Abstract
Background:
Previous studies demonstrated increased digestive tract cancers among individuals with cystic fibrosis (CF), particularly among lung transplant recipients. We describe cancer incidence among CF and non-CF lung recipients.
Methods:
We used data from the US transplant registry and 16 cancer registries. Standardized incidence ratios (SIRs) compared cancer incidence to the general population, and competing risk methods were used for the cumulative incidence of colorectal cancer.
Results:
We evaluated 10,179 lung recipients (1,681 with CF). Risk was more strongly increased in CF recipients than non-CF recipients for overall cancer (SIR 9.9 vs. 2.7) and multiple cancers including colorectal cancer (24.2 vs. 1.7), esophageal cancer (56.3 vs. 1.3), and non-Hodgkin lymphoma (61.8 vs. 9.4). At five years post-transplant, colorectal cancer was diagnosed in 0.3% of CF recipients aged <50 at transplant and 6.4% aged ≥50.
Conclusions:
CF recipients have increased risk for colorectal cancer, suggesting a need for enhanced screening.
Keywords: cancer, transplant
Introduction
Cystic fibrosis (CF) is one of the most common life-shortening, genetic disorders and occurs in approximately one in 3,500 Caucasian births in the US (1). Mutations in the cystic fibrosis transmembrane regulator (CFTR) gene lead to malfunctioning or absent CFTR protein, which impairs mucosal clearance mechanisms causing recurring lung infections, inflammation, and airflow obstruction. Over time there have been tremendous improvements in CF treatment. In 2014, the median predicted survival in the US was 39.3 years, reflecting steady increases in survival since the early 1960s (2). Bilateral lung transplantation is a treatment option for some individuals with CF with severe lung disease. Currently, approximately 200–250 individuals with CF receive a lung transplant each year in the US (2).
Cancer is emerging as a long-term complication of CF. During the 1980s, numerous case reports described individuals with CF developing malignancies of the digestive tract, pancreas, and gallbladder (3–7). These observations led to single center and registry-based studies. While these studies consistently showed that people with CF did not have elevated overall cancer risk, they demonstrated 3–6 fold increased risks for cancers of the digestive tract (8–11).
Specifically, cancer risk was found to be elevated among CF patients who received a lung transplant, with almost 3-fold increased risk for cancer overall and 17-fold increased risk for digestive tract cancers (11). Maisonneuve et al. reported a 6-fold increased risk of colon cancer among CF patients without a transplant and a 30-fold increase among CF patients with a lung transplant (11). Transplant recipients have increased risk for a number of cancers, especially those caused by viruses, as a result of immunosuppression administered to prevent rejection of the transplanted organ (12, 13). However, the strong increase in digestive tract cancers, especially colon cancer, observed in transplant recipients with CF is not typical of other transplant recipients (12–14).
The primary objective of this study is to describe cancer incidence among lung transplant recipients with CF and compare cancer incidence to that in lung recipients without CF. We focused on colorectal cancer and other gastrointestinal tract cancers. Strengths of our study include the evaluation of a large representative population of lung transplant recipients in the US and systematic ascertainment of incident cancer diagnoses.
Methods
We used data from the Transplant Cancer Match (TCM) Study (13). The TCM Study links the US solid organ transplant registry (i.e., Scientific Registry of Transplant Recipients, SRTR) to data from 16 state and metropolitan region cancer registries (13). The SRTR includes data from the Organ Procurement and Transplant Network on all US transplant recipients, including demographic, clinical, and transplant characteristics, and patient and graft survival. Cancer registries collect data on all malignancies other than basal and squamous cell skin cancers in their defined geographic area. Individuals are eligible for the TCM Study based on whether their residence when listed for transplant or transplanted was covered by a participating cancer registry. The linked data in the TCM Study thus capture incident cancers for 45% of all US transplants during 1987–2011. The TCM Study was approved by institutional review boards at the National Cancer Institute and, as required, participating cancer registries.
We included all lung recipients in the TCM Study with post-transplant follow-up transplant during a period covered by a participating cancer registry. We excluded patients who received a combined lung and other organ transplant (n=41 among the CF subgroup). The cohort was restricted to non-Hispanic whites, non-Hispanic blacks, Hispanics, and Asian/Pacific Islanders, since data on expected cancer rates were available for these groups. The analysis excluded American Indians/Native Alaskans, multiracial individuals, and those with unknown race.
Analyses were conducted using the following SRTR variables: reason for transplant (CF vs. other), age at transplant, sex, race/ethnicity, type of lung transplant (bilateral sequential, en-bloc double, single right lung, single left lung), calendar year of transplant, baseline Epstein-Barr virus serostatus (negative, positive, missing), and cigarette use (no, yes, not collected during transplant year, collected but missing). Cancers were identified using cancer registry data and categorized according to a modified version of the Surveillance, Epidemiology and End Results (SEER) site recode (13).
Follow-up was calculated separately for each transplant (3.6% of individuals with more than one transplant were evaluated in successive intervals). Individuals were followed for cancer from lung transplantation or start of cancer registry coverage, whichever came later, until the first of the following events: death (50% of recipients exited for this reason) graft failure or a subsequent transplant (5%), loss to follow-up by the SRTR (4%), or end of cancer registry coverage (40%).
We calculated standardized incidence ratios (SIRs) to evaluate cancer risk of lung recipients compared to the general population. Expected cancer rates were calculated in strata defined by age, sex, race/ethnicity, calendar year and registry region. SIRs were calculated for all cancers combined and for specific cancers, separately for CF and non-CF lung recipients. For non-Hodgkin lymphoma (NHL), the most common cancer among CF recipients, we used Poisson regression to calculate SIR ratios comparing risk in CF and non-CF recipients, adjusting for age and EBV serostatus at time of transplant.
For colorectal cancer, we sought to understand how absolute risk among lung recipients compared to risk in the general population at age 50 (the recommended age for routine colorectal cancer screening) (15). We therefore calculated the cumulative incidence of colorectal cancer, stratified by CF status and age at transplant (<50 vs. 50+ years) (16). Follow-up was measured from transplant until the first of: colorectal cancer diagnosis, graft failure, re-transplantation, death, or loss to follow-up. Cumulative incidence accounts for the competing risk of death (17). This method could not accommodate delayed entry, so we excluded a small number of recipients whose transplant occurred before cancer registry coverage (23 CF recipients and 171 non-CF recipients).
Results
We evaluated 10,179 lung recipients comprising 1,681 CF recipients and 8,498 non-CF recipients. Patient characteristics are displayed in Table 1. Age at transplant differed substantially as 77% of CF recipients were younger than age 35, compared with only 7% of non-CF recipients. CF recipients primarily received a bilateral transplant versus fewer than half of non-CF recipients (94% versus 43%). CF recipients were more likely than non-CF recipients to be EBV seronegative at the time of transplant (although there was substantial missing data). In addition, although data were not collected for the entire time period and there were also missing data, only 1% of CF recipients were reported to have smoked >10 pack years, compared to 30% of non-CF recipients. Use of induction and maintenance immunosuppressive medications did not differ between CF and non-CF recipients (data not shown).
Table 1.
Characteristics of U.S. Lung Transplant Recipients
| Characteristic | CF recipient N (% of total) | Non-CF recipient N (% of total) | p-value | |
|---|---|---|---|---|
| Total | 1,681 (100) | 8,498 (100) | ||
| Sex | Male | 846 (50.3) | 4,522 (53.2) | 0.03 |
| Female | 835 (49.7) | 3,976 (46.8) | ||
| Age at Transplant, years | 0–19 | 381 (22.7) | 234 ( 2.8) | <0.001 |
| 20–34 | 915 (54.4) | 386 (4.5) | ||
| 35–49 | 335 (19.9) | 1,778 (20.9) | ||
| 50–64 | 48 ( 2.9) | 5,150 (60.6) | ||
| 65+ | 2 ( 0.1) | 950 (11.2) | ||
| Race/Ethnicity | White, Non-Hispanic | 1,580 (94.0) | 7,121 (83.8) | <0.001 |
| Black, Non-Hispanic | 17 ( 1.0) | 719 ( 8.5) | ||
| Hispanic | 81 ( 4.8) | 526 ( 6.2) | ||
| Asian/Pacific Islander | 3 ( 0.2) | 132 ( 1.6) | ||
| Transplant Procedure Type | Bilateral Sequential | 1,575 (93.7) | 3,660 (43.1) | <0.001 |
| En-Bloc Double Lung | 57 ( 3.4) | 259 ( 3.0) | ||
| Single Left Lung | 22 ( 1.3) | 2,305 (27.1) | ||
| Single Right Lung | 27 ( 1.6) | 2,274 (26.8) | ||
| Year of Transplant | 1987–1994 | 174 (10.4) | 980 (11.5) | 0.02 |
| 1995–1999 | 405 (24.1) | 1,808 (21.3) | ||
| 2000–2004 | 472 (28.1) | 2,257 (26.6) | ||
| 2005–2009 | 562 (33.4) | 3,062 (36.0) | ||
| 2010–2011 | 68 ( 4.0) | 391 ( 4.6) | ||
| EBV Serostatus | Negative | 268 (15.9) | 658 (7.7) | <0.001 |
| Positive | 761 (45.3) | 4,383 (51.6) | ||
| Missing | 652 (38.8) | 3,457 (40.7) | ||
| Cigarette use > 10 pack-years years | No | 456 (27.1) | 734 (8.6) | <0.001 |
| Yes | 20 (1.2) | 2585 (30.4) | ||
| Missing | 455 (27.1) | 919 (10.8) | ||
| Not Collected1 | 750 (44.6) | 4260 (50.1) |
Data on smoking status were only collected for tranplants during 1995–2004.
During 35,514 person-years of follow-up (mean 3.7 and 3.4 person-years for CF and non-CF recipients, respectively), 845 cancers were diagnosed (85 among CF recipients and 760 in non-CF recipients). This reflects an unadjusted rate of 1.3 per 100 person years among CF recipients and 2.6 per 100 person years among non-CF recipients. Compared with the general population, CF recipients had an almost 10-fold elevation in overall cancer risk (SIR=9.9, 95% confidence interval (CI)=7.9, 12.3) and non-CF recipients had a 2.7-fold elevation (SIR=2.7, 95%CI= 2.5, 2.9). Among the cancer types evaluated, there were 24 with at least one case diagnosed among a CF lung transplant recipient. SIRs for these are displayed in Figure 1, and observed counts and SIRs for all cancers are presented in Supplemental Table 1. The most common cancers among CF recipients were NHL (n=34, 40% of the total) and colorectal cancer (n=15, 18%). For non-CF recipients, the most common cancers were lung cancer (n=215, 25%) and NHL (n=128, 17%).
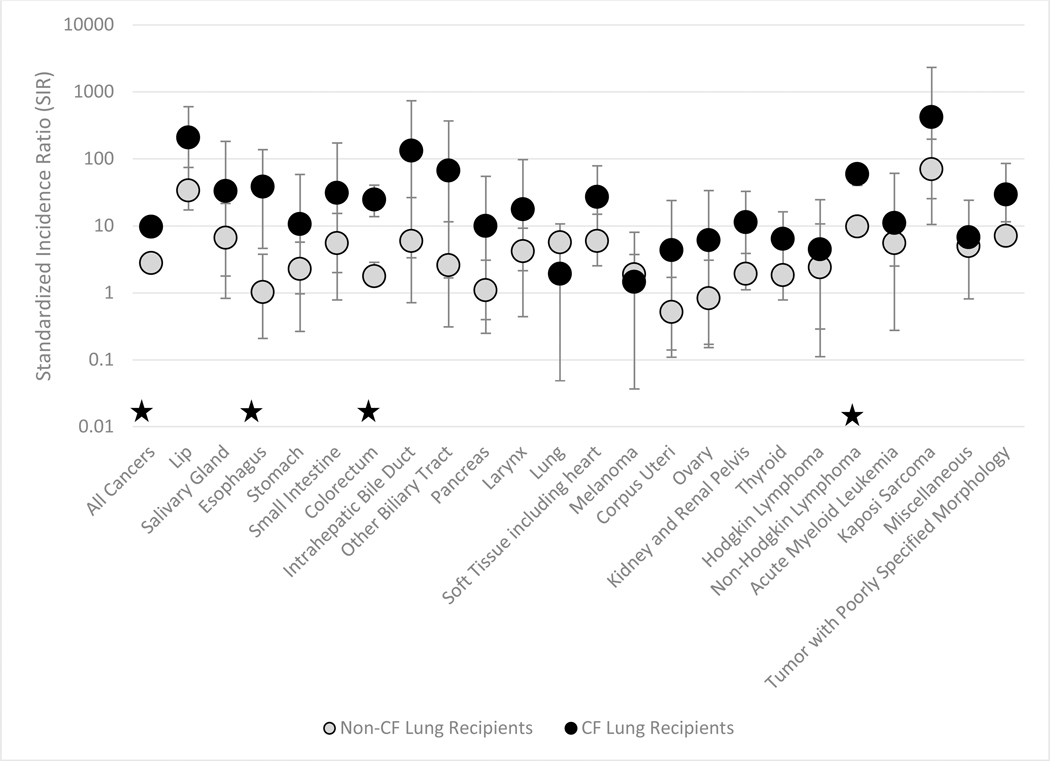
Figure 1: Standardized Incidence Ratios for Selected Cancers among CF and Non-CF Lung Recipients.
The figure shows standardized incidence ratios (circles) and 95% confidence intervals (vertical lines) among lung recipients with and without cystic fibrosis (CF). Results are shown for cancer for which there was at least 1 observed case among recipients with CF. Cancers for which the SIR for CF recipients is statistically significantly different from the SIR for non-CF recipients are marked by a ★
Compared to the general population, CF recipients had significantly elevated incidence for cancers of the lip (n=3, SIR=203, 95%CI=41.8, 592), esophagus (n=3, SIR=56.3, 95%CI=11.6, 165), colorectum (n=15, SIR=24.2, 95%CI=13.5, 39.8), soft tissue (n=3, SIR=26.5, 95%CI=5.5, 77.4), kidney and renal pelvis (n=3, SIR=11.0, 95%CI=2.3, 32.3), and thyroid (n=4, SIR=6.2, 95%CI=1.7, 16.0), as well as for NHL (n=34, SIR=61.8, 95%CI=42.8, 86.3) and poorly specified tumors (n=3, SIR=28.8, 95% CI=5.9, 84.3). In addition, elevated risk was observed for the following cancers with only one observed case: intrahepatic bile duct (SIR=130, 95%CI=3.3, 727), other biliary tract excluding gall bladder (SIR=65.5, 95%CI=1.7, 365), acute monocytic leukemia (SIR=178, 95%CI=4.5, 991) and Kaposi sarcoma (SIR=409, 95%CI=10.3, 2280).
As indicated in Figure 1 by lack of overlap in the confidence intervals, the SIRs in CF recipients were significantly higher than in non-CF recipients for esophageal cancer, colorectal cancer, and NHL. We further explored these cancers by combining esophageal cancer with other upper digestive tract cancers (consistent with previous studies) (11), and we subdivided colorectal cancers by subsite and NHLs by histologic subtype (Supplemental Table 1). Risk for upper digestive tract cancer was significantly higher in CF recipients compared to non-CF recipients (SIR=27.5, 95%CI=8.9, 64.1 vs. SIR=2.3, 95%CI=1.3, 3.6).
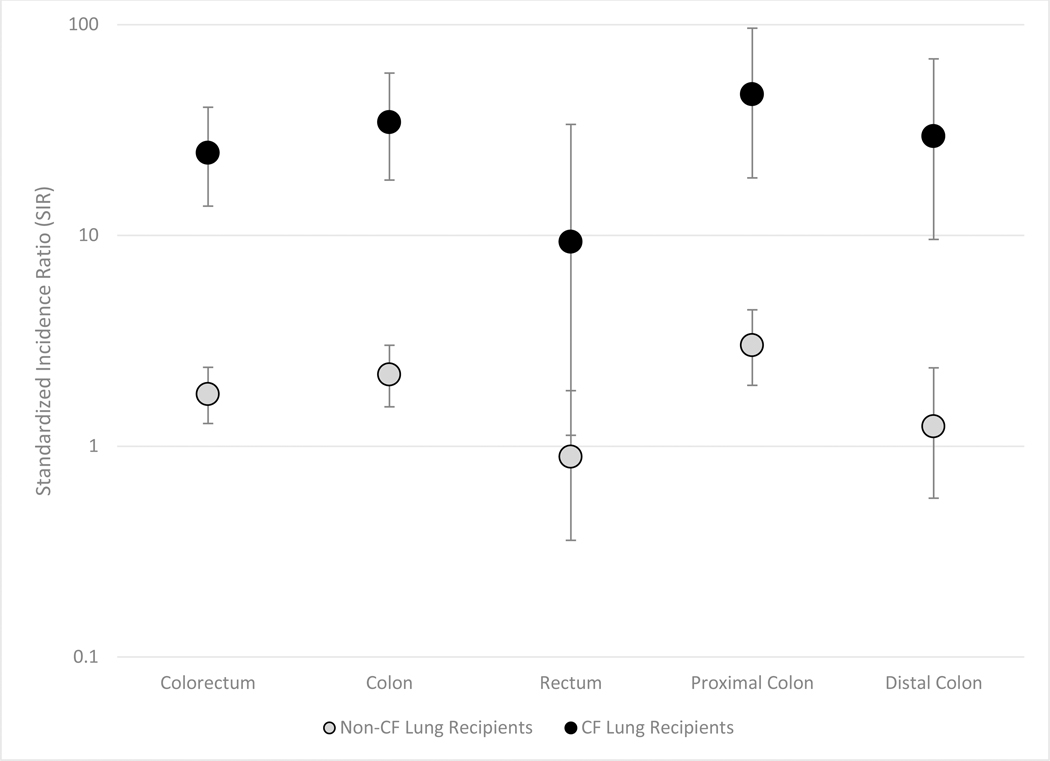
Risks for all subsites of the colorectum (proximal colon, distal colon and rectum) were significantly higher among CF recipients than in the general population, and the SIRs were also higher than among non-CF recipients (Figure 2, Supplemental Table 1). Of the 15 cases of colorectal cancer in CF recipients, only 2 were in patients transplanted after age 50. The median age at colorectal cancer diagnosis was 43 years and the median time from transplant to colorectal cancer was 6.4 years. Among CF recipients, 6 colorectal cancers were diagnosed at local stage, 5 were regional stage, and 4 were distant stage. There was no apparent pattern between the timing of cancer diagnosis and cancer stage. Of eight CF recipients with colorectal cancer who died, 6 died from their cancer, and their survival time ranged from 0.3 to 4.4 years after cancer diagnosis.
Figure 2: Standardized Incidence Ratios of Colorectal Cancer for CF and Non-CF Lung Recipients, by Colorectal Subsite.
The figure shows standardized incidence ratios (circles) and 95% confidence intervals (vertical lines) among lung recipients with and without cystic fibrosis (CF). Results are shown for colorectal cancer overall and for cancers arising in colorectal subsites
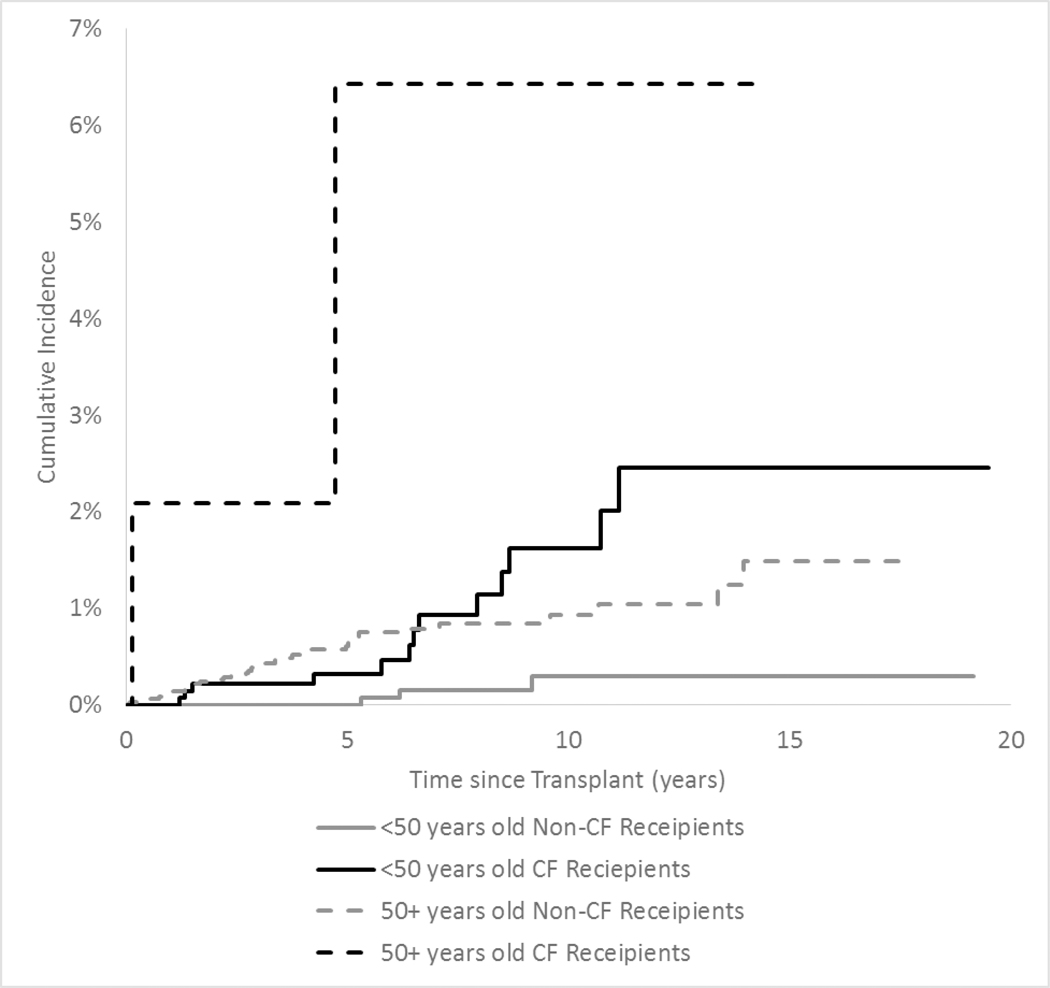
Cumulative incidence of colorectal cancer in CF and non-CF recipients is displayed in Figure 3. Among recipients under age 50 at transplant, those with CF had a five-year absolute risk of 0.3% of developing colorectal cancer, compared with a zero risk among non-CF recipients. Among CF recipients transplanted when they were younger than age 50, the cumulative incidence of colorectal cancer continued to rise over time, reaching 1.6% at 10 years after transplant. Among individuals who received a transplant after age 50, CF recipients had a five-year absolute risk of 6.4% for colorectal cancer compared to 0.6% among non-CF recipients.
Figure 3: Cumulative Incidence of Colorectal Cancer in Lung Recipients, by CF Status and Age at Transplant.
The figure shows the cumulative incidence of colorectal cancer among lung recipients according to reason for transplant and age at transplant. Black lines correspond to estimates for recipients with cystic fibrosis (CF) and gray lines correspond to estimates for recipients without CF.
NHL was the most commonly observed cancer among CF patients, and diffuse large B-cell lymphoma was the most common subtype (Supplemental Table 1). In univariate analyses, the ratio of SIRs for NHL among CF recipients vs. non-CF recipients was 6.5 (95%CI: 4.4, 9.4) (Table 2). Because the age and baseline EBV serostatus distribution of CF and non-CF recipients differed, we adjusted for these variables and observed that the difference in NHL risk was attenuated and no longer significant (adjusted SIR ratio: 1.6, 95%CI 0.9, 2.7) (Table 2).
Table 2:
Associations of Age and CF Status with NHL Risk
| Characteristic | Univariate Analysis SIR Ratio (95% CI) | Multivariate Analysis* SIR Ratio (95% CI) | |
|---|---|---|---|
| Age At Transplant, years | |||
| 0–19 | 22.4 (12.4, 37.6) | 13.1 (6.7, 24.2) | |
| 20–34 | 8.9 (5.5, 13.8) | 5.6 (2.9, 10.3) | |
| 35–49 | 1.9 (1.2, 2.8) | 1.7 (1.1, 2.6) | |
| 50–64 | Reference | Reference | |
| 65+ | 0.7 (0.3, 1.2) | 0.8 (0.4, 1.4) | |
| Baseline EBV Serostatus | |||
| Negative | 6.6 (4.1, 10.7) | 5.0 (3.1, 8.1) | |
| Positive | Reference | Reference | |
| Unknown | 2.5 (1.7, 3.8) | 2.2 (1.5, 3.3) | |
| Cystic Fibrosis | |||
| No | Reference | Reference | |
| Yes | 6.5 (4.4, 9.4) | 1.6 (0.9, 2.8) |
Multivariate model includes age at transplant, baseline EBV serostatus and CF status.
Discussion
In this large, population-based study of cancer among lung transplant recipients, individuals with CF had a 10-fold elevation in overall cancer risk compared to the general population, but this risk varied substantially for different cancer types. Notably, risk was 24-fold elevated for colorectal cancer, which was the second most common malignancy in CF lung recipients. NHL was most common, but the increased risk compared to other lung recipients was primarily due to the younger age and EBV serostatus of the CF group, as the difference largely disappeared after adjustment for these factors. There were numerous cancers with no cases among CF patients; however, small numbers prevent us from determining whether these represent true decreases in risk compared to the general population.
This study confirms previous observations that CF patients have markedly high overall risk of cancer, especially digestive tract cancers (11). Overall cancer risk was higher in this study than observed previously (SIR of 9.9 versus 2.7) (11), which might be due to more complete ascertainment of cancers. Specifically, we observed 3.3 times more cancers while only having 76% of the person-time compared to the Maisonneuve study, which relied on reports from CF clinicians. We had sufficient sample size to document significantly elevated risks for cancers at a number of digestive tract sites, including the esophagus and colorectum subsites. We also observed non-significant elevations for stomach and small intestine cancers. A previous single center study examined risk specifically for colorectal cancer and, similar to this study, reported higher colorectal cancer incidence among CF than non-CF lung recipients (18).
Several factors other than CF itself may contribute to differences in cancer risks. Compared with other lung recipients, CF recipients were substantially younger, less likely to smoke, and more frequently received a bilateral lung transplant. The impact of the difference in age is apparent, in that CF recipients had a lower incidence of overall cancer than non-CF recipients. Lung cancer was the most common cancer in non-CF recipients in our study, but risk was not increased in CF recipients. Among non-CF recipients, many of whom only receive a single lung, lung cancers typically arise in the native lung, which has been damaged by the disease process leading to transplantation. Lung transplants in CF patients are nearly all bilateral, which provides them with two relatively healthy lungs that are unlikely to harbor substantial pre-neoplastic damage. Furthermore, the very low smoking rates among CF recipients contribute to their low risk of developing lung cancer.
The apparently high risk of NHL in CF recipients relative to non-CF recipients is largely explained by their young age and greater likelihood of baseline EBV seronegative status, since the difference in NHL risk between CF and non-CF recipients was attenuated and no longer significant after adjustment for these differences. NHL and other types of post-transplant lymphoproliferative disorder are often related to primary infection with Epstein-Barr virus, which occurs most commonly among young transplant recipients.
The remarkably high risk of upper gastrointestinal tract and colorectal cancers among CF recipients has been reported previously (11, 14, 18, 19). Our study demonstrates that colorectal cancer risk is elevated for all subsites (i.e., proximal and distant colon, rectum). CFTR mutations may directly contribute to colorectal cancer since CFTR may act as a tumor suppressor gene (20). Indirectly, impaired function of CFTR is associated with intestinal stasis, chronic gut inflammation, and malabsorption of micronutrients. Additionally, individuals with CF receive repeated prolonged courses of antibiotics to treat pulmonary infections, which alter the gut microbiota, possibly contributing to colorectal carcinogenesis (21). These processes might be exacerbated by the immunosuppression associated with transplantation.
In the US, it is recommended that all individuals aged 50–75 receive colorectal cancer screening via fecal occult blood testing, colonoscopy or sigmoidoscopy (15). High-risk groups, including people with a personal or family history of colorectal cancer or those with familial polyps or inflammatory bowel disease, are singled out for earlier and more intensive screening, since their risk is 2–3 times higher than the general population (15). In the general population, the probability of a person aged 50 developing colorectal cancer within 5 years and 10 years is 0.3% and 1.0%, respectively (statistics available at http://seer.cancer.gov/canques/). In comparison, we show that CF patients who received a lung transplant before age 50 reached a risk of 0.3% by 5 years and 1.6% by 10 years. Gory et al. observed a 10-fold increase in colorectal cancer found during screening of CF patients compared to non-CF controls (22). Furthermore, in a single site initiative to perform colonoscopy on CF patients (aged 40 and older), Billings et al. documented adenomatous polyps in 8 of 27 (30%) CF patients who had not received a lung transplant and 3 of 7 (43%) CF patients who had received a transplant (23). These data suggest the need to include CF lung recipients in the high-risk category for colorectal screening, including initiation of screening before age 50 and perhaps at increased frequency.
Transplant recipients differ from the general population with regard to the presence of immunosuppression and their medical comorbidity. The present study is the largest study to date that compared CF and non-CF recipients. An added strength is that we utilized data from the SRTR (which captures data on all US transplants) and 16 population-based cancer registries (which ascertain all reportable cancers in their catchment regions). Previous studies were primarily conducted within the Cystic Fibrosis Foundation (CFF) Patient Registry (8–11). This registry captures data on care received at centers within the CFF Care Center Network, but outside care received at transplant or oncology centers is not consistently available.
Despite our study’s large size, the number of cases expected for specific cancers was limited in the CF group. Thus, we may have lacked power to observe true increases in risk for some cancers, confidence intervals were wide for some estimates, and we could not examine all cancer subtypes. When interpreting results from this study, because cancer incidence is rare, there can be large relative differences in risk that are associated with more modest changes in absolute risk.
We also could not capture cancers in individuals who migrated out of the state in which they received their transplant; however, this under-ascertainment is small (16). We focused only on lung recipients and therefore excluded individuals who received multiple organs. It was felt that individuals who received multiple organs would be different than single organ recipients, but the numbers were too small to examine them separately.
Given the small number of such recipients, this exclusion would not have materially affected our results. While our study provides evidence of an increased risk of cancer in lung recipients with CF, our population did not include CF patients who had not undergone lung transplantation, and so we could not determine whether the excess can be attributed solely to the underlying CF or if there is a synergistic effect of CF and transplantation.
In summary, our large population-based study showed that CF patients who have received a lung transplant have an increased risk of cancer, which is especially notable for colorectal cancer. These data support the need for investigations into colorectal cancer surveillance among CF lung transplant recipients. CF patients may benefit from screening colonoscopies beginning at an earlier age or at increased frequency compared to screening recommended for the general population.
Supplementary Material
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Cancer Institute. The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California (Tina Clarke), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa, Illinois (Lori Koch), Kentucky (Jaclyn Nee), Michigan), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Leticia Nogueria), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
The SRTR is currently operated under contract number HHSH250201500009C (Health Resources and Services Administration) by the Minneapolis Medical Research Foundation, Minneapolis, MN. Previously the SRTR was managed under contracts HHSH250201000018C and HHSH234200537009C. The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013-00021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5U58DP003883-03), Maryland (U58DP12-1205 3919-03), Michigan (5U58DP003921-03), New Jersey (5U58/DP003931-02), New York (U58DP003879), North Carolina (U58DP000832) and Texas (5U58DP000824-04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey, New York (including the Cancer Surveillance Improvement Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
References
- 1.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foundation CF. Annual Data Report to the Center Directors. Bethesda, MD: Cystic Fibrosis Foundation, 2014. [Google Scholar]
- 3.McIntosh JC, Schoumacher RA, Tiller RE. Pancreatic adenocarcinoma in a patient with cystic fibrosis. The American journal of medicine. 1988;85(4):592. [DOI] [PubMed] [Google Scholar]
- 4.Tedesco FJ, Brown R, Schuman BM. Pancreatic carcinoma in a patient with cystic fibrosis. Gastrointestinal endoscopy. 1986;32(1):25–6. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JA, Tullett WM, Thomas JS, Galloway D, Stack BH. Bowel adenocarcinoma in a patient with cystic fibrosis. Scottish medical journal. 1986;31(2):109. [DOI] [PubMed] [Google Scholar]
- 6.Redington AN, Spring R, Batten JC. Adenocarcinoma of the ileum presenting as non-traumatic clostridial myonecrosis in cystic fibrosis. British medical journal (Clinical research ed). 1985;290(6485):1871–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdul-Karim FW, King TA, Dahms BB, Gauderer MW, Boat TF. Carcinoma of extrahepatic biliary system in an adult with cystic fibrosis. Gastroenterology. 1982;82(4):758–62. [PubMed] [Google Scholar]
- 8.Neglia JP, FitzSimmons SC, Maisonneuve P, Schoni MH, Schoni-Affolter F, Corey M, et al. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med. 1995;332(8):494–9. [DOI] [PubMed] [Google Scholar]
- 9.Neglia JP, Wielinski CL, Warwick WJ. Cancer risk among patients with cystic fibrosis. J Pediatr. 1991;119(5):764–6. [DOI] [PubMed] [Google Scholar]
- 10.Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW, 3rd, Lowenfels AB. Cancer risk in nontransplanted and transplanted cystic fibrosis patients: a 10-year study. J Natl Cancer Inst. 2003;95(5):381–7. [DOI] [PubMed] [Google Scholar]
- 11.Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013;105(2):122–9. [DOI] [PubMed] [Google Scholar]
- 12.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. The Lancet. 2007;370(9581):59–67. [DOI] [PubMed] [Google Scholar]
- 13.Engels EA, Pfeiffer RM, Fraumeni JF Jr., , Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safaeian M, Robbins HA, Berndt SI, Lynch CF, Fraumeni JF, Jr., Engels EA. Risk of Colorectal Cancer After Solid Organ Transplantation in the United States. Am J Transplant. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137(2):129–31. [DOI] [PubMed] [Google Scholar]
- 16.Hall EC, Pfeiffer RM, Segev DL, Engels EA. Cumulative incidence of cancer after solid organ transplantation. Cancer. 2013;119(12):2300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–10. [DOI] [PubMed] [Google Scholar]
- 18.Meyer KC, Francois ML, Thomas HK, Radford KL, Hawes DS, Mack TL, et al. Colon cancer in lung transplant recipients with CF: increased risk and results of screening. J Cyst Fibros. 2011;10(5):366–9. [DOI] [PubMed] [Google Scholar]
- 19.Johannesson M, Askling J, Montgomery SM, Ekbom A, Bahmanyar S. Cancer risk among patients with cystic fibrosis and their first-degree relatives. Int J Cancer. 2009;125(12):2953–6. [DOI] [PubMed] [Google Scholar]
- 20.Than BL, Linnekamp JF, Starr TK, Largaespada DA, Rod A, Zhang Y, et al. CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gory I, Brown G, Wilson J, Kemp W, Paul E, Roberts SK. Increased risk of colorectal neoplasia in adult patients with cystic fibrosis: a matched case-control study. Scandinavian journal of gastroenterology. 2014;49(10):1230–6. [DOI] [PubMed] [Google Scholar]
- 23.Billings JL, Dunitz JM, McAllister S, Herzog T, Bobr A, Khoruts A. Early colon screening of adult patients with cystic fibrosis reveals high incidence of adenomatous colon polyps. J Clin Gastroenterol. 2014;48(9):e85–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.