed in formalin and embedded in paraffin. 5 μm thick sections were stained with hematoxylin-eosin (HE).
Western blotting
Liver tissues were lysed in RIPA lysis buffer and were centrifuged to collect protein lysates. Protein (60μg) was separated by 4–20% Tris-Glycine protein gel before transfer to a PVDF membrane. Membranes were probed with antibodies against IL-1β (Invitrogen, India) and actin (LI-COR Biosciences, NE, USA) as loading control, followed by IRDye® 800 CW Donkey anti-Rabbit IgG (LI-COR Biosciences, NE, USA) or IRDye® 680RD Goat anti-mouse IgG (Invitrogen, IL, USA). Fluorescence signals were analyzed with Odyssey Imaging System (Odyssey CLx, LI-COR, Inc, USA).
Glutathione Assay
Liver glutathione levels were measured with Glutathione Fluorescent Detection Kit (Arbor Assays, MI, USA) according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed using GraphPad Prism 8 Software and were presented as mean ± standard error of the mean. The Shapiro-Wilk test was used to assess the normality of distribution of investigated parameters. F test or the Brown-Forsythe test was used for testing the equality of variances. Results were analyzed using unpaired Student’s t test for comparison between two groups. For multiple comparisons, results were analyzed using one-way or two-way ANOVA followed by Turkey post-test analysis. If normality test failed, results were further analyzed using the Mann-Whitney U test or Kruskal-Wallis test, followed by Dunn post hoc test. Results were considered significant at *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
Results
Metabolic parameters following diet-induced obesity
Following 10 weeks of a high fat, high sucrose diet, both wild type mice (WT DIO) and IL-1R−/− mice (IL-1R−/− DIO) showed elevated body weight, fasting blood glucose and fasting insulin levels compared to wild type mice on standard diet (WT lean) (Table 1). HOMA-IR level in WT DIO and IL-1R−/− DIO mice were increased about 5-fold compared to WT lean mice, indicating insulin resistance after dietary challenge.
Table 1. Metabolic parameters of WT lean, WT DIO and IL-1R−/−DIO mice following 10 weeks’ dietary challenge.
Both WT DIO and IL1-R−/− DIO mice showed increased body weight, higher insulin and glucose level, higher HOMA-IR compared to WT lean mice. compared to WT lean mice.
| WT Lean | WT DIO | IL1-R−/− DIO | |
|---|---|---|---|
| Body weight (g) | 31.43 ± 0.364 | 45.16 ± 0.676* | 43.04 ± 0.626* |
| Fasting glucose (mg/dL) | 196.4 ± 15.72 | 267.1 ± 9.15* | 251.0 ± 15.16* |
| Fasting insulin (mU/L) | 10.96 ± 3.149 | 41.2 ± 8.117* | 46.87 ± 4.993* |
| HOMA-IR | 5.798 ± 1.977 | 27.71 ± 6.026* | 26. 80 ± 3.305* |
P<0.05
Comparison of clopidogrel AM generation in WT lean, WT DIO and IL-1R−/− DIO mice
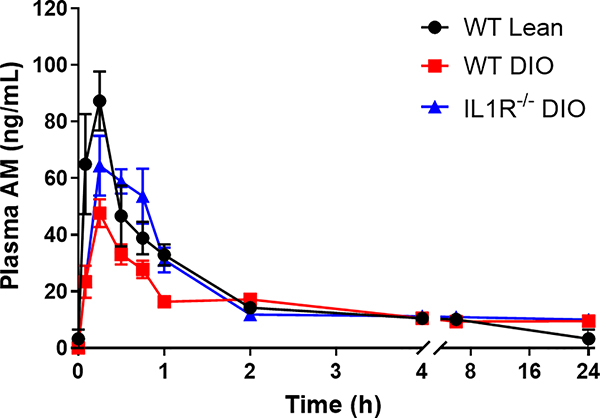
To determine whether DIO affected generation of the clopidogrel AM, WT lean, WT DIO and IL-1R−/− DIO mice were treated with clopidogrel by oral gavage and then plasma was collected at multiple time points (Figure 1). A dose of 5mg/kg was chosen to provide a consistent prolongation of occlusion times in WT lean mice. After a single oral dose of 5mg/kg clopidogrel, the peak plasma AM concentrations in WT DIO mice were approximately half that of WT lean mice (WT DIO= 47.64 ± 4.902 vs. WT lean= 95.32 ± 18.40 ng/ml, p <0.05). The area under the concentration-time curve (AUC), representing the average AM concentration over the time period measured, was approximately 40% lower in WT DIO mice compare to WT lean mice 2 hours after dosing (WT DIO= 40.04 ± 2.549 vs. WT lean= 69.93 ± 8.513 ng*h/ml, p <0.05). These data suggest WT DIO mice had lower circulating AM exposure after clopidogrel treatment compared to WT lean mice. Although the peak concentrations of AM in IL-1R−/− DIO mice were not different from WT DIO mice (IL-1R−/− DIO= 67.03 ± 9.766 vs. WT DIO= 47.64 ± 4.902 ng/ml, p =0.4645), IL-1R−/− DIO mice continuously released the AM after it reached peak concentration, which resulted in a larger AUC 2 hours after dosing compared to WT DIO mice (IL-1R−/− DIO= 69.79 ± 6.274 vs. WT DIO= 40.04 ± 2.549 ng*h/ml, p <0.05). IL-1R−/− DIO mice thus displayed higher AM exposure after clopidogrel treatment compared to WT DIO mice.
Figure 1. Mean plasma concentration-time curve of AM in WT lean, WT DIO and IL-1R−/− DIO mice after a single oral dose of 5mg/kg clopidogrel.
Plasma AM (n=3–5 each) was quantified as described in Materials and Methods. The PK parameters were obtained by fitting the data to a non-compartmental model using PKSolver. WT DIO mice showed lower peak AM concentrations (WT DIO= 47.64 ± 4.902 vs. WT lean= 95.32 ± 18.40 ng/ml, p <0.05) and lower AUC (WT DIO= 40.04 ± 2.549 vs. WT lean= 69.93 ± 8.513 ng*h/ml, p <0.05) 2 hours after dosing compared to WT lean mice. IL-1R−/− DIO mice showed higher AUC compared to WT DIO mice 2 hours after dosing (IL-1R−/− DIO= 69.79 ± 6.274 vs. WT DIO= 40.04 ± 2.549 ng*h/ml, p <0.05). AM= active metabolite, AUC= area under curve.
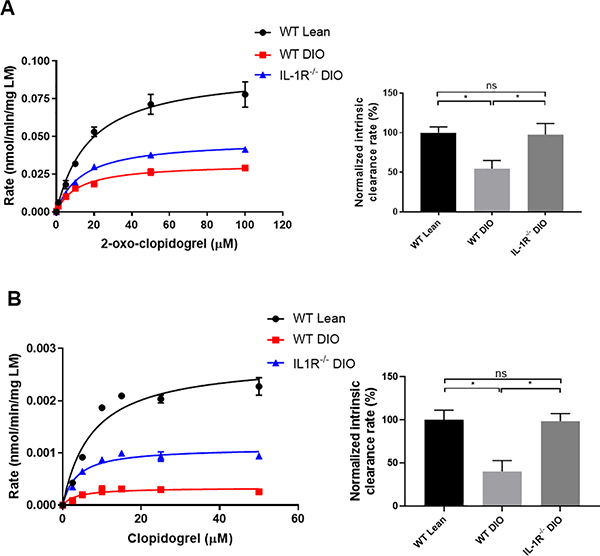
To determine whether the difference of AM exposure was related to variable hepatic clopidogrel activation, liver microsomes were isolated from WT lean, WT DIO and IL-1R−/− DIO mice to study the generation of clopidogrel AM by hepatic CYP enzymes. Data were fit into a Michaelis-Menten curve.25 The catalytic efficiency of CYP enzymes was determined by intrinsic clearance (Vmax/KM) and normalized to WT lean liver microsomes. Liver microsomes from WT DIO mice showed impaired efficiency in generating the AM from 2-oxo-clopidogrel or clopidogrel compared to microsomes from WT lean mice (Figure 2). The intrinsic clearance of WT DIO mice liver microsomes was about half of WT lean mice, whereas IL-1R−/− DIO mice had the same intrinsic clearance compared to WT lean mice. These results suggest that the reduced AM exposure observed in the PK profile in WT DIO mice is due to impaired hepatic enzymatic bioactivation of clopidogrel.
Figure 2. Production of clopidogrel AM from 2-oxo-clopidogrel (A) and clopidogrel (B) metabolism by mouse liver microsomes.
Kinetic data were fit into a Michaelis-Menten curve (Left Panel), whereas the catalytic efficiency of CYP enzymes was determined by intrinsic clearance (Vmax/KM) and normalized to WT lean liver microsomes (Right Panel). n=3–7 each. Data were analyzed using one-way ANOVA followed by Turkey post-test analysis. ns=not significant, *p<0.05.
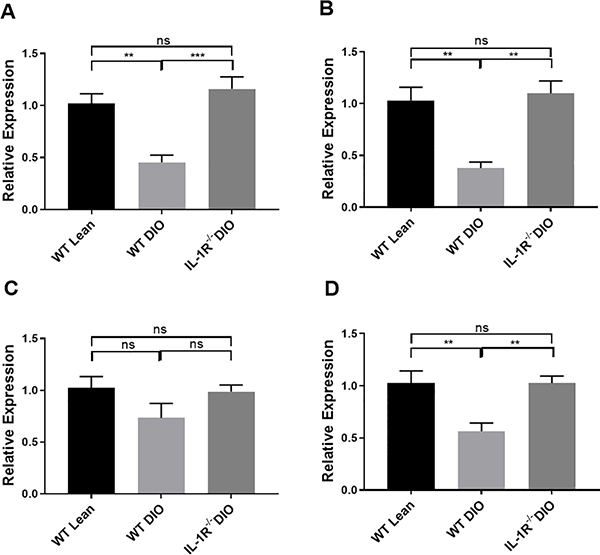
To further explore mechanisms underlying differences in clopidogrel AM generation by liver microsomes from WT lean, WT DIO and IL-1R−/− DIO mice, relative mRNA expression of cyp2C genes related to clopidogrel metabolism were analyzed. Bioactivation of clopidogrel to the AM follows similar mechanism(s) in rodents and humans.26,27 Therefore we analyzed mRNA expression of cyp2C genes in WT lean, WT DIO and IL-1R−/− DIO mice. Due to expansion of the cyp2C genes in mice,28 mRNA expression of 4 isoforms of cyp2C genes in the liver known to be responsible for bioactivation of clopidogrel, including cyp2c29, cyp2c37, cyp2c50 and cyp2c54 were analyzed.29 Among 4 analyzed genes, cyp2c29, cyp2c37, cyp2c54 were all down-regulated in WT DIO mice compared to WT lean mice, whereas IL-1R−/− DIO mice showed higher expression of the analyzed cyp2C genes compared to WT DIO mice and similar expression compared to WT lean mice (Figure 3). These results suggest that the regulation of cyp2C genes by DIO may contribute to impaired hepatic enzymatic bioactivation of clopidogrel in WT DIO mice, and that the altered cyp2C enzyme expression is mediated by IL-1 receptor signaling. In addition to the cyp2C gene, other major forms of CYP genes related to clopidogrel activation such as cyp3a11 and cyp1a2 were also down-regulated in WT DIO mice except for cyp2b10 (see Supplemental Figure I in the Supplemental Materials).
Figure 3. Hepatic mRNA expression of cyp2C genes consisting of cyp2c29 (A), cyp2c37 (B), cyp2c50 (C) and cyp2c54 (D) in WT lean, WT DIO and IL-1R−/− DIO mice.
n=5 each. Data were analyzed using one-way ANOVA followed by Turkey post-test analysis. ns=not significant, **p<0.01, ***p<0.001.
Effects of clopidogrel on platelet aggregation and thrombosis formation in WT lean, WT DIO and IL-1R−/− DIO mice
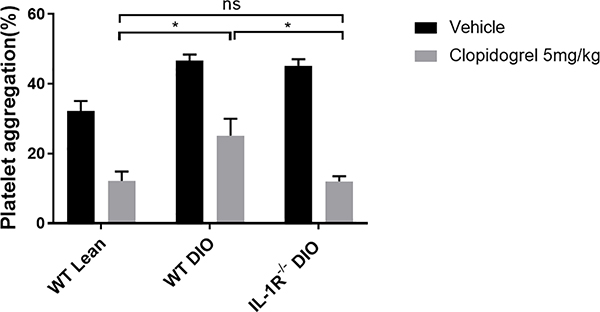
To determine if the observed impaired AM generation in WT DIO mice would lead to reduced clopidogrel-mediated platelet inhibition, platelet aggregation studies were performed (Figure 4). At the baseline, platelets from WT DIO mice showed a higher aggregation response to ADP compared to WT lean mice (WT DIO= 46.6 ± 1.81% vs. WT lean= 32.2 ± 2.89%, p<0.05). Two hours following clopidogrel treatment (5mg/kg), both WT lean and WT DIO groups showed reduced ADP-induced platelet aggregation. However, the residual platelet aggregation response to ADP in WT DIO mice was higher compared to WT lean mice (WT DIO= 25.2 ± 4.76% vs. WT lean= 12.2 ± 2.63%, p<0.05). Similar to WT lean mice, IL-1R−/− DIO mice showed less residual platelet aggregation response to ADP compared to WT DIO mice (IL-1R−/− DIO= 12.0 ± 1.52% vs. WT DIO= 25.2 ± 4.76%, p<0.05). These findings indicate that platelets from IL-1R−/− DIO mice are inhibited to a greater extent compared to WT DIO mice after clopidogrel treatment.
Figure 4. Effects of clopidogrel on platelet aggregation in WT lean, WT DIO and IL-1R−/− DIO mice.
A single oral dose of 5mg/kg clopidogrel or vehicle was given to WT lean, WT DIO and IL-1R−/− DIO mice (n=5 each). Platelet aggregation in platelet rich plasma in response to ADP (10 μM) was measured 2 hours after dosing. Data were analyzed using two-way ANOVA followed by Turkey post-test analysis. ns=not significant, *p<0.05.
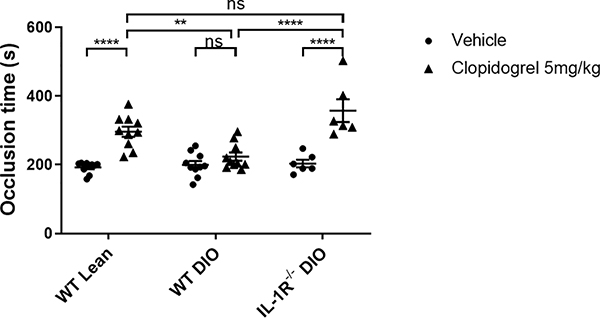
Since in vitro platelet aggregation results may not predict thrombotic responses in the arterial vasculature, clopidogrel efficacy was tested in the ferric chloride carotid thrombosis model (Figure 5). In this model, clopidogrel treatment (5mg/kg) prolonged the time to occlusion from 192.3 ± 5.17 to 296.1 ± 14.90 seconds (p<0.0001) in WT lean mice, but did not significantly prolonged the time to occlusion in WT DIO mice (199.9 ± 10.82 (vehicle) to 221.6 ± 11.40 seconds (clopidogrel), p=0.8097). The time to carotid artery occlusion following clopidogrel treatment in WT DIO mice was significantly shorter than the time to occlusion in WT lean mice (WT DIO= 221.6 ± 11.40 vs. WT lean= 296.1 ± 14.90 seconds, p <0.01), suggesting WT DIO mice were resistant to the anti-thrombotic effects of clopidogrel following arterial injury. In IL-1R−/− DIO mice, the effect of clopidogrel in prolonging carotid occlusion times was not attenuated compared to WT lean mice (IL-1R−/− DIO= 357.3 ± 33.17 vs. WT lean= 296.1 ± 14.90 seconds, p=0.076). IL-1R−/− DIO mice showed improved clopidogrel response compared to WT DIO mice (IL-1R−/− DIO= 357.3 ± 33.17 vs. WT DIO= 221.6 ± 11.40 seconds, p<0.0001). These findings indicate resistance to the antithrombotic effects of clopidogrel in DM is not observed in mice deficient in IL-1R.
Figure 5. Determination of the occlusion times in WT lean, WT DIO and IL-1R−/− DIO mice after FeCl3-induced arterial injury.
A single oral dose of 5mg/kg clopidogrel or vehicle was given to WT lean (n=10), WT DIO (n=10) and IL-1R−/− DIO mice (n=6), and occlusion times were measured 2 hours after dosing. Data were analyzed using two-way ANOVA followed by Turkey post-test analysis. ns=not significant, **p<0.01, ****p<0.0001.
Liver histology and IL-1β expression in WT lean, WT DIO and IL-1R−/− DIO mice
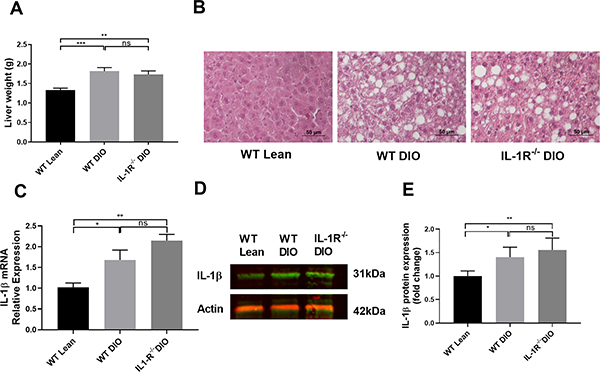
After dietary challenge, both WT DIO and IL-1R−/− DIO mice showed increased liver weight compared to WT lean mice (Figure 6A). Livers sections from both WT DIO and IL-1R−/− DIO mice showed extensive lipid accumulation compared to WT lean mice (Figure 6B). To further study the mechanism(s) of different clopidogrel responses between WT DIO and IL-1R−/− DIO mice, hepatic IL-1β mRNA and protein expression were measured. Both WT DIO and IL-1R−/− DIO mice showed higher IL-1β expression at mRNA (Figure 6C) and protein (Figure 6D, 6E) level compared to WT lean mice, suggesting DIO promotes hepatic IL-1β expression.
Figure 6. Liver histology and IL-1β expression in WT lean, WT DIO and IL-1R−/− DIO mice.
(A) Liver weight of WT lean, WT DIO and IL-1R−/− DIO mice (n=10 each). (B) HE-stained liver sections from WT lean, WT DIO and IL-1R−/− DIO mice. WT DIO and IL-1R−/− DIO mice showed extensive lipid accumulation compared to WT lean mice. Scale bar: 50μm. (C) Hepatic IL-1β mRNA expression in WT DIO and IL-1R−/− DIO mice (n=5 each). (D) Representative image and (E) Quantification of IL-1β western blot (n=6 each). Data were analyzed using one-way ANOVA followed by Turkey post-test analysis or by Kruskal-Wallis test, followed by Dunn post hoc test. ns=not significant, *p<0.05, **p<0.01, ***p<0.001.
Effects of DT-678 in WT DIO mice
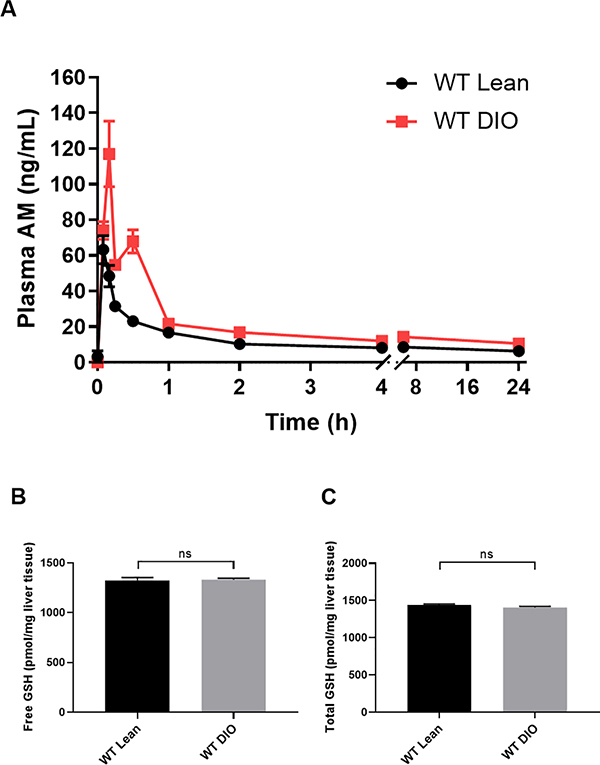
Our results suggest clopidogrel resistance in WT DIO mice is related to low clopidogrel AM exposure caused by down regulation of CYP enzymes, and this resistance can be reversed by IL-1 receptor deficiency. We then tested the P2Y12 inhibitor, DT-678, which does not require hepatic CYP enzyme activation. A dose of 0.5mg/kg was chosen which had comparable effects on occlusion times to 5mg/kg clopidogrel in WT lean mice (DT-678= 317.3 ± 14.90 vs. clopidogrel= 296.1 ± 14.90 seconds, p=0.3276). In contrast to clopidogrel, WT DIO mice showed no resistance to DT-678 treatment (WT DIO= 340.5 ± 32.74 vs. WT lean= 317.3 ± 14.90 seconds, p =0.9705). The Pharmacokinetics of DT-678 is shown in Figure 7A. DT-678 (0.5mg/kg) led to faster release of the AM with higher AUC (WT DIO= 48.03 ± 3.286 vs. WT lean= 27.01 ± 1.351 ng*h/ml, p <0.001) and higher peak concentrations (WT DIO= 127.9.2 ± 11.72 vs. WT lean= 63.22 ± 7.862 ng/ml, p <0.01) in WT DIO mice compared to WT lean mice. DT-678 can release the AM in the presence of GSH, however there was no significant difference in free or total GSH between WT lean and WT DIO mice (Figure 7B, 7C). These findings indicate that resistance to clopidogrel in DM is not observed when mice are treated with DT-678 which does not require bioactivation by CYP enzymes, and support the hypothesis that clopidogrel resistance in DM is due to reduced CYP-mediated AM generation.
Figure 7.
(A) Mean plasma concentration-time curve of AM in WT lean and WT DIO mice after a single oral dose of 0.5mg/kg DT-678. Plasma AM (n=6 each) was quantified as described in Materials and Methods. The PK parameters were obtained by fitting the data to a non-compartmental model using PKSolver. WT DIO mice showed higher peak AM concentrations (WT DIO= 127.9.2 ± 11.72 vs. WT lean= 63.22 ± 7.862 ng/ml, p <0.01) and higher AUC (WT DIO= 48.03 ± 3.286 vs. WT lean= 27.01 ± 1.351 ng*h/ml, p <0.001) compared to WT lean mice. (B, C) Liver GSH content in WT lean and WT DIO mice. n=5 each. Data were analyzed by unpaired t-test. ns=not significant. GSH= glutathione.
Discussion
Clopidogrel is a second generation thienopyridine for patients with acute coronary syndromes and has been widely used for over a decade. As a prodrug, it requires hepatic conversion, leading to delayed onset of action and wide variability among individuals. Approximately one-third of patients are resistant to clopidogrel which is related to diabetes, CYP genetic variation, and drug-drug interactions, leading to reduced protection against thrombotic events.30,31
Previous studies have shown that diabetes is a risk factor, independent of CYP polymorphisms, for diminished clopidogrel response.11,32 In this study, we used a mouse model of DIO as a model for type 2 diabetes. The DIO model has been shown by many investigators to develop reproducible obesity, chronic inflammation, along with elevated fasting insulin and glucose levels consistent with non-insulin-dependent (type 2) diabetes due to insulin resistance.33,34 Evidence of vascular dysfunction has also been demonstrated in this model.35
It has been demonstrated that diabetic patients have low clopidogrel responsiveness and plasma AM concentration compared to non-diabetic patients,5,13 however the mechanism remains unknown. Hypotheses include platelet hyper-reactivity,36 decreased gastrointestinal absorption, increased clopidogrel prodrug hydrolysis to inactive carboxylic acid metabolites, and increased AM hydrolysis.5 Our results suggest DM can influence clopidogrel responses by down-regulating CYP enzymes, leading to lower circulating concentrations of AM, resulting in reduced anti-platelet effects. DM is associated with biomarkers of inflammation.37 Inflammatory cytokines, including IL-1β, have been shown to down-regulate CYP mRNA levels in human hepatocytes.16 After dietary challenge, DIO mice showed elevated hepatic IL-1β expression in the current study, which may promote the down-regulation of CYP genes in WT DIO mice. Consistent with this hypothesis, IL-1R−/− DIO mice showed increased CYP genes expression, increased AM exposure, and improved antithrombotic activity after clopidogrel administration compared to WT DIO mice. In the CANTOS Trial,38 in which IL-1β inhibition was tested in patients at high CVD risk, approximately 40% of recruited patients were diabetic and 95% patients were on anti-thrombotic treatment including clopidogrel. The beneficial change in clinical outcome after IL-1β antagonism treatment could be related in part to improved antithrombotic activity of clopidogrel. In addition to DM, there are other conditions that could affect hepatic and systemic inflammation with resultant effects on clopidogrel responses.39,40
To further clarify if clopidogrel resistance in WT DIO mice is related to reduced hepatic CYP activation, DT-678 was tested. DT-678 is a conjugate of the clopidogrel active metabolite, which releases the same clopidogrel AM without the necessity of bioactivation by hepatic CYP enzymes. WT DIO mice showed no resistance to DT-678 treatment, suggesting hepatic CYP enzyme activity is related to clopidogrel resistance in WT DIO mice. DT-678 releases the active metabolite of clopidogrel via a thiol-exchange reaction that may be accelerated by thioreduxin and glutaredoxin.4,41 On the contrary, the release of AM from DT-678 was more efficient in WT DIO mice compared to WT lean mice, resulting in even larger drug exposure. This is not due to variation in the GSH levels in the liver (Figure 7). The exact mechanisms remain to be elucidated. DT-678 was previously shown to exhibit efficient platelet inhibition in a rabbit model but with less prolongation of bleeding times compared to clopidogrel.42 For diabetic patients, a more efficient anti-platelet drug without increased bleeding risk may be beneficial.
Limitations of this study include use of a mouse model of diet-induced obesity which may not fully mimic the conditions of diabetes mellitus in humans. In addition, the thrombosis model is induced by application of ferric chloride which may not mimic the triggers of thrombosis in patients with atherosclerosis.
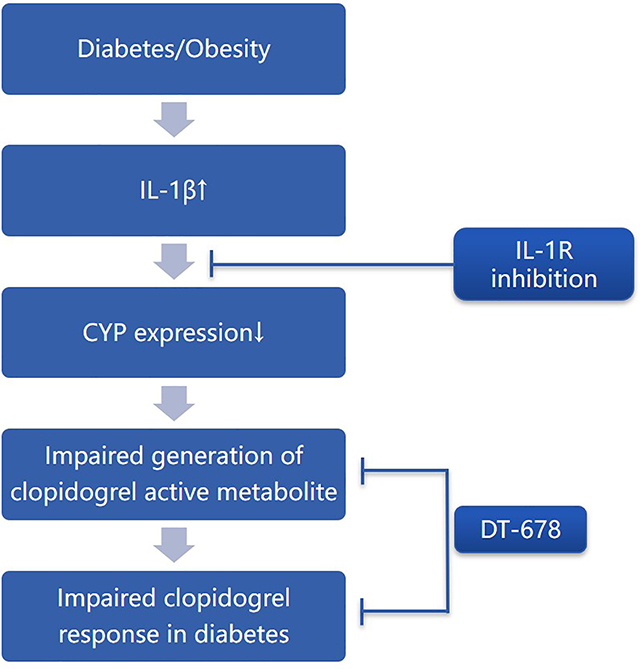
In summary, this study reveals that clopidogrel displays reduced anti-thrombotic effects in WT DIO mice due to down-regulation of CYP genes with resultant lower circulating AM in plasma. This resistance to clopidogrel can be reduced by eliminating IL-1 receptor signaling or reduced by a non-CYP mediated conjugate of clopidogrel active metabolite, DT-678.
Supplementary Material
Acknowledgments
Source of Funding
This work was supported by a grant from University of Michigan- Peking University Joint Institute for Translational and Clinical Research (UM/PUSC Award) to D.T.E and H.Z and the China Scholarship Council to Y.S.
Abbreviations
- CYP
cytochrome P450
- AM
active metabolite
- DM
diabetes mellitus
- IL-1β
interleukin-1β
- IL-1R
Interleukin-1 receptor
- DIO
diet-induced obesity
- WT
wild type
- GSH
Glutathione
- AUC
Area under curve
Footnotes
Disclosures
Dr. Haoming Zhang is the inventor of DT-678 (US patent 10,173,980).
References
- 1.Clark MG, Beavers C, Osborne J. Managing the acute coronary syndrome patient: Evidence based recommendations for anti-platelet therapy. Heart Lung. 2015;44:141–149. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial I. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 4.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello-Montoliu A, Rollini F, Franchi F, Ueno M, Darlington A, Desai B, Moser BA, Sugidachi A, Guzman LA, Bass TA. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64:1005–1014. [DOI] [PubMed] [Google Scholar]
- 6.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS 2nd, Lachno DR, Salazar D, Winters KJ. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. [DOI] [PubMed] [Google Scholar]
- 7.Cavender MA, Steg PG, Smith SC Jr., Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL, Investigators RR. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years From the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923–931. [DOI] [PubMed] [Google Scholar]
- 8.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014–1019. [DOI] [PubMed] [Google Scholar]
- 9.Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SR, Forouhi NG, Sigurdsson G, Danesh J, Gudnason V. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 2010;7:e1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuisset T, Frere C, Quilici J, Morange PE, Camoin L, Bali L, Lambert M, Juhan-Vague I, Alessi MC, Bonnet JL. Relationship between aspirin and clopidogrel responses in acute coronary syndrome and clinical predictors of non response. Thromb Res. 2009;123:597–603. [DOI] [PubMed] [Google Scholar]
- 11.Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Buttner HJ, Neumann FJ. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55:2427–2434. [DOI] [PubMed] [Google Scholar]
- 12.Erlinge D, Varenhorst C, Braun OO, James S, Winters KJ, Jakubowski JA, Brandt JT, Sugidachi A, Siegbahn A, Wallentin L. Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol. 2008;52:1968–1977. [DOI] [PubMed] [Google Scholar]
- 13.Pankert M, Quilici J, Loundou AD, Verdier V, Lambert M, Deharo P, Bonnet G, Gaborit B, Morange PE, Valero R, Dutour A, Bonnet JL, Alessi MC, Cuisset T. Impact of obesity and the metabolic syndrome on response to clopidogrel or prasugrel and bleeding risk in patients treated after coronary stenting. Am J Cardiol. 2014;113:54–59. [DOI] [PubMed] [Google Scholar]
- 14.Sibbing D, von Beckerath O, Schomig A, Kastrati A, von Beckerath N. Impact of body mass index on platelet aggregation after administration of a high loading dose of 600 mg of clopidogrel before percutaneous coronary intervention. Am J Cardiol. 2007;100:203–205. [DOI] [PubMed] [Google Scholar]
- 15.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32–42. [DOI] [PubMed] [Google Scholar]
- 16.Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmentier JH, Batt AM, Kremers P. Interleukin 1 beta and interleukin 6 repress clofibric acid induction of different P450 isoforms in cultured foetal rat hepatocytes. Xenobiotica. 1996;26:1181–1193. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Lauver DA, Wang H, Sun D, Hollenberg PF, Chen YE, Osawa Y, Eitzman DT. Significant Improvement of Antithrombotic Responses to Clopidogrel by Use of a Novel Conjugate as Revealed in an Arterial Model of Thrombosis. J Pharmacol Exp Ther. 2016;359:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol. 2014;20:8525–8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilford BL, Parson JC, Grote CW, Vick SN, Ryals JM, Wright DE. Increased FNDC5 is associated with insulin resistance in high fat-fed mice. Physiol Rep. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99:306–314. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Wang Q, Kleiman K, Guo C, Eitzman DT. Hematopoietic Deficiency of miR-223 Attenuates Thrombosis in Response to Photochemical Injury in Mice. Sci Rep. 2017;7:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series). Arterioscler Thromb Vasc Biol. 2007;27:2079–2093. [DOI] [PubMed] [Google Scholar]
- 25.Houston JB, Kenworthy KE. In vitro-in vivo scaling of CYP kinetic data not consistent with the classical Michaelis-Menten model. Drug Metab Dispos. 2000;28:246–254. [PubMed] [Google Scholar]
- 26.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 27.Hagihara K, Kazui M, Ikenaga H, Nanba T, Fusegawa K, Takahashi M, Kurihara A, Okazaki O, Farid NA, Ikeda T. Comparison of formation of thiolactones and active metabolites of prasugrel and clopidogrel in rats and dogs. Xenobiotica. 2009;39:218–226. [DOI] [PubMed] [Google Scholar]
- 28.Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. [DOI] [PubMed] [Google Scholar]
- 29.Tai T, Mi QY, Ji JZ, Yin Q, Pan YQ, Zhang MR, Huang BB, Xie HG. Enhanced Platelet Response to Clopidogrel in Abcc3-deficient Mice Due to Its Increased Bioactivation. J Cardiovasc Pharmacol. 2016;68:433–440. [DOI] [PubMed] [Google Scholar]
- 30.Jiang XL, Samant S, Lesko LJ, Schmidt S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet. 2015;54:147–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Zhou ZY, Chen YB, Li JL, Yu WB, Chen XM, Zhao M, Zhao YQ, Cai YF, Jin J, Huang M. Associations of CYP3A4, NR1I2, CYP2C19 and P2RY12 polymorphisms with clopidogrel resistance in Chinese patients with ischemic stroke. Acta Pharmacol Sin. 2016;37:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price MJ, Murray SS, Angiolillo DJ, Lillie E, Smith EN, Tisch RL, Schork NJ, Teirstein PS, Topol EJ, Investigators G. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. J Am Coll Cardiol. 2012;59:1928–1937. [DOI] [PubMed] [Google Scholar]
- 33.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. [DOI] [PubMed] [Google Scholar]
- 34.Heydemann A An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J Diabetes Res. 2016;2016:2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatta A, Yao L, Xu Z, Toque HA, Chen J, Atawia RT, Fouda AY, Bagi Z, Lucas R, Caldwell RB, Caldwell RW. Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovasc Res. 2017;113:1664–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rollini F, Franchi F, Muniz-Lozano A, Angiolillo DJ. Platelet function profiles in patients with diabetes mellitus. J Cardiovasc Transl Res. 2013;6:329–345. [DOI] [PubMed] [Google Scholar]
- 37.Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohemy A, Karmali M. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;3:173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 39.Iizuka N, Oka M, Hamamoto Y, Mori N, Tamesa T, Tangoku A, Miyamoto T, Uchimura S, Nakayama H, Hamada K, Yamada-Okabe H. Altered Levels of Cytochrome P450 Genes in Hepatitis B or C Virus-infected Liver Identified by Oligonucleotide Microarray. Cancer Genomics Proteomics. 2004;1:53–58. [PubMed] [Google Scholar]
- 40.Ying L, Wang F, Zhang J, Yang L, Gong X, Fan Y, Xu K, Li J, Lu Y, Mei L, Zhou Z, Li C. Impact of hepatitis B virus (HBV) infection on platelet response to clopidogrel in patients undergoing coronary stent implantation. Thromb Res. 2018;167:119–124. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Lauver DA, Lucchesi BR, Hollenberg PF. Formation, reactivity, and antiplatelet activity of mixed disulfide conjugates of clopidogrel. Mol Pharmacol. 2013;83:848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauver DA, Kuszynski DS, Christian BD, Bernard MP, Teuber JP, Markham BE, Chen YE, Zhang H. DT-678 inhibits platelet activation with lower tendency for bleeding compared to existing P2Y12 antagonists. Pharmacol Res Perspect. 2019;7:e00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.