Key Points
Question
What are the effects of hookworm treatment compared with placebo on relapsing multiple sclerosis?
Findings
In this randomized clinical trial that included 71 patients, the median cumulative numbers of new magnetic resonance imaging lesions were not significantly different between the groups, but approximately half of participants treated with hookworm vs approximately a quarter of those receiving placebo had no detectable magnetic resonance activity. Hookworm significantly increased T regulatory cell counts in peripheral blood.
Meaning
The data from this study suggest a possible, albeit mild, therapeutic effect of hookworm infection in relapsing multiple sclerosis that warrants further study.
Abstract
Importance
Studies suggest gut worms induce immune responses that can protect against multiple sclerosis (MS). To our knowledge, there are no controlled treatment trials with helminth in MS.
Objective
To determine whether hookworm treatment has effects on magnetic resonance imaging (MRI) activity and T regulatory cells in relapsing MS.
Design, Setting, and Participants
This 9-month double-blind, randomized, placebo-controlled trial was conducted between September 2012 and March 2016 in a modified intention-to-treat population (the data were analyzed June 2018) at the University of Nottingham, Queen’s Medical Centre, a single tertiary referral center. Patients aged 18 to 61 years with relapsing MS without disease-modifying treatment were recruited from the MS clinic. Seventy-three patients were screened; of these, 71 were recruited (2 ineligible/declined).
Interventions
Patients were randomized (1:1) to receive either 25 Necator americanus larvae transcutaneously or placebo. The MRI scans were performed monthly during months 3 to 9 and 3 months posttreatment.
Main Outcomes and Measures
The primary end point was the cumulative number of new/enlarging T2/new enhancing T1 lesions at month 9. The secondary end point was the percentage of cluster of differentiation (CD) 4+CD25highCD127negT regulatory cells in peripheral blood.
Results
Patients (mean [SD] age, 45 [9.5] years; 50 women [71%]) were randomized to receive hookworm (35 [49.3%]) or placebo (36 [50.7%]). Sixty-six patients (93.0%) completed the trial. The median cumulative numbers of new/enlarging/enhancing lesions were not significantly different between the groups by preplanned Mann-Whitney U tests, which lose power with tied data (high number of zeroactivity MRIs in the hookworm group, 18/35 [51.4%] vs 10/36 [27.8%] in the placebo group). The percentage of CD4+CD25highCD127negT cells increased at month 9 in the hookworm group (hookworm, 32 [4.4%]; placebo, 34 [3.9%]; P = .01). No patients withdrew because of adverse effects. There were no differences in adverse events between groups except more application-site skin discomfort in the hookworm group (82% vs 28%). There were 5 relapses (14.3%) in the hookworm group vs 11 (30.6%) receiving placebo.
Conclusions and Relevance
Treatment with hookworm was safe and well tolerated. The primary outcome did not reach significance, likely because of a low level of disease activity. Hookworm infection increased T regulatory cells, suggesting an immunobiological effect of hookworm. It appears that a living organism can precipitate immunoregulatory changes that may affect MS disease activity.
Trial Registration
ClinicalTrials.gov Identifier: NCT01470521
This randomized clinical trial assesses the effect of hookworm treatment on magnetic resonance imaging activity and T regulatory cells in British patients with relapsing multiple sclerosis.
Introduction
Multiple sclerosis (MS) is an immune-mediated central nervous system disease characterized clinically by neurological deficits with relapsing-remitting and progressive patterns and pathologically by inflammation, demyelination, and loss and damage to neurons. It is associated with an imbalance between inflammatory (eg, proinflammatory cytokines) and immunoregulatory factors (eg, anti-inflammatory cytokines and regulatory T cells [Tregs]).1
Multiple sclerosis is unevenly distributed across populations.1 The hygiene hypothesis postulates that certain infectious agents, including gastrointestinal helminths, protect against inflammatory diseases, including MS.2 These agents induce Tregs, which prevent autoimmunity.3 There is an inverse relationship between the prevalence and incidence of MS and of infections with the nematode Trichuris and other intestinal parasites.4,5 Patients with MS naturally infected with gastrointestinal parasites have milder disease than matched uninfected MS controls over 5 years; this is associated with indicators of higher Treg activity.6 There are trials of Trichuris suis ova or Necator americanus (N americanus) larvae in autoimmune and allergic conditions.7 In MS, pilot studies with Trichuris ova show a good safety profile and clinical, radiological, and immunological effects.8,9,10 These trials included few participants, were not placebo-controlled, and mostly had a short duration.11 The largest study (16 patients) reported moderate favorable magnetic resonance imaging (MRI) and immunological outcomes.11
N americanus is a hookworm infecting only humans, an evolutionary “old friend” that has accompanied humans during historical migration.12 Infection is generally benign but can cause anemia in cases of high parasite burdens.13 N americanus and MS show inverse geographical distributions.14 N americanus induces a mixed peripheral T-helper (Th) cell response with Th2 and Treg-like dominance.15 For therapy, larvae are applied to the skin, mimicking natural infection. N americanus establishes a controlled localized infection that can last longer than 5 years without need for repeated exposure and shows no aberrant extraintestinal migration or risk of transmission to others in good hygiene conditions.16 A dose-ranging program at the University of Nottingham in healthy volunteers indicated that infection with 10 to 50 N americanus larvae is safe and induces Th2 responses with eosinophilia.17,18 The pilot trials with N americanus in asthma, allergic rhinoconjunctivitis, and inflammatory bowel disease showed the safety of controlled infection in these conditions.19
To our knowledge, there are no published placebo-controlled helminth therapy trials in MS. We performed a phase 2, randomized, double-blind, placebo-controlled trial of N americanus (hookworm [HW]) in relapsing MS to determine whether therapeutic infection is safe, upregulates immunoregulatory mechanisms (Treg cells, eosinophils), and has effect on radiological and clinical activity.
Methods
Study Design and Participants
We performed this phase 2, single-center, randomized, blinded, placebo-controlled study of live HW infection with infective third-stage larvae (L3) of N americanus manufactured according to current good manufacturing practice regulations in patients with MS at Queen’s Medical Centre (QMC), University of Nottingham (Nottingham, England) (trial protocol in Supplement 1). Ethical approval was obtained (East Midlands National research ethics committee11). Patients were identified and recruited from the MS clinic at QMC or referred from other UK centers and provided written informed consent.
Clinically stable patients aged 18 to 64 years with relapsing-remitting MS or secondary progressive MS (SPMS) with relapses, fulfilling McDonald criteria, Expanded Disability Status Scale (EDSS) score of 0 to 5.5, 1 or more relapses in the last 12 months or 2 in the last 24 months, an MRI consistent with MS, and without immunomodulatory treatment were eligible for screening.20,21 Patients were excluded if they had received treatment with corticosteroids for fewer than 4 weeks, interferon β/glatiramer acetate/immunosuppressive drugs or any investigational agent 8 to 12 weeks or less before baseline, or bone marrow transplant or monoclonal antibodies at any time.
Randomization and Masking
After screening, patients were randomized with equal probability to the treatment arms using a computer-generated pseudorandom code using random stratified permuted blocks of randomly varying size, created by the Nottingham Clinical Trials Unit and held on a secure server. The research nurses and investigators administering the treatment, participants, and those assessing outcomes were masked to group assignment. The data monitoring committee was granted access to unmasked data but had no contact with the study participants. The unmasked pharmacists had no involvement or contact with study participants.
Procedures
Consenting patients fulfilling inclusion criteria were assessed pretreatment (screening and baseline) for anemia and stool parasitology. After a 7-day screening period, patients entered a 36-week treatment period followed by a 12-week safety follow-up (eAppendices 1 and 2 in Supplement 2). At baseline (month 0), they were randomized 1:1 to either receive experimental infection with 25 N americanus L3s or placebo (pharmacopoeial-grade water) pipetted onto a gauze pad and placed on the arm for longer than 30 minutes. eAppendix 3 in Supplement 2 shows the choice of larvae number, manufacture, and procedure. Participants were contacted by telephone after 1 week to check for well-being and seen at 2 weeks, 1 month, every month for 10 months, and at month 12. Concomitant medications, adverse events (AEs), and symptom diaries were checked and recorded at each visit. Routine blood cell counts, urine pregnancy test results, and stool samples were collected at specified points (eAppendices 1 and 2 in Supplement 2). Because of the unpredictability of microscopy egg counts at low infection intensity and the small sample volume, fecal N americanus egg presence was determined by DNA-targeting polymerase chain reaction (PCR; eAppendix 3 in Supplement 2) instead of microscopy as initially planned.
Magnetic resonance imaging was performed on 1.5-T General Electric Signa HDx at QMC Nottingham. The MRI protocol is provided in eAppendix 4 in Supplement 2. The patients were assessed clinically with monthly EDSS from months 1 to 10 and Multiple Sclerosis Functioning Composite scores at screening and month 9 (eradication of HW).22
Neurologic assessment was performed by the team’s examining physician. Symptoms and AEs were monitored by a treating physician. Relapses were defined as new or recurrent neurologic symptoms with objective signs not associated with fever or infection lasting longer than 24 hours. If needed, relapses were treated with methylprednisolone, 2500 to 3000 mg, over 3 to 5 days. At month 9, all patients were offered mebendazole, 100 mg, twice daily for 3 days to eradicate the parasite and were reassessed blindly at month 10. To rule out the theoretical possibility of inflammatory rebound after eradication, a final MRI and hematological test and physical and neurologic examination results were obtained at month 12.23 After the trial, participants were followed up in MS clinics. If the patients withdrew early, they were contacted and offered mebendazole.
Outcomes
The primary outcome measure was the cumulative number of new or enlarging T2 lesions or newly enhancing lesions over months 4 to 9 postinfection. Lesions that were simultaneously new/enlarged and enhancing were only counted once. T2 spin-echo images were registered using rigid registration and visualized slice by slice with automated switching between the 2 images. This highlighted new and enlarging lesions, which were counted. T1-weighted images acquired 13 minutes postgadolinium were registered to the equivalent image from the previous visit. The images were inspected slice by slice and the 2 visits automatically switched periodically to highlight newly enhancing lesions on the latter visit, which were counted.
The secondary outcome was the change at month 9 from month 0 in the percentage of cluster of differentiation (CD4) +CD25highFoxp3+T cells (marker of total Treg) from total CD4 + lymphocytes and the percentage of suppression in the Treg assay. CD4+CD25highCD127negT cells are surrogates of suppressor Treg subpopulations. We amended the protocol and replaced the percentage of suppression in the Treg assay with change at month 9 from month 0 in the percentage of T cells expressing CD4+CD25highCD127negT from total CD4 + lymphocytes, during the trial before unmasking for reproducibility and feasibility with steering committee approval. Treg cells were detected/counted using flow cytometry (eAppendix 5 in Supplement 2).
Relapses, EDSS scores, and MSFC score changes at month 9 were some of the exploratory outcomes. We obtained safety laboratory measures, including full blood cell count and hemoglobin, transaminase, and creatinine levels throughout the trial. Adverse events were recorded at each visit.
Statistical Analysis
Sample size calculation was based on Tubridy et al24 for frequent gadolinium MRI (primary end point). Assuming new lesion distribution similar to that study, using the Mann-Whitney U test to compare placebo and HW groups, 36 patients per arm (1:1 randomization) are needed to show 70% reduction (relative risk 0.3) between month 3 and month 9 with approximately 95% power (2-tailed significance of 5%).24
The primary efficacy statistic was the number of new, enlarging, or newly enhancing lesions by month 9. The threshold for statistical significance by Mann-Whitney U test was set at P < .05 (2-tailed). Statistical analyses were performed using R (R Foundation).
Missing values were compensated for using imputation separately by treatment arm. Two cases were considered: (1) replace missing values with means of other within-patient values; for any patient randomized but with all data missing, use the mean outcome from the group; and (2) replace missing values with maximum of other within-patient values; for any patient randomized but with all data missing, use the maximum outcome from the group. Safety analyses were performed on a safety set (ie, all randomized participants who received study treatment).
A post hoc analysis using a binary logistic regression assessed the odds of MRI activity in patients with complete MRI data. Baseline variables assessed for prediction of MRI activity were T2 lesion count, enhancing lesion count, age, sex, disease duration, Treg percentage (of total CD4+T cells), number of relapses in the preceding year, and treatment group.
Results
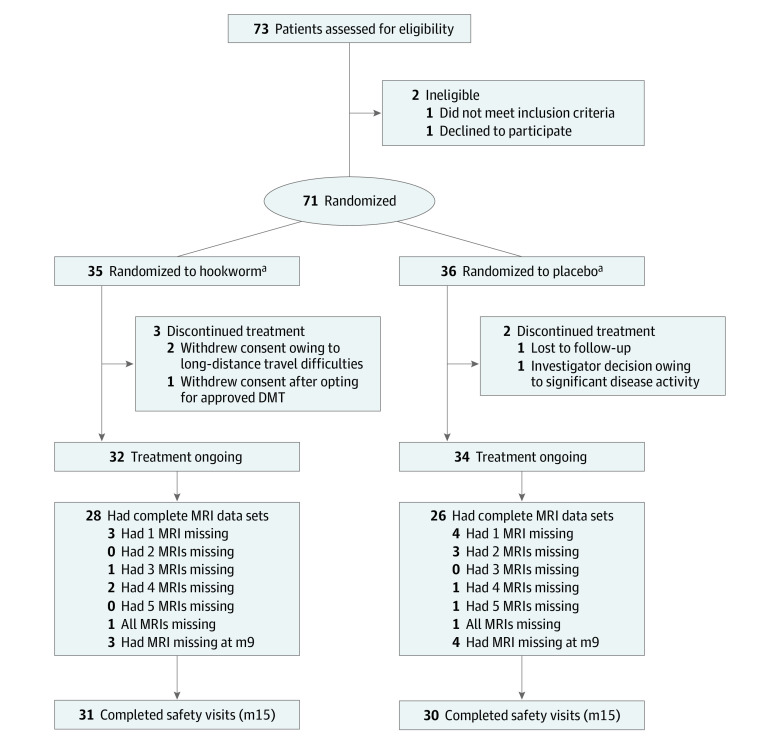
Of 73 participants screened between September 17, 2012, and March 26, 2015, 71 (97.2%) were randomly assigned to receive HW (35 [49.3%]) or placebo (36 [50.7%]) (modified intention-to-treat population) (Figure 1). In total, 66 (93%; 32 HW [48.9%], 34 placebo [51.1%]) patients completed the 9-month treatment period and 61 (86%; 31 HW [50.8%], 30 placebo [49.2%]) completed the 3-month safety follow-up (Figure 1). Baseline demographic and clinical characteristics were not different between the HW and placebo groups (Table 1).
Figure 1. Trial Profile.
Flow chart of participants and visits during the trial. At month 9 (m9), the primary end point, eradication offered. DMT indicates disease-modifying therapy; m15, month 15; MRI, magnetic resonance imaging.
aIntention-to-treat population.
Table 1. Demographic and Baseline Clinical Characteristics of All Randomized Patients.
| Patient characteristics | Hookworm (n = 35) | Placebo (n = 36) |
|---|---|---|
| Demographic characteristics | ||
| Age, mean (SD), y | 45 (8.7) | 44.7 (10.4) |
| Sex, No. (%) | ||
| Women | 25 (71) | 26 (71) |
| Men | 10 (29) | 10 (29) |
| Clinical characteristics | ||
| Type of relapsing MS, No. (%) | ||
| RR | 31 (88.6) | 27 (75) |
| SP with superimposed relapses | 4 (11.4) | 9 (25) |
| Time, mean (SD), y | ||
| From first symptoms of MS | 11.7 (8.3) | 11.1 (9.2) |
| From MS diagnosis | 6 (5.9) | 6.5 (5.7) |
| From last relapse, d | 195 (119) | 205 (172) |
| Relapses, mean (range) | ||
| In the previous year | 1.66 (1-4) | 1.33 (1-3) |
| Previous 2 y | 2.3 (1-5) | 2.1 (1-5) |
| Previous 3 y | 2.85 (1-7) | 2.55 (1-7) |
| EDSS score, mean (range) | 3 (1.5-5) | 3 (1.5-5) |
| Previous DMT | ||
| Glatiramer acetate | 2 | 2 |
| IFN-β | 7 | 8 |
| Other | 1 (Azathioprine) | 1 (Fingolimod) |
| MRI characteristics | ||
| Baseline T2 lesion No. | 15.3 (9.3) | 16.5 (8.4) |
| Median (range) | 14 (1-33) | 15 (1-36) |
| Gadolinium-enhancing lesion No. at baseline | ||
| Mean (range) | 1.15 (2.5) | 0.8 (1.5) |
| Median (range) | 0 (0-11) | 0 (0-6) |
| MRI scans without gadolinium-enhancing lesions at baseline | 23 | 21 |
Abbreviations: DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; IFN, interferon; MRI, magnetic resonance imaging; MS, multiple sclerosis; RR, relapsing-remitting; SP, secondary progressive.
There were no differences in the T2 lesion load on MRI results at the baseline run-in scan between participants assigned to receive HW or placebo (Table 1). Screening EDSS scores were not different between the groups (Table 1). The 25th percentile, median, and 75th percentile EDSS scores were 2, 3, and 4 in the HW and placebo groups.
The MRI and clinical outcomes are presented in Table 2. Fifty-four patients (76.1%) had complete data sets (28 HW [51.9%]; 26 placebo [48.1%]); 10 (14.1%) missed 1 or more MRI scans (Figure 1). At month 9, the cumulative number of new T2 lesions, newly enhancing lesions, or enlarging lesions was 154 in the HW group and 164 in the placebo group (mean-based imputation). This was not statistically significant. In the 71 randomized participants, the estimated difference in median range (placebo–HW) was 0 to 2 (P = .19; Mann-Whitney U tests results adjusted for ties) when means were imputed and 0 to 3 (P = .26; Mann-Whitney U test results adjusted for ties) when maximum values were imputed.
Table 2. MRI, Treg, and Clinical End Points.
| Characteristic | Hookworm | Placebo |
|---|---|---|
| Total No. of new T2 lesions, newly enhancing T1-weighted lesions, and T2 enlarging lesions from months 3-9 (participants with complete data) | 28 | 26 |
| Mean (SD) | 4.1 (6.75) | 3.8 (4.6) |
| Median (range) | 0 (0-22) | 1.5 (0-14) |
| Detectable MRI activity OR (95% CI) vs placebo (participants with complete data MRI sets [ n = 54]) | OR: 0.33 (0.11-1.02) | |
| HW: 16 (no MRI activity), 12 (active MRI) | ||
| Placebo: 8 (no MRI activity), 18 (active MRI) | ||
| No. of new T2 lesions from months 3-9, mean (SD) | 2.4 (4.3) | 2.4 (3.3) |
| No. of T2 enlarging lesions from months 3-9, mean (SD) | 0.8 (1.5) | 0.8 (1.1) |
| No. of newly enhancing T1-weighted lesions from months 3-9, mean (SD) | 0.9 (2.2) | 0.6 (1.4) |
| Patients with new gadolinium-enhanced T1-weighted lesions by visit, No./total No. | ||
| Month 4 (first visit after baseline) | 5/33 | 3/34 |
| Month 5 | 6/33 | 2/33 |
| Month 6 | 3/32 | 1/32 |
| Month 7 | 2/31 | 2/30 |
| Month 8 | 2/29 | 2/30 |
| Month 9 (end point) | 2/32 | 5/32 |
| Individual relapses per patientsa | No./total No. (%): 5/35 (14.3) | No./total No. (%): 11/36 (30.6) |
| 1 Patient had 2 relapses | 1 Patient had 2 relapses | |
| EDSS at month 9, mean (range) | 2.8 (1- 6) | 3.45 (1.5-6.5) |
| Change in EDSS score at month 9 vs month 3, mean (SD) | −0.17 (0.71) | 0.13 (0.74) |
| Change in MSFC at month 9 vs screening, mean (SD) | 0.1 (0.48) | 0.1 (0.67) |
| Percentage of CD4+CD25highCD127negT cells of total CD4+T cells | ||
| Screening | ||
| % (SE) | 3.5 (0.28) | 3.9 (0.24) |
| No. | 35 | 36 |
| Month 3 | ||
| % (SE) | 3.7 (0.23) | 4.2 (0.18) |
| No. | 34 | 36 |
| Month 9 | ||
| % (SE) | 4.4 (0.26) | 3.9 (0.24) |
| No. | 32 | 34 |
| CD4+CD25highCD127negT cells of total CD4+T cells at mo 9 vs screening | ||
| % (SE) | 1.0 (0.24) | −0.1 (0.3) |
| P value | NA | .01 2-Sided t test |
| Mean difference (95% CI) | NA | 0.97 (0.21-1.73) |
| Percentage of CD4+CD25highFoxp3+T cells of total CD4+T cells m9 vs screening | ||
| Screening | ||
| % (SE) | 2.9 (0.28) | 3.27 (0.24) |
| No. | 35 | 36 |
| Month 3 | ||
| % (SE) | 2.8 (0.22) | 3.44 (0.19) |
| No. | 34 | 36 |
| Month 9 | ||
| % (SE) | 3.3 (0.25) | 3.10 (0.18) |
| No. | 32 | 34 |
| Mean difference in change in % of CD4+CD25highFoxp3+T cells of total CD4+T cells | ||
| % (SE) | 0.5 (0.25) | −0.2 (0.27) |
| P value | NA | .09; 2-Sided t test |
| Mean difference | NA | 0.69 (−0.09 to 1.380) |
| Absolute eosinophil counts/month of evaluation, No (SD), μL | ||
| Month 1 | 0.32 (0.40) | 0 (0.09) |
| Month 3 | 0.71 (0.66) | 0 (0.09) |
| Month 6 | 0.36 (0.33) | 0.02 (0.09) |
| Month 9 | 0.21 (0.22) | 0.01 (0.09) |
Abbreviations: CD, cluster of differentiation; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; MSFC, multiple sclerosis functional composite; NA, not applicable; OR, odds ratio; Treg, T regulatory cells.
SI conversion factor: To convert eosinophils to ×109/L, multiply by 0.001.
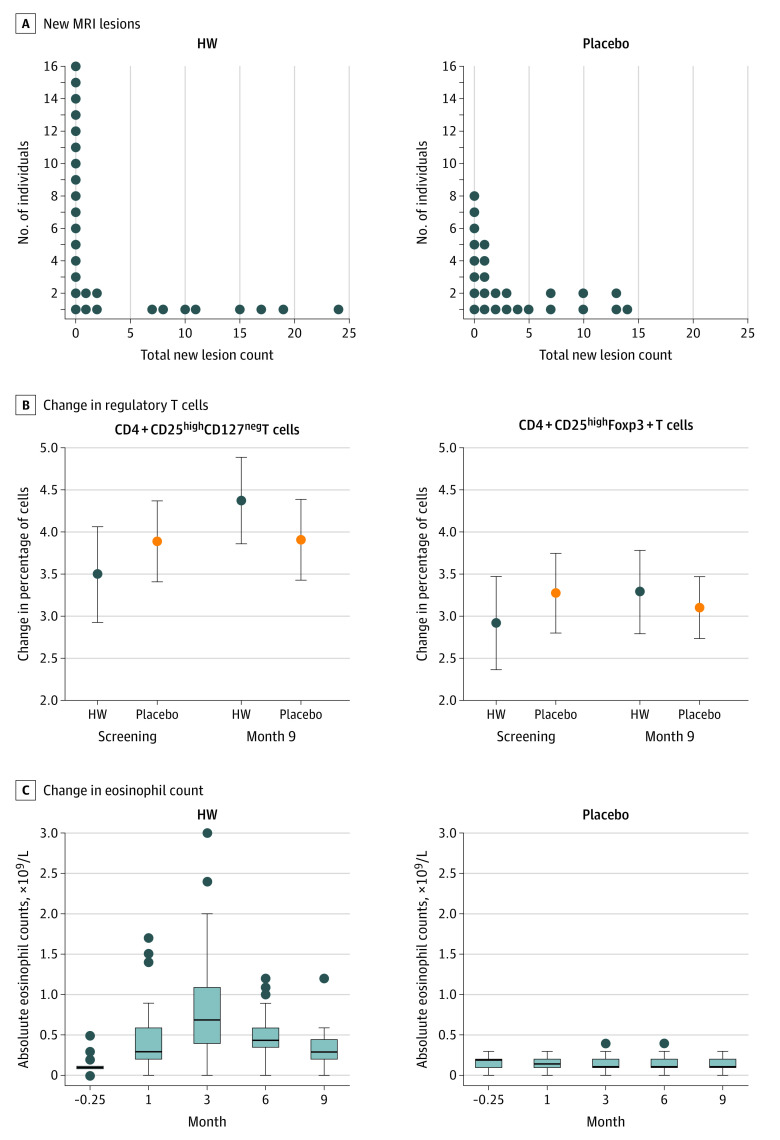
Eighteen of the patients treated with HW (51%) vs only 10 (28%) treated with placebo had no detectable MRI activity. In the per-protocol analysis of the 54 patients with complete data sets, 16 (57%) in the HW group vs 8 (31%) in the placebo had no MRI changes (Figure 2A). This is a higher number of tied zero-activity counts than expected in the HW arm given the sample used to power the study, in which of 59 patients, only 12 (20%; 95% CI, 11%-33%) showed no new activity over the trial period.24 This rendered the planned Mann-Whitney U test inappropriate, since it loses power in the presence of ties.25 This high rate of no detectable MRI activity in the HW, while reducing the study power, suggests a treatment effect.
Figure 2. New Magnetic Resonance Imaging (MRI) Lesions, Changes in Treg Cells, and Eosinophils During the Trial.
A, New MRI lesions during the trial. Total new lesion count (new, newly enhancing, and enlarging lesions) in the placebo participants (26 [74%]) and the participants who received hookworm (HW) (28 [78%]) with complete MRI data. B, Change in cluster of differentiation (CD) 4+CD25highCD127negT cells and CD4+CD25highFoxp3+T cells in the 2 groups during the trial. The y-axis indicates the change in the percentage of CD4+CD25highCD127negT cells of total CD4+T cells. The x-axis indicates the time (months). All participants with measurement used. The solid circle marker is HW (n = 35). The hollow circle is the placebo (n = 36). The error bars are ± 2 SE. C, Changes in eosinophil counts during the trial.
To explore reasons for the high number of participants having no MRI activity, in the post hoc analysis, the odds of MRI activity were assessed using a binary logistic regression on patients with complete MRI data. Of the variables assessed for predicting MRI activity, only baseline T2 was significant, with odds of 1.1 (95% CI, 1.0-1.2). Backward elimination to remove noncontributing terms to the model removed all significance. Adding HW treatment as a dependent predictor of odds reduced the model to just HW and baseline enhancing lesion count as contributing predictors with odds of 0.27 (95% CI, 0.08-0.90) and 2.4 (95% CI, 0.98-5.70). This analysis hints at larger baseline enhancing lesion counts increasing the odds and, independently, HW treatment lowering the odds of subsequent detectable MRI activity.
The number of newly enhancing lesions and patients with newly enhancing lesions was higher in the HW arm vs placebo at month 4 but shifted downward between months 4 and 9 while shifting upwards (suggesting a robust reduction to the mean) in the placebo arm (eAppendix 6 in Supplement 2). Accepting that numbers are small, one can hypothesize a trend toward reducing MRI activity with treatment duration in the HW arm.
At baseline, higher percentages of CD4+CD25highCD127negT cells and CD4+CD25highFoxp3+ were detected in the peripheral blood cell counts of the placebo than the HW arm (Figure 2b). There was an increase in the CD4+CD25highCD127negT cell percentage from total CD4+T cells from screening to month 9 in the HW group and a decrease in the placebo group (P = .01). The percentages of CD4+CD25highFoxp3+ from total CD4+lymphocytes increased in the HW group from screening to month 9 and decreased in the placebo group without reaching significance. In the post hoc analysis, percentages of CD4+foxp3+CD127neg were significantly increased in the HW group and decreased in the placebo group from months 0 to 9 (mean [SD], 3.04 [1.5]-3.41 [1.5]; n = 36; vs mean [SD], 3.62 [1.4]-3.25 [1.0]; n = 35).
Four participants (11.4%) in the HW arm and 10 (27.8%) in the placebo experienced relapses during the active period of the trial (5 relapses for HW; 11 relapses for placebo; Table 2). Six relapses (3 per /group) were treated with steroids, of which 2 (1 in each group) occurred during the outcome phase (months 3-9) of the trial. There were no steroid-attributable AEs. One patient from each arm was excluded from the study because of relapses.
The absolute eosinophil counts were unchanging in the placebo group but increased in all but 3 (for whom it stayed the same) participants treated with HW. They were maximal at month 3 posttreatment and remained elevated for most participants at month 9 (Figure 2C).
Twenty-three of 35 patients with infection (65.7%) were identified as positive for N americanus DNA by PCR and amplicon sequencing (eAppendix 3 in Supplement 2). No HW DNA was detected in placebo fecal samples. There was an increased eosinophil count in all but 3 patients who were assigned to the HW group and were PCR-negative.
Of the 305 AEs recorded (153 HW [50.2%], 152 placebo [49.8%]), 281 (92.1%) were nonrecurrent (137 HW [48.8%], 144 placebo [51.2%]) (Table 3). There were 9 serious AEs (SAE) in 7 patients (5 placebo [71.4%]; 2 HW [28.6%]). The SAEs in the HW arm were type 1 diabetes in 1 patient and a hysterectomy for menorrhagia in another. Two patients in the placebo arm had 2 SAEs each. There were no deaths. The most common AEs were diarrhea, skin reaction at the plaster site, and infections (urinary tract and upper respiratory tract) (Table 3). Hookworm infection showed a satisfactory safety profile. The only apparent differences were more common plaster-site reactions in the HW arm; however, this was also reported by one-third of patients in the placebo arm.
Table 3. Treatment-Emergent Adverse Effects.
| Summary of treatment-emergent adverse events | No./total No. (%) | |
|---|---|---|
| Hookworm | Placebo | |
| Any serious treatment-emergent adverse event | 2/35 (5.71) | 4/36 (11.11) |
| Any nonserious treatment-emergent adverse event | 35/35 (100.00) | 33/36 (91.67) |
| Death | 0 | 0 |
| Discontinuation of treatment because of a treatment-emergent adverse event | 0 | 0 |
| Serious treatment-emergent adverse events | Treated | Placebo |
| Total participants affected by serious adverse events | 2/35 (5.71) | 4/36 (11.11) |
| Surgical and medical procedures | ||
| Hysterectomy | 1/35 (2.86) | 0/36 (0) |
| Thyroidectomy | 0/35 (0) | 1/36 (2.78) |
| Nervous system disorders | ||
| Multiple sclerosis relapse with hospital admission | 0/35 (0) | 1/36 (2.78) |
| Brain hypoxia due to cardiac arrest | 0/35 (0) | 1/36 (2.78) |
| Pregnancy | 0/35 (0) | 1/36 (2.78) |
| Skin disorders | ||
| Allergy to synthetic fabric | 0/35 (0) | 1/36 (2.78) |
| Back pain | 0/35 (0) | 2/36 (5.56) |
| Diabetes | 1/35 (2.86) | 0/36 (0) |
| Common treatment-emergent nonserious adverse events affecting ≥5% of patients | ||
| Gastrointestinal tract disorders | ||
| Diarrhea | 9/35 (25.71) | 9/36 (25.00) |
| Abdominal discomfort | 8/35 (22.86) | 6/36 (16.67) |
| Gastroenteritis | 4/35 (11.43) | 5/36 (13.89) |
| Constipation | 1/35 (2.86) | 3/36 (8.33) |
| Nausea | 3/35 (8.57) | 0/36 (0.00) |
| Urinary tract infection | 7/35 (20.00) | 6/36 (16.67) |
| Respiratory | ||
| Upper tract infections | 16/35 (45.71) | 18/36 (50.00) |
| Nasopharyngitis | 3/35 (8.57) | 2/36 (5.56) |
| Lower tract infections | 3/35 (8.57) | 3/36 (8.33) |
| Cough | 2/35 (5.71) | 2/36 (5.56) |
| Chest pain | 2/35 (5.71) | 0/36 (0.00) |
| Nervous system | ||
| Headache | 1/35 (2.86) | 6/36 (16.67) |
| Dizziness | 0/35 (0) | 2/36 (5.56) |
| Depression | 2/35 (5.71) | 1/36 (2.78) |
| Skin | ||
| Reaction at plaster site | 29/35 (82.86) | 10/36 (27.78) |
| Rash | 3/35 (8.57) | 3/36 (8.33) |
| Pain of skin | 1/35 (2.86) | 2/36 (5.56) |
| Musculoskeletal | ||
| Pain in extremity | 2/35 (5.71) | 7/36 (19.44) |
| Back pain | 3/35 (8.57) | 2/36 (5.56) |
| Other | ||
| Ear infection | 0/35 (0) | 3/36 (8.33) |
| Tooth abscess | 3/35 (8.57) | 3/36 (8.33) |
| Hay fever | 1/35 (2.86) | 3/36 (8.33) |
Discussion
In this 36-week, phase 2 trial of HW in relapsing MS we did not find a difference between the cumulative number of active MRI lesions in the 2 groups (the primary outcome). However, the higher proportion of scans with no new activity in the HW group suggests a beneficial effect.
The characteristics of relapsing-remitting MS trial populations have changed over time, largely reflecting changes in the diagnostic process and availability of treatments. Patients in recent trials presented with lower disease activity and slower clinical progression. Despite this, the percentage of inactive scans in our study’s placebo arm was similar to that in the placebo arm of the Tubridy et al study24 that was used to power this study and of other trials.26,27
In contrast, 51% of patients treated with HW had no new, enlarging, or enhancing lesions during the trial. The 2 arms in the Worms for Immune Regulation in Multiple Sclerosis (WIRMS) trial were matched for clinical and MRI activity at baseline. This suggests that the HW had an anti-inflammatory effect.24
The change in Treg suggested an immunobiological effect for HW. CD4+CD25highCD127negT define functional human Tregs. Their percentage of the total CD4+ cells was used in this study as a surrogate for Treg suppressive ability.28,29 The percentage of CD4+CD25highCD127negT cells of total CD4+T cells relative to the baseline in the HW arm significantly increased compared with the placebo arm, in which it decreased. This is consistent with the expected immunological effects of helminths.30 The increase in CD4+CD25highFoxp3+T cells in the HW arm did not reach significance. Given that Foxp3+ has been considered a criterion standard, this could mean that the effect of HW on Treg is modest. However, Foxp3+ T cells are a less specific marker of Treg and can include activated T cells.31 The proportion of CD4+foxp3+CD127neg cells, markers that when combined may be more specific for true Treg cells, was significantly increased by HW (this analysis was done post hoc).
The HW infection was very well tolerated and safe. None of the patients who received HW discontinued because of AE, supporting tolerability at 25 larvae. The study population had, by and large, similar clinical and demographic characteristics to those enrolled in other phase 2 trials of other disease-modifying treatments (DMTs) in MS.27
In MS, there is a numerical or functional deficit of Treg cells, and DMT increases Treg cell number and activity.32 Pathogenic T cells in MS may be of Th1, Th17, or Th1/Th17 mixed phenotype. Elevated inflammatory responses may reflect defective immunoregulation.33 It is plausible that enhancing immunoregulatory mechanisms (eg, by increasing Treg) is beneficial. Direct effects on inflammatory T-cell subsets, induction of regulatory B cells, or HW-induced gut microbiota changes are other potential immunological effects that will be explored in secondary analyses.34,35
Natural and therapeutic helminth infections have been associated with immune deviation, representing a switch from proinflammatory, predominantly Th1 to a Th2 response.36 Most DMTs in MS have also been reported to induce a Th2 switch, although Th1 and Th2 responses in MS are not mutually exclusive. A marker and possible mediator of a protective Th2 response may be an increase in eosinophil counts. A blunted helminth-induced eosinophil response in experimental autoimmune encephalomyelitis reduces protection against neuroinflammation.37 An absent eosinophil response to Trichuris suis by concomitant interferon treatment may be associated with a lack of beneficial effect.10 Our study demonstrated a robust eosinophil response in the range of eosinophilia observed in patients with MS with natural helminth infection.6,18 This was associated with a Treg cell response that was sustained even longer and peaked later in the trial, suggesting that a Th2 switch leads to an increase in Treg cells. However, only 1 of 12 patients treated with HW with an active MRI had no eosinophilia, suggesting that even with eosinophilia HW hyporesponsiveness can occur.
The disproportionately higher number of participants in the HW arm showing no MRI activity suggests that these patients are responsive to HW treatment. A few patients in the HW arm had persistent disease activity; it is interesting to speculate about potential nonresponders, but there were no clear demographic, clinical, or immunological (eg, Treg, eosinophilia) distinguishing characteristics for these patients. Likewise, although we cannot rule out absent or insufficient infection in the 3 patients treated with HW without eosinophilia, they showed no distinguishing clinicoradiological features. Changes in gut microbiota will be important to explore in the future, as they may explain the mechanisms of action and the differential responses to HW in MS.
Besides being, to our knowledge, the largest MS helminth trial, our study has other merits. It demonstrated the feasibility and safety of controlled HW infection in MS. The large amount of data generated can contribute to the understanding of MS immunopathogenesis in secondary analysis through a correlation between clinical, radiological, immunological, and microbiological findings. If the biological effect and the induced immunoregulatory mechanisms can be more efficiently harnessed, it may enhance therapeutic opportunities in MS, making this inexpensive, long-lived treatment potentially beneficial. The study encourages efforts to activate endogenous immunoregulatory mechanisms and/or immune deviation in inflammatory disease. The data and samples can improve the understanding of some properties of the parasite, still a major cause of disease worldwide, and of the immunity against it. This may facilitate the development of therapies and vaccines against HW.
Limitations
Our study has some limitations. Logistical and regulatory aspects led to a lag between conceptualization and design and its implementation. The assumptions of the design and sample size calculations with monthly MRI scans may not be entirely up to date. A design and sample size calculation using the proportion of patients with no disease activity may be a better choice for an outcome measure. The treatment duration, 36 weeks, was relatively short. Longer time (1-2 years) is needed to assess clinical response. The MRI inclusion criteria were based on previous clinical scans. We acknowledge that it would have been preferable to have the screening MRI at a defined point before the baseline. However, reassuringly, patients were matched at baseline for T2 lesion numbers.
Despite the size of the study, its feasibility as a multicenter trial has not been tested. Although some patients were referred from other centers, recruitment was from a single center because of the logistical limitations of the immunoparasitology component of the study (point of manufacture; Medicines and Healthcare Products Regulatory Agency rules regarding transport of the placebo). However, given the appropriate infrastructure, such a trial could be run as a multicenter trial.
Although some participants had SPMS, the short duration of the trial and the relatively low proportion (18%) do not allow generalization of the findings to progressive MS. On the other hand, no specific safety or tolerability issues were noted in the SPMS cohort, suggesting the feasibility of future trials.
Conclusions
This phase 2, randomized, double-blind, placebo-controlled trial of N americanus in relapsing MS has shown the safety and tolerability of the treatment and a biological effect compatible with studies in which natural helminth infection has a protective effect in MS. Infection with HW increased eosinophil counts and some Treg cell markers. The trial can inform future trials with therapeutic HW, which may include different MRI outcome measures (eg, the proportion of patients with no detectable disease activity on MRI), interval booster redosing, higher numbers of larvae, or longer treatment periods, allowing assessment of the effects on disease progression.
Trial Protocol
eAppendix 1. Study overview
eAppendix 2. Study assessments
eAppendix 3. Number of larvae choice, manufacturing, storage and molecular confirmation of Necator americanus infection
eAppendix 4. MRI protocol for WIRMS trial
eAppendix 5. Treg cells measurement
eAppendix 6. New T1 enhancing MRI lesions during the trial
Data Sharing Statement
References
- 1.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. doi: 10.1016/S0140-6736(18)30481-1 [DOI] [PubMed] [Google Scholar]
- 2.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911-920. doi: 10.1056/NEJMra020100 [DOI] [PubMed] [Google Scholar]
- 3.Maizels RM, Smith KA. Regulatory T cells in infection. Adv Immunol. 2011;112:73-136. doi: 10.1016/B978-0-12-387827-4.00003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming JO, Cook TD. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67(11):2085-2086. doi: 10.1212/01.wnl.0000247663.40297.2d [DOI] [PubMed] [Google Scholar]
- 5.de Cássia Ribeiro Silva R, Barreto ML, Assis AM, et al. The relative influence of polyparasitism, environment, and host factors on schistosome infection. Am J Trop Med Hyg. 2007;77(4):672-675. doi: 10.4269/ajtmh.2007.77.672 [DOI] [PubMed] [Google Scholar]
- 6.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61(2):97-108. doi: 10.1002/ana.21067 [DOI] [PubMed] [Google Scholar]
- 7.Elliott DE, Weinstock JV. Nematodes and human therapeutic trials for inflammatory disease. Parasite Immunol. 2017;39(5). doi: 10.1111/pim.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming JO, Isaak A, Lee JE, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler. 2011;17(6):743-754. doi: 10.1177/1352458511398054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benzel F, Erdur H, Kohler S, et al. Immune monitoring of Trichuris suis egg therapy in multiple sclerosis patients. J Helminthol. 2012;86(3):339-347. doi: 10.1017/S0022149X11000460 [DOI] [PubMed] [Google Scholar]
- 10.Voldsgaard A, Bager P, Garde E, et al. Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Mult Scler. 2015;21(13):1723-1729. doi: 10.1177/1352458514568173 [DOI] [PubMed] [Google Scholar]
- 11.Fleming J, Hernandez G, Hartman L, et al. Safety and efficacy of helminth treatment in relapsing-remitting multiple sclerosis: Results of the HINT 2 clinical trial. Mult Scler. 2017;25(1):81-91 doi: 10.1177/1352458517736377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fumagalli M, Pozzoli U, Cagliani R, et al. The landscape of human genes involved in the immune response to parasitic worms. BMC Evol Biol. 2010;10:264. doi: 10.1186/1471-2148-10-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard DI, Quinnell RJ, Moustafa M, et al. Hookworm (Necator americanus) infection and storage iron depletion. Trans R Soc Trop Med Hyg. 1991;85(2):235-238. doi: 10.1016/0035-9203(91)90038-Z [DOI] [PubMed] [Google Scholar]
- 14.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19(12):547-551. doi: 10.1016/j.pt.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Quinnell RJ, Bethony J, Pritchard DI. The immunoepidemiology of human hookworm infection. Parasite Immunol. 2004;26(11-12):443-454. doi: 10.1111/j.0141-9838.2004.00727.x [DOI] [PubMed] [Google Scholar]
- 16.Hotez PJ, Pritchard DI. Hookworm infection. Sci Am. 1995;272(6):68-74. doi: 10.1038/scientificamerican0695-68 [DOI] [PubMed] [Google Scholar]
- 17.Mortimer K, Brown A, Feary J, et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am J Trop Med Hyg. 2006;75(5):914-920. doi: 10.4269/ajtmh.2006.75.914 [DOI] [PubMed] [Google Scholar]
- 18.Blount D, Hooi D, Feary J, et al. Immunologic profiles of persons recruited for a randomized, placebo-controlled clinical trial of hookworm infection. Am J Trop Med Hyg. 2009;81(5):911-916. doi: 10.4269/ajtmh.2009.09-0237 [DOI] [PubMed] [Google Scholar]
- 19.Croese J, O’neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55(1):136-137. doi: 10.1136/gut.2005.079129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127. doi: 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 21.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120(pt 11):2059-2069. doi: 10.1093/brain/120.11.2059 [DOI] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 23.Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233(1-2):6-11. doi: 10.1016/j.jneuroim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 24.Tubridy N, Ader HJ, Barkhof F, Thompson AJ, Miller DH. Exploratory treatment trials in multiple sclerosis using MRI: sample size calculations for relapsing-remitting and secondary progressive subgroups using placebo controlled parallel groups. J Neurol Neurosurg Psychiatry. 1998;64(1):50-55. doi: 10.1136/jnnp.64.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fay BR. Various methods of resolving ties for six distribution-free tests of location. J Mod Appl Stat Methods. 2006;5(1):22-40. doi: 10.22237/jmasm/1146456180 [DOI] [Google Scholar]
- 26.Traboulsee A, Li DKB, Cascione M, Fang J, Dangond F, Miller A. Effect of interferon beta-1a subcutaneously three times weekly on clinical and radiological measures and no evidence of disease activity status in patients with relapsing-remitting multiple sclerosis at year 1. BMC Neurol. 2018;18(1):143. doi: 10.1186/s12883-018-1145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kappos L, Radue EW, O’Connor P, et al. ; FREEDOMS Study Group . A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387-401. doi: 10.1056/NEJMoa0909494 [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta A, Mahapatra M, Saxena R. Flow cytometric immunophenotyping of regulatory T cells in chronic lymphocytic leukemia: comparative assessment of various markers and use of novel antibody panel with CD127 as alternative to transcription factor FoxP3. Leuk Lymphoma. 2013;54(4):778-789. doi: 10.3109/10428194.2012.730614 [DOI] [PubMed] [Google Scholar]
- 29.Ruitenberg JJ, Boyce C, Hingorani R, Putnam A, Ghanekar SA. Rapid assessment of in vitro expanded human regulatory T cell function. J Immunol Methods. 2011;372(1-2):95-106. doi: 10.1016/j.jim.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 30.Weinstock JV, Elliott DE. Helminth infections decrease host susceptibility to immune-mediated diseases. J Immunol. 2014;193(7):3239-3247. doi: 10.4049/jimmunol.1400927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129-138. doi: 10.1002/eji.200636435 [DOI] [PubMed] [Google Scholar]
- 32.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259(1):231-244. doi: 10.1111/imr.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constantinescu CS, Gran B. The essential role of T cells in multiple sclerosis: a reappraisal. Biomed J. 2014;37(2):34-40. doi: 10.4103/2319-4170.128746 [DOI] [PubMed] [Google Scholar]
- 34.Cantacessi C, Giacomin P, Croese J, et al. Impact of experimental hookworm infection on the human gut microbiota. J Infect Dis. 2014;210(9):1431-1434. doi: 10.1093/infdis/jiu256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correale J, Equiza TR. Regulatory B cells, helminths, and multiple sclerosis. Methods Mol Biol. 2014;1190:257-269. doi: 10.1007/978-1-4939-1161-5_18 [DOI] [PubMed] [Google Scholar]
- 36.Smith DR, Balashov KE, Hafler DA, Khoury SJ, Weiner HL. Immune deviation following pulse cyclophosphamide/methylprednisolone treatment of multiple sclerosis: increased interleukin-4 production and associated eosinophilia. Ann Neurol. 1997;42(3):313-318. doi: 10.1002/ana.410420307 [DOI] [PubMed] [Google Scholar]
- 37.Finlay CM, Stefanska AM, Walsh KP, et al. Helminth products protect against autoimmunity via innate type 2 cytokines IL-5 and IL-33, which promote eosinophilia. J Immunol. 2016;196(2):703-714. doi: 10.4049/jimmunol.1501820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Study overview
eAppendix 2. Study assessments
eAppendix 3. Number of larvae choice, manufacturing, storage and molecular confirmation of Necator americanus infection
eAppendix 4. MRI protocol for WIRMS trial
eAppendix 5. Treg cells measurement
eAppendix 6. New T1 enhancing MRI lesions during the trial
Data Sharing Statement