Key Points
Question
What are the associations of prenatal illicit drug exposure to marijuana, cocaine, and methadone and/or heroin with measures of brain organization in newborns?
Findings
In this cohort study of 118 economically disadvantaged mothers and their newborns, prenatal exposure to marijuana, cocaine, or opioids was associated with measures of brain structure, tissue organization, and metabolite concentrations; the associations were similar to those of advancing age with brain measures in unexposed newborns.
Meaning
Prenatal drug exposure to marijuana, cocaine, and opioids is associated with measures of newborn brain tissue in patterns that suggest an exaggeration of normal fetal brain maturation.
Abstract
Importance
Increasing rates of illicit drug use during pregnancy may be associated with risk for long-term health problems in prenatally exposed children.
Objective
To identify the associations of prenatal exposure to illicit drugs with organization of the newborn brain.
Design, Setting, and Participants
For this cohort study, a volunteer sample of 210 illicit drug–using and nonusing mothers and their newborns was enrolled from prenatal clinics and drug abuse treatment programs in New York, New York. Enrollment, scanning, and long-term follow-up occurred from September 2004 through February 2012, and image processing and statistical analyses continued through fall 2018. In addition to 26 participants with incomplete data, a total of 64 mothers were lost to follow-up during pregnancy, and 13 newborns were lost to follow-up at birth because of perinatal complications.
Exposures
Newborns were assigned to 1 of 4 primary exposure groups based on the history of most frequent maternal drug use: marijuana, cocaine, methadone maintenance, and/or heroin. Unexposed newborns were controls.
Main Outcomes and Measures
Unsedated magnetic resonance imaging (MRI) of newborn brains was performed shortly after birth. Infant neurodevelopmental outcomes were assessed at age 12 months. MRI modalities included anatomical imaging, diffusion tensor imaging, T2 relaxometry, and magnetic resonance spectroscopic imaging. Infant neurodevelopmental outcomes included Bayley scales of infant development–III and Vineland Adaptive Behavior Scales. Statistical analyses were performed with results represented on the brain images.
Results
Of 118 mothers, 42 (35%) were in the control group (mean [SD] age, 25.9 [6.1] years), 29 (25%) were in the cocaine group (mean [SD] age, 29.0 [6.1] years), 29 (25%) were in the marijuana group (mean [SD] age, 24.3 [5.5] years), and 18 (15%) were in the methadone and/or heroin group (mean [SD] age, 30.9 [5.7] years). Not all newborns could be scanned successfully; therefore, usable MRIs were acquired for 118 newborns from predominantly minority groups and with economically disadvantaged mothers. Anatomic abnormalities were detected in similar locations across all 3 drug exposures and included smaller volumes in the dorsal, medial, and ventral surfaces of the frontal lobe and dose-related increases in volumes in the lateral temporal lobe, dorsal parietal lobe, and superior frontal gyrus. Dose-related increases in diffusion tensor measures of tissue organization, decreases in T2 relaxometry times, and increases in spectroscopy metabolite concentrations were similar across exposures. These associations of exposures with brain measures were similar to the associations of newborn age with brain measures. The anatomic and diffusion tensor imaging measures suppressively mediated the associations of prenatal exposure with poorer 12-month infant outcomes.
Conclusions and Relevance
The findings suggest that prenatal drug exposure is associated with measures of newborn brain tissue in patterns that may indicate that exposures accelerated normal fetal brain maturation, which in turn mediated the associations with poorer 12-month infant outcomes.
This cohort study assesses the association of prenatal maternal drug exposure to marijuana, cocaine, and opioids with organization of the newborn brain and infant neurodevelopmental outcomes at age 12 months.
Introduction
Substance use during pregnancy has become commonplace and may be associated with risk for long-term cognitive, behavioral, and emotional health problems in prenatally exposed children. More than 60% of pregnant women report using illegal substances, and 15% have a substance use disorder1,2; these women are often younger and are experiencing material and social deprivation.3 Owing partially to its decriminalization, marijuana use during pregnancy has increased nearly 7-fold in the past decade.4,5,6 The US opioid crisis has been associated with a concomitant epidemic of use among pregnant women and with withdrawal syndromes in their prenatally exposed newborns.7,8,9 Cocaine use remains relatively stable, with approximately 750 000 pregnant US women using this drug annually.10
Long-term outcome studies face the challenge of disentangling the effects of prenatal illicit drug exposure from those of co-occurring substance use, poverty, maternal stress, and poor prenatal care. Nevertheless, consensus is emerging that prenatal cocaine exposure is associated with preterm birth,11,12,13 sustained newborn jitteriness,11,13,14,15,16,17 slowed fetal and postnatal somatic growth,18,19,20 delayed language development,21,22,23 impaired self-regulation,24 and poor sustained attention.25,26,27 Prenatal marijuana exposure slows somatic growth28; impairs memory, verbal reasoning, and attention; and increases impulsivity, hyperactivity, and oppositional behaviors.29,30,31,32,33 Prenatal methadone exposure produces motor and cognitive impairments,34,35 inattention, impulsivity, and hyperactivity.36,37,38 Prenatal alcohol and tobacco exposure can lead to similar problems in childhood.39 Thus, although differing exposures are associated with unique developmental problems, they have in common an association with the development of inattention, hyperactivity, and impulsivity in later childhood.39,40
This study evaluated the associations of prenatal exposure to marijuana, opioids, or cocaine with magnetic resonance imaging (MRI) measures in the neonatal brain as soon after exposure as possible. Given the common associations that prenatal cocaine, marijuana, and opioid exposure have with reducing brain growth and increasing attention-deficit/hyperactivity disorder–like symptoms, we hypothesized that we would detect exposure-associated reductions in brain volumes in anatomic MRI scans localized to the frontal lobe and other higher-order association areas. Other MRI modalities were acquired for post hoc analyses to help identify the likely cellular bases for volume reductions.
Methods
For this cohort study, illicit drug–using pregnant women were recruited from prenatal clinics and drug abuse treatment programs in Manhattan, New York City. Healthy pregnant women were recruited from prenatal clinics at New York Presbyterian Hospital, New York, New York. All women provided informed written consent for participation. Procedures were approved by the institutional review board of the New York State Psychiatric Institute; participants were protected by a certificate of confidentiality. Mothers were paid for participation in gift cards for newborn supplies. Statistical analyses were performed with results represented on the brain images.
Inclusion and Exclusion Criteria
Inclusion criteria consisted of (1) maternal age of 18 to 45 years; (2) maternal use of marijuana, cocaine, or methadone and/or heroin for the exposed groups or no history of illicit drug or alcohol use during pregnancy for the control group; and (3) newborn gestational age at birth of at least 37 weeks.
Exclusion criteria consisted of (1) maternal HIV infection or AIDS, seizure disorder, malignant tumors, diabetes, or use of psychoactive medications; (2) infant congenital anomalies, genetic syndromes, or significant neonatal complications; (3) maternal alcohol use of more than 2 ounces per day for the exposed group or more than 1 ounce per day in any trimester for the control group; and (4) urine toxicology or self-reports indicating use of other illicit substances.
Illicit drug use history was obtained using drug use questions from the Addiction Severity Index41,42,43 administered at enrollment, during each subsequent trimester, and at birth. Drug use was confirmed with random urine toxicology screens for the mother during pregnancy and for the newborn at delivery and through maternal and medical record reviews. Exposed newborns were divided into 3 groups based on most frequent maternal drug use: marijuana, cocaine, or methadone maintenance therapy for narcotic addiction with or without concurrent use of heroin (methadone and/or heroin). Every effort was made to recruit mothers who were using only a single drug.
Sample
We enrolled 210 mother and newborn pairs: controls (n = 63), cocaine users (n = 53), marijuana users (n = 55), and methadone and/or heroin users (n = 39). During pregnancy, 64 of 210 (30.5%) were lost to follow-up because of disinterest, maternal incarceration, changes in residence, or spontaneous abortions. At birth, 13 of the remaining 146 newborns (8.9%) were lost to follow-up owing to prematurity (methadone and/or heroin [n = 2], cocaine [n = 1]), major congenital heart or pulmonary disease (cocaine [n = 1], methadone and/or heroin [n = 1], and marijuana [n = 1]), abnormal anatomic scan findings reflecting infarction (cocaine [n = 1], marijuana [n = 1]), or foster care (cocaine [n = 2], methadone and/or heroin [n = 2], and marijuana [n = 1]).
We acquired anatomical MRIs to study brain structure, diffusion tensor imaging and T2 time relaxometry to study brain tissue organization, and magnetic resonance spectroscopic imaging to study chemical metabolites. MRIs were obtained for 133 unsedated newborns at 37 to 46 weeks’ postmenstrual age.
Usable MRI data in at least 1 modality were obtained in 118 newborns: marijuana (n = 29), cocaine (n = 29), methadone and/or heroin (n = 18), and unexposed controls (n = 42). Not all MRI modalities could be acquired for every newborn, leaving a subset with usable MRIs in each modality. We group matched each drug-exposed group to a randomly sampled subset of the unexposed newborns, with the constraint that groups did not differ significantly by sex or postmenstrual age at the time of MRI, yielding the following: (1) anatomic MRI: marijuana (n = 23) and controls (n = 37), cocaine (n = 25) and controls (n = 37), methadone and/or heroin (n = 17) and controls (n = 33); (2) diffusion tensor imaging: marijuana (n = 20) and controls (n = 26), cocaine (n = 16) and controls (n = 21), methadone and/or heroin (n = 13) and controls (n = 20); (3) T2 relaxometry: cocaine (n = 17) and controls (n = 20), marijuana (n = 14) and controls (n = 18), methadone and/or heroin (n = 11) and controls (n = 18); and (4) 1H-multi-planar chemical shift imaging44: marijuana (n = 22) and controls (n = 29), cocaine (n = 18) and controls (n = 29), and methadone and/or heroin (n = 13) and controls (n = 29). Enrollment, scanning, and long-term follow-up occurred from September 2004 through February 2012, and image processing and statistical analyses continued through fall 2018.
Assessments
English- or Spanish-language interviews were completed to collect maternal demographics, psychiatric history,45 socioeconomic status,46 depression severity,47 and anxiety severity.48 Maternal IQ was assessed prenatally.49 Newborn assessments at age 12 months included Bayley Scales of Infant Development–III50 and Vineland Adaptive Behavior Scales.51 Race/ethnicity was self-reported. MRI, pulse sequences, and image processing procedures are detailed in the eMethods in the Supplement.
Statistical Analyses
We applied multiple linear regression at each voxel in template space within each MRI modality to test our a priori hypothesis that prenatal drug exposure would be associated with alterations in the newborn brain. The general model for testing exposure correlates in each modality was
| Imaging Measure = β0 + (β1 × Drug Exposure) + (β2 × Alcohol) + (β3 × Tobacco) + (β4 × Postmenstrual Age) + (β5 × Sex) + ϵ. |
The dependent measure for anatomical analyses was the signed euclidean distance at each point on the surface of each newborn brain from the corresponding point on the surface of the template, which we designate for simplicity here as a measure of local volume. For diffusion tensor imaging, the dependent measure was either fractional anisotropy (FA) or average diffusion coefficient (ADC), and for relaxometry, it was T2 relaxation time; for magnetic resonance spectroscopic imaging, it was N-acetylaspartate (NAA) concentration. The independent variable (drug exposure) was prenatal exposure to a particular drug in the specific exposure group and its matched unexposed controls, with exposure coded as a continuous variable representing cumulative exposure throughout pregnancy based on maternal reports of daily use, converted to either grams of cocaine, joints of marijuana, grams of methadone, cigarette packs, or ounces of alcohol (Table 1).52,53 Cumulative exposure distributions were highly skewed, with most values being small but some large. Preliminary analyses showed that drug exposure had logarithmic rather than linear associations with brain measures. We considered various log transformations, but the one that could best address 0 values was the inverse hyperbolic sine (IHS)54,55:
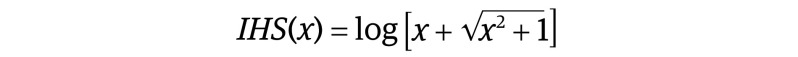 |
where the transformed value approaches the log of twice the exposure for large values and is 0 for no exposure. Covariates in all analyses were newborn postmenstrual age at MRI, newborn sex, and cumulative maternal tobacco and alcohol use during pregnancy. We also assessed the effects of including as additional covariates in our models maternal age, socioeconomic status, race/ethnicity, depression severity, anxiety severity, or prenatal stress.
Table 1. Maternal Demographic Characteristics.
| Characteristic | Groupa | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls (n = 42) | Cocaine use (n = 29)b | Marijuana use (n = 29) | Methadone and/or heroin use (n = 18) | df | F statistic | P value | ||
| Maternal age, y | 25.9 (6.1) | 29.0 (6.1)c | 24.3 (5.5) | 30.9 (5.7)d,e,f | 3,1,114 | 6.51 | <.001 | |
| Race/ethnicity, No. (%) | ||||||||
| White non-Hispanic | 1 (2) | 3 (10) | 1 (3) | 2 (11) | 9 | 16.7g | .053 | |
| African American | 5 (12) | 11 (38) | 12 (42) | 4 (22) | ||||
| Hispanic | 33 (79) | 12 (42) | 14 (48) | 9 (50) | ||||
| Biracial | 3 (7) | 3 (10) | 2 (7) | 3 (17) | ||||
| Educational level, y | 12.5 (2.7) | 11.0 (2.2)c | 11.7 (1.7) | 11.0 (2.0)c | 3,1,112 | 3.30 | .02 | |
| Hollingshead socioeconomic status | 34.3 (10.9) | 28.2 (7.6)d | 30.2 (6.5) | 28.2 (7.1)c | 3,1,111 | 3.56 | .02 | |
| Full-scale IQ | 88.3 (14.7) | 86.3 (10.9) | 87.8 (14.7) | 86.5 (16.6) | 3,1,97 | 0.13 | .95 | |
| Depression severity (HAMD) | 5.4 (8.0) | 6.2 (5.7) | 8.5 (8.0) | 7.1 (7.0) | 3,1,101 | 1.01 | .39 | |
| Anxiety severity (HAMA) | 3.5 (4.5) | 5.0 (5.7) | 7.0 (6.4)f | 4.2 (4.4) | 3,1,101 | 2.3 | .08 | |
| Cumulative drug use in pregnancy | ||||||||
| Cocaine equivalent, mean (SD) [range], g | ||||||||
| T1 | 0 | 610.9 (1064.0) [0.1-5400.0] | 3.4 (17.4) [0-93.6] | 51.4 (193.7) [0-825.0] | NA | NA | NA | |
| T2 | 0 | 437.2 (1035.2) [0-5400.0] | 0.03 (0.2) [0-1.0] | 46.2 (194.4) [0-825.0] | NA | NA | NA | |
| T3 | 0 | 124.8 (333.8) [0-1170.0] | 0 | 0.3 (1.4) [0-6.0] | NA | NA | NA | |
| Sum | 0 | 1173.0 (2162.4) [0.1-10 800.0] | 3.5 (17.4) [0-93.6] | 98.0 (387.7) [0-1650.0] | NA | NA | NA | |
| Marijuana, mean (SD), median No. of joints | ||||||||
| T1 | 0 | 44.0 (135.6) [0-720.0] | 205.1 (256.2) [0-1080.0] | 27.7 (886.1) [0-360.0] | NA | NA | NA | |
| T2 | 0 | 32.1 (134.1) [0-720.0] | 99.4 (113.3) [0-360.0] | 20.5 (84.8) [0-360.0] | NA | NA | NA | |
| T3 | 0 | 2.1 (11.1) [0-60.0] | 27.1 (55.5) [0-180.0] | 0 | NA | NA | NA | |
| Sum | 0 | 78.2 (270.3) [0-1440.0] | 331.7 (330.6) [9.0-1260.0] | 48.2 (169.4) [0-720.0] | NA | NA | NA | |
| Methadone equivalents, mean (SD) [range], mg | ||||||||
| T1 | 0 | 17.7 (93.5) [0-495.0] | 0 | 63 669.7 (97 768.4) [0-290 000.0] | NA | NA | NA | |
| T2 | 0 | 1724.0 (9189.1) [0-49 500.0] | 0 | 37 121.3 (94 914.6) [0-404 100.0] | NA | NA | NA | |
| T3 | 0 | 0 | 0 | 7506.1 (4909.7) [0-23 400.0] | NA | NA | NA | |
| Sum | 0 | 1741.1 (9186.2) [0-49 500.0] | 0 | 108 297.2 (143 632.4) [3030.0-473 100.0] | NA | NA | NA | |
| Cigarettes, mean (SD) [range], No. | ||||||||
| T1 | 0 | 993.5 (860.3) [0-3600.0] | 303.3 (432.6) [0-1800.0] | 1534.1 (2104.2) [0-8174.0] | NA | NA | NA | |
| T2 | 2.1 (13.9) [0-90.0] | 734.0 (832.8) [0-3600.0] | 247.5 (435.3) [0-1800.0] | 800.0 (1323.0) [0-5400.0] | NA | NA | NA | |
| T3 | 4.3 (27.8) [0-180.0] | 311.9 (405.0) [0-1800.0] | 214.1 (409.2) [0-1800.0] | 425.3 (589.4) [0-1800.0] | NA | NA | NA | |
| Sum | 6.4 (41.7) [0-270.0] | 2039.3 (1781.4) [0-7650.0] | 764.9 (1113.4) [0-4500.0] | 2759.1 (3209.2) [0-11 700.0] | NA | NA | NA | |
| Cumulative alcohol equivalents, mean (SD) [range], oz | ||||||||
| T1 | 0.1 (0.4) [0-1.8] | 12.5 (30.8) [0-144.0] | 5.5 (17.7) [0-86.4] | 0.5 (2.2) [0-9.0] | NA | NA | NA | |
| T2 | 0.2 (1.4) [0-9.0] | 2.0 (8.5) [0-45.0] | 0.4 (1.2) [0-5.4] | 0 | NA | NA | NA | |
| T3 | 0.3 (1.3) [0-6.3] | 0.1 (0.4) [0-2.4] | 0.06 (0.3) [0-1.8] | 0 | NA | NA | NA | |
| Sum | 0.6 (2.4) [0-14.4] | 14.6 (34.7) [0-144.0] | 5.9 (18.2) [0-88.8] | 0.5 (2.1) [0-9.0] | NA | NA | NA | |
| Comorbid drug useh | ||||||||
| Any | 0 | 4 (13.8) | 1 (3.5) | 6 (33.3) | NA | NA | NA | |
| Cocaine use | 0 | NA | 1 (3.5) | 5 (27.8) | NA | NA | NA | |
| Marijuana use | 0 | 3 (10.3) | NA | 2 (11.1) | NA | NA | NA | |
| Opioid use | 0 | 2 (7) | 0 | NA | NA | NA | NA | |
| Cigarettes more than half pack per d | 0 | 6 (20.7) | 2 (6.9) | 6 (33.3) | NA | NA | NA | |
| Any alcohol use ≥2 oz/d | 0 | 0 | 0 | 0 | NA | NA | NA | |
| Obstetrical history | ||||||||
| Gravida | 3.1 (1.8) | 4.9 (2.9)d,e | 3.0 (2.0) | 4.6 (3.2)c,i | 3,1,112 | 5.14 | .002 | |
| Para | 1.1 (1.1) | 2.4 (2.0)d,e | 0.7 (0.9) | 2.8 (2.5)d,e | 3,1,112 | 10.60 | <.001 | |
| Abortions | 1.1 (1.3) | 1.5 (1.7) | 1.3 (1.4) | 0.9 (1.2) | 3,1,112 | 0.85 | .47 | |
| Obstetric complications, %j | 9 | 5 | 0 | 15 | 3 | 3.5g | .32 | |
| Delivery mode, % | ||||||||
| Vaginal | 72 | 68 | 70 | 62 | 3 | 0.63g | .89 | |
| Cesarean | 28 | 32 | 30 | 38 | ||||
Abbreviations: HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Scale; NA, not applicable.
Data are presented as mean (SD) values unless otherwise indicated.
In the cocaine exposure group, 10 were exclusively crack users, 8 were exclusively powder cocaine users, and 11 used both forms of cocaine.
P < .05 vs control.
P < .005 vs control.
P < .005 vs marijuana.
P < .005 vs cocaine.
χ2 Statistic.
Any comorbid drug use: any use of cocaine, marijuana, or methadone and/or heroin. Alcohol use was required to be 2 oz or less on any day in the exposed groups. Only 1 control mother had any tobacco exposure (less than 30 cigarettes total in the first trimester only). Assumptions used in converting doses for drugs into equivalent units: 1 g of crack equals 1 g of cocaine, 1 dime bag of cocaine equals 0.15 mg of cocaine, 1 rock of crack equals 0.1 mg of cocaine, 1 vial of crack equals 0.5 g of cocaine, 1 crack dime bag equals 0.13 mg of cocaine, 1 g of heroin equals 1.65 mg of methadone, 1 bundle of heroin equals 10 bags, 1 bag of heroin equals 50 mg of heroin,52,53 1 beer equals 1 glass of wine equals 1 shot of liquor equals 0.6 oz of alcohol, 1 pack equals 20 cigarettes.
P < .05 vs marijuana.
Infections, fevers, hypertension, preeclampsia, cesarean delivery for maternal emergency indications.
P values in each statistical map within each imaging modality were corrected for the number of statistical comparisons. We applied the Benjamini–Yekutieli procedure56 for false discovery rate to control for the expected number of false-positive findings. False discovery rate–corrected, 2-sided P values were color coded and displayed on the template brain.
We assessed dose-response associations by correlating cumulative drug use with imaging measures within each exposure group excluding the unexposed controls and covarying for postmenopausal age and sex.
Testing brain-based mediation of the associations of exposures with 12-month infant outcomes was performed voxelwise using the Sobel test (eFigure 15 in the Supplement), and we assessed whether mediation was complementary or suppressive (eMethods and eFigure 15 in the Supplement).57,58
Results
Of 118 mothers whose newborns had usable MRI data, 42 (35%) were in the control group (mean [SD] age, 25.9 [6.1] years), 29 (25%) were in the cocaine group (29.0 [6.1] years), 29 (25%) were in the marijuana group (24.3 [5.5] years), and 18 (15%) were in the methadone and/or heroin group (30.9 [5.7] years). The 133 mothers with newborns who underwent MRI did not differ significantly in demographics from the 77 enrolled mothers with newborns who did not undergo MRI (eResults in the Supplement). Among the 118 mothers of newborns with usable MRI scans, polydrug use was present in 4 of 29 (13.8%) in the cocaine group, 1 of 29 (3.5%) in the marijuana group, and 6 of 18 (33.3%) in the methadone and/or heroin group (Table 1). By design, reported alcohol use was minimal in each exposure group for the duration of pregnancy. The number of women who smoked more than half a pack per day of cigarettes was 6 (20.7%) in the cocaine group, 2 (11.1%) in the marijuana group, and 6 (33.3%) in the methadone and/or heroin group (Table 1). Additional maternal and newborn characteristics are shown in eFigure 12 and the eResults in the Supplement.
Anatomical MRI
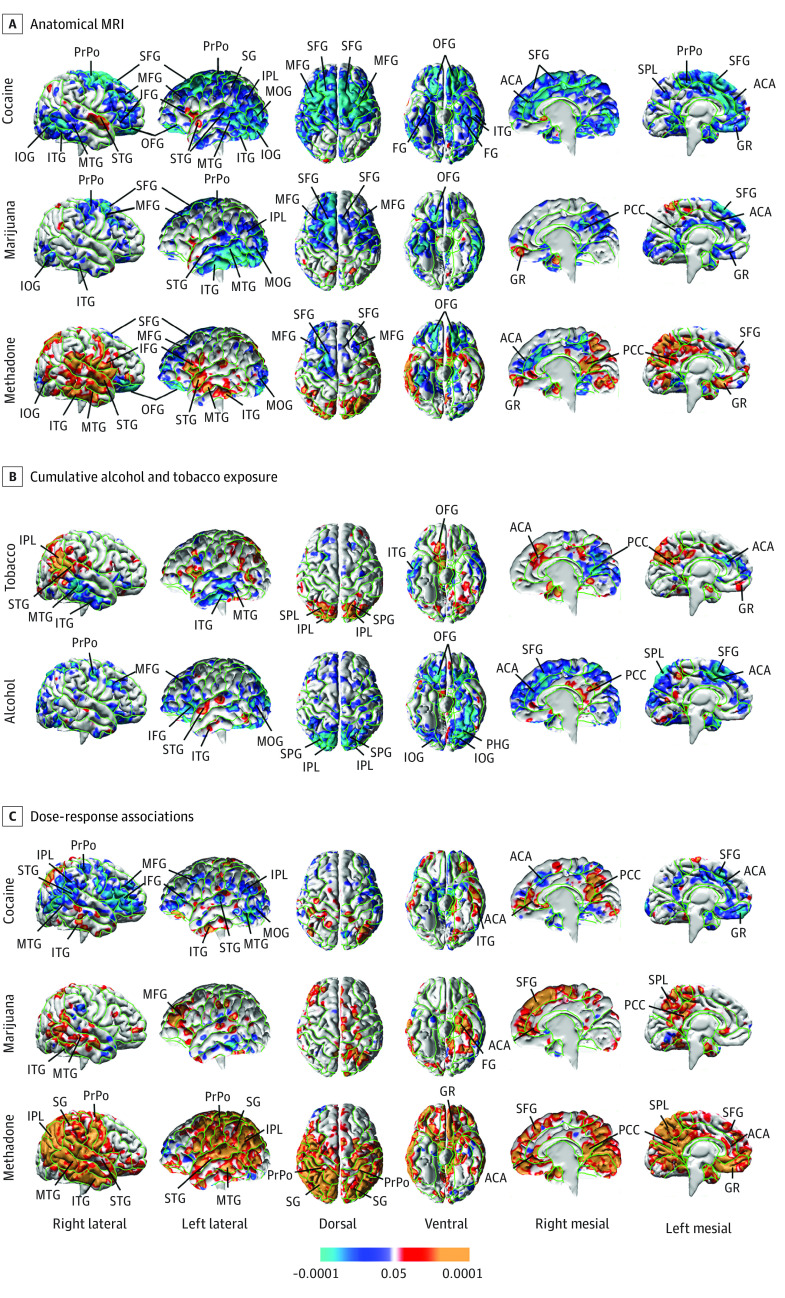
MRI data quality is shown in eFigure 1 in the Supplement. Compared with their matched, unexposed controls, all 3 exposure groups had dose-related volume reductions in the same general locations, including dorsal and lateral surfaces of the frontal lobe and mesial and inferior cerebral surfaces. The cocaine and marijuana groups also had smaller volumes in most of the lateral surface of the temporal lobe, extending to the inferolateral occipital surface (Figure 1). Reduced volumes associated with co-occurring alcohol use were present in similar locations although smaller in spatial extent (Figure 1).
Figure 1. Statistical Maps of Prenatal Drug Exposure Associations With Anatomical Magnetic Resonance Imaging (MRI) Measures.
A, The scale represents false discovery rate–corrected P values for the statistical significance of the correlations of cumulative prenatal exposure to each drug with measures of local volume at each point on the surface of the brain in statistical models that included newborns separately in each drug-exposed group and their matched unexposed controls. B, Association of cumulative tobacco and alcohol exposure across all newborns in the cohort (N = 118). C, False discovery rate–corrected statistical maps correlating cumulative drug exposure with local volumes in a linear regression model including all newborns in the drug-exposed group but excluding the unexposed controls. ACA indicates anterior cingulate cortex; FG, fusiform gyrus; GR, gyrus rectus; IFG, inferior frontal gyrus; IOG, inferior occipital gyrus; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; OFG, orbitofrontal gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; SPL, superior parietal lobule; PrPo, pre- and postcentral gyrus; SFG, superior frontal gyrus; SG, supramarginal gyrus; STG, superior temporal gyrus.
Prenatal exposure was also associated with regional enlargement, particularly of the lateral temporal and inferolateral frontal lobes and mesial wall in the methadone and/or heroin group, with smaller foci in similar locations in the cocaine group (Figure 1).
Findings were similar when assessing exposure correlates for all newborns entered into a single exposure model and when treating individual drug exposures as dichotomous variables for any exposure during pregnancy (eFigures 3-7 in the Supplement). Regional volume reductions exhibited linear associations with IHS-transformed measures of cumulative exposure during pregnancy within each exposure group (eFigure 22 in the Supplement).
Diffusion Tensor Imaging
Prenatal cocaine, marijuana, and methadone and/or heroin exposure were all significantly associated with increased FA (Figure 2) and reduced ADC (Figure 2) throughout frontal and parietal white matter. Marijuana was additionally associated with increased FA in the anterior limb of the internal capsule and marijuana and cocaine with reduced FA in the posterior limb of the internal capsule. Scattered significant voxels at the interfaces of gray matter, white matter, and cerebrospinal fluid may represent partial volume effects, but large clusters in regions in deep white matter distant from tissue boundaries likely represent findings in only white matter.
Figure 2. Statistical Maps of Prenatal Drug Exposure Associations With Diffusion Tensor Imaging (DTI), Relaxometry, and Spectroscopy Measures.
The scale represents false discovery rate–corrected P values for the statistical significance of the correlations of cumulative prenatal exposure to each drug with brain imaging measures at each voxel. The model was applied separately to each exposure group with its matched unexposed controls. Significant correlations of cumulative exposure with each imaging measure were similar in location across exposures. aCR indicates anterior corona radiata; aCS, anterior centrum semiovale; ALIC, anterior limb of the internal capsule; BG, basal ganglia; EC, external capsule; NAA, N-acetyl aspartate; pCR, posterior corona radiata; pCS, posterior centrum semiovale; PLIC, posterior limb of the internal capsule; Th, thalamus.
Relaxometry and Magnetic Resonance Spectroscopic Imaging
All 3 exposures were associated with reduced T2 relaxation times in frontal and parietal white matter; cocaine and methadone and/or heroin were associated with increased T2 times in ventral striatum and thalamus (Figure 2). Cocaine and marijuana exposure but not methadone and/or heroin were associated with increased NAA in deep white matter of the frontal and parietal lobes (Figure 2). Post hoc analyses showed that choline and creatine concentrations exhibited similar associations as NAA (eFigures 12-14 in the Supplement).
Age Correlates
Advancing newborn age was associated with smaller volumes of frontal, posterior, and inferior brain surfaces when controlling for overall brain size, suggesting slower growth compared with overall brain growth. Newborn age was also associated with widespread increases in FA and reductions in ADC and T2 times throughout the brain, and with increased NAA concentrations in basal ganglia and thalamus (Figure 3).
Figure 3. Statistical Maps Showing the Correlations of Newborn Age With Imaging Measures.
The color scale represents false discovery rate–corrected P values for the statistical significance of the voxelwise correlations of age with each imaging measure. Sex was included as a covariate. A, Correlation of age with local volumes when brains were not rescaled to a common volume. Nearly all regions correlated positively with age; thus, we inferred that all regions were growing through this period of development (postmenstrual ages of 37-46 weeks). Exceptions included regions that did not vary significantly with age (small portions of the inferior frontal gyri [IFG], superior frontal gyri [SFG], and orbital frontal gyri [OFG]) or that declined with age (parahippocampal gyrus [PHG]). B, Correlation of age with local volumes are shown for brains when rescaled to a common volume. We inferred that regions growing more slowly than overall brain volume included those at the dorsal (pre- and postcentral gyrus [PrPo], SFG), inferior (orbitofrontal gyrus [OFG] and PHG), and medial (SFG) brain surfaces, and that regions growing faster than overall brain volume included those over the lateral surface of the temporal (superior temporal gyrus [STG], middle temporal gyrus, [MTG], and inferior temporal gyrus [ITG]) and parietal (inferior parietal lobe [IPL]) lobes, as well as the medial posterior (posterior cingulate cortex [PCC]) and orbital (gyrus rectus [GR]) surfaces. Note that the prenatal exposure associations in Figure 1 map onto these age correlations, suggesting that the exposures are associated with accelerated growth of regions that normally grow quickly and with slowed growth of regions that normally grow slowly compared with overall brain volume. ADC indicates average diffusion coefficient; FA, fractional anisotropy; NAA, N-acetyl aspartate.
Influences of Potential Confounders
Findings were similar when maternal age, socioeconomic status, race/ethnicity, depression severity, anxiety severity, or prenatal stress were included as additional covariates in our statistical models.
Brain-Based Mediation
Twelve-month Bayley and Vineland scores were lower for exposed infants (eTable in the Supplement). Exposure-associated reductions in dorsal, inferior, and medial frontal volumes partially mediated the associations of any prenatal drug exposure with 12-month Vineland measures of adaptive behavior (eFigure 16 in the Supplement) and communication (eFigure 17 in the Supplement), and reduced volumes in premotor regions partially mediated exposure associations with lower Bayley gross motor scores (eFigure 18 in the Supplement). In all regions, mediation was suppressive rather than complementary (eFigures 16-18 in the Supplement).57,58 Regional enlargements did not significantly mediate outcomes. Lower mean diffusivity measures partially mediated exposure associations with lower scores for Vineland adaptive behavior and living skills and Bayley receptive communication (eFigures 19-21 in the Supplement).
Intermodality Correlations
We assessed the intercorrelations of imaging measures across MRI modalities for all available newborns in regions exhibiting a commonality of findings across exposures. Scatterplots for imaging measures adjusted for newborn postmenstrual age and sex showed significant linear correlations between measures in all MRI modalities (eFigure 26 in the Supplement).
Discussion
In this study, we detected significant associations of prenatal illicit drug exposure with measures of brain structure, tissue organization, and metabolites in newborns. Overall head circumference and brain volume were smaller in drug-exposed newborns (significantly in the methadone and/or heroin group) when controlling for length as a scaling effect and postmenstrual age as a maturational index (Table 2) and thus exceeded the anticipated association between illicit drug use and slowing of overall somatic growth.18,19,20,28,39 Smaller overall brain volumes derived from smaller regions in similar locations across all 3 exposures, including dorsal, medial, and ventral surfaces of the frontal lobe (Figure 1). These findings were present when comparing newborns in individual exposure groups with unexposed controls, when controlling for co-occurring exposures in a single exposure model that included all newborns, and when treating individual drug exposures as either dichotomous variables (eFigures 6-9 in the Supplement) or continuous measures of cumulative exposure (eFigures 2-7 and 10-14 in the Supplement). Each exposure was also associated with regional enlargements (lateral temporal lobe, dorsal parietal lobe, and superior frontal gyrus) in direct proportion to cumulative exposure measures and in similar locations across exposures, reaching statistical significance for cocaine and methadone and/or heroin (Figure 1), especially when adjusting for overall brain volume (eFigures 2, 5, and 7 in the Supplement).
Table 2. Newborn Demographic Characteristics.
| Characteristic | Groupa | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 42) | Cocaine use (n = 29) | Marijuana use (n = 29) | Methadone and/or heroin use (n = 18) | df | F statistic | P value | |||
| Sex, No. (%) | |||||||||
| Female | 16 (38) | 10 (34) | 17 (59) | 6 (33) | 3 | 4.78b | .19 | ||
| Male | 26 (62) | 19 (66) | 12 (41) | 12 (67) | |||||
| Apgar score, 1 min | 8.6 (0.9) | 8.6 (0.8) | 8.6 (0.9) | 8.5 (1.1) | 3,1,103 | 0.13 | .94 | ||
| Apgar score, 5 min | 9.0 (0.2) | 9.1 (0.3) | 9.0 (0.4) | 9.0 (0.4) | 3,1,103 | 0.45 | .72 | ||
| Gestational age at birth, wk | 39.2 (1.2) | 38.9 (1.5) | 38.6 (1.5) | 39.2 (1.4) | 3,1,114 | 1.11 | .35 | ||
| Birth weight, g | 3327 (469) | 3128 (487) | 3015 (454)c | 3077 (564) | 3,1,114 | 2.75 | .046 | ||
| Percentiled | 40.7 (22.0) | 31.1 (21.5) | 32.2 (24.6) | 27.5 (22.6) | 3,1,114 | 1.93 | .13 | ||
| Head circumference at time of birth, cm | 34.4 (1.3) | 34.0 (1.6) | 33.1 (1.5)e,f | 32.9 (1.3)e,f | 3,1,113 | 7.07 | <.001 | ||
| Percentiled | 33.8 (20.4) | 29.7 (22.1) | 23.5 (20.4)c | 17.5 (17.3)c | 3,1,113 | 3.18 | .03 | ||
| Length at time of birth, cm | 50.6 (2.6) | 48.7 (6.2) | 49.0 (2.5) | 48.7 (2.8) | 3,1,113 | 2.00 | .12 | ||
| Percentiled | 53.4 (25.6) | 44.6 (22.2) | 40.6 (27.1)c | 31.2 (26.1)e | 3,1,113 | 3.55 | .02 | ||
| PMA at MRIg | 41.3 (6.4) | 42.7 (2.9) | 41.3 (3.3) | 45.2 (5.6)c | 3,1,114 | 5.22 | .002 | ||
| Adjusted cerebral volumes, mean (SE), cm3h | 415.5 (6.1) | 405.6 (7.5) | 404.4 (8.3) | 379.3 (10.3)e | 6,1,1,1,1,3,94 | 2.97 | .04 | ||
Abbreviations: MRI, magnetic resonance imaging; PMA, postmenstrual age.
Data are presented as mean (SD) unless otherwise indicated.
χ2 Statistic.
P < .05 vs control values.
Percentiles are plotted against gestational age.
P < .005 vs control values.
P < .05 vs cocaine.
Magnetic resonance imaging was performed at a later PMA in the methadone and/or heroin group because severe narcotic abstinence syndrome often precluded earlier scanning.
Adjusted cerebral volumes are estimated marginal means from a general linear model that included as covariates postmenstrual age and newborn length at the time of scan and sex.
These common associations of prenatal exposures with brain structure are similar in direction and location to the overall correlates of advancing age on brain structure, which included slower growth of frontal, posterior, and inferior brain surfaces and more rapid growth of lateral temporal surfaces compared with overall brain growth (Figure 3). Prenatal drug exposure therefore seemed to be associated with slowed brain growth disproportionately in regions that grow more slowly and with accelerated growth in regions that grow more quickly. Accelerated growth in lateral temporal cortices was associated with cumulative exposure (eFigure 25 in the Supplement). Because most drug exposure occurred in the first trimester, however, dose-related acceleration in growth could instead represent a timing-specific consequence of exposure early in gestation. Regions of exaggerated slowing of growth were not associated with cumulative dose and may be independent of both exposure timing and dose.
Developmental interpretations of our anatomical findings are supported by findings in each of the other modalities. Advancing age was associated with widespread increases in FA and reductions in ADC and T2 times in our data and previous studies59,60,61 and in our findings of exposure-related increases in NAA concentrations in subcortical white and gray matter (Figure 2). All 3 exposures were associated, to varying degrees, with abnormalities in these same regions and in the same direction, supporting interpretation of the anatomic findings that exposures may be associated with accelerated fetal brain maturation. Each exposure, for example, was associated with increased FA and reduced ADC (Figure 2), indicating a dose-related greater restriction on the diffusion of water perpendicular to the direction of axons within white matter, most likely from more myelin or greater axon density62 throughout frontal and parietal white matter and in proportion to cumulative exposure (eFigure 23 in the Supplement). The dose-related decrease in T2 relaxation times in these same regions for each exposure (Figure 2) was also consistent with more myelin or greater cell density because both reduce brain free water content and thereby reduce T2 times with advancing age in early postnatal life.60,63,64 Finally, previous studies have reported that NAA concentrations and axon density increase rapidly in white matter after birth.65,66,67,68 In our data, higher NAA concentrations were associated with newborn age and with prenatal cocaine and marijuana exposure in large white matter fiber bundles and basal ganglia (Figure 2 and eFigure 12 in the Supplement), suggesting that increased density or metabolism of neurons69,70 accompanies advancing newborn age and higher exposures.
Regions of slow anatomic maturation suppressively mediated lower infant 12-month Vineland social communication and adaptive behavior scores (eFigures 16 and 17 in the Supplement). Significant suppressive mediation indicates that slowed regional maturation was likely an adaptive response to prenatal illicit drug exposure, attenuating what would otherwise have been worse 12-month outcomes.57,58 We speculate that slowed maturation in dorsal and inferior frontal brain regions is a response to the deleterious consequences of illicit drug exposure elsewhere in the brain.
Although the specific biochemical consequences of each drug would indicate specific brain effects (eDiscussion in the Supplement),39,71 our findings instead suggest mostly a commonality of drug effects, presumably operating through a final common pathway. The commonality of drug effects is underscored by similar findings across exposures in each MRI modality and by the significant correlations of imaging measures across modalities (eFigure 25 in the Supplement) showing that newborns who had the largest exposure correlates in one modality also had the largest exposure correlates in another modality. Preclinical studies (eDiscussion in the Supplement) suggest that the reductions in neuronal growth, differentiation, dendritic complexity, synaptic density, and neurotransmitter signaling that all 3 illicit drugs produce may be associated with slowed development in regions where growth is normally slower than in the rest of the brain. Regional accelerations in growth and increased density or myelination of white matter, in contrast, may be associated with general drug effects on increasing cellular excitation, which would in turn influence the activity-dependent survival of axons, dendrites, and synapses66 and proliferation of oligodendrocyte precursors72,73,74,75 during circuit formation. Activity-dependent processes would account for the dose-dependent increases in volumes of the lateral temporal, posterior parietal, and dorsal medial surfaces (Figure 1).
Limitations
This study has limitations. Co-exposure is a possible reason for the commonality of findings across exposure groups, particularly because women with substance abuse typically use a cocktail of drugs, making differentiation of specific effects difficult even with very large samples. Nevertheless, we strove to exclude women using illicit drugs other than those in the exposure groups, rates of co-occurring drug use were relatively low (Table 1), and findings persisted when all newborns were entered into a single model for all concurrent exposures. The most common co-exposures were tobacco or alcohol use, and all statistical analyses covaried for them. Moreover, alcohol use was minimal and tobacco use was limited in each exposure group (Table 1). We also modeled in each MRI modality the correlates of drug, alcohol, and tobacco use (Figure 1) (eFigures 4-7 and 10-14 in the Supplement). Tobacco correlates were dissimilar to the correlates of the other exposures; alcohol correlates included some regions of reduced volume in anatomic images overlapping with drug associations but did not show the regional enlargements associated with drug exposures. Furthermore, the associations of tobacco and alcohol with FA, ADC, and NAA measures were few and were not similar to the drug correlates (Figure 1). In addition, we demonstrated a dose-response association of drug exposures with brain measures, even when controlling for co-occurring tobacco and alcohol exposures, when using continuous measures of drug exposure in models for the correlates of co-occurring exposures and in scatterplots displaying the dose-response associations (eFigures 22-25 in the Supplement).
We also cannot exclude the possibility that other potential confounders, such as socioeconomic status, prenatal nutrition, or stress, contributed to the commonality of findings, especially because the sample size limited our ability to model confounder effects. However, covarying for these factors had negligible effects on our findings. In addition, the demographics of the exposure and control groups were similar and controlled for many of these potential confounders.
Sample sizes for individual exposure were modest, limiting our statistical power to discern associations. Moreover, study participation, sample size, and eligibility criteria limit the generalizability of our findings. Many mothers discontinued illicit drug use early in their pregnancies, limiting exposures in the second and third trimesters and the brain correlates specific to those periods of fetal development. Unassessed postnatal exposures could have contributed to poorer 12-month neurodevelopmental outcomes in the drug-exposed groups but would not have contributed to neonatal MRI measures. Furthermore, inconsistent follow-up assessments limited our analyses of brain-based mediators of the associations of exposure with long-term outcomes, which should be regarded as heuristic rather than conclusive. In addition, inferring developmental correlates from cross-sectional data such as ours can be hazardous; our inference that exposure associations represent an exaggeration of normal brain maturation requires confirmation with repeated scanning in a long-term study that defines growth trajectories in the newborn brain.
Conclusions
The findings suggest that prenatal illicit drug exposure is associated with measures of newborn brain tissue and that these associations may represent an exaggeration of normal fetal brain maturation, which in turn may mediate the association of prenatal exposures with poorer 12-month infant outcomes.
eMethods. MRI Pulse Sequences, Image Processing Methods, Post Hoc Statistical Analyses
eResults. Additional Maternal Characteristics
eDiscussion. Neurochemical Action of Exposure, Drugs Relation to Prior Studies, and Additional Limitations
eReferences.
eTable. Infant Outcomes at Postnatal Age 12 Months
eFigure 1. Examples of MPCSI Data Quality, T2 Relaxometry Image Quality, DTI Data Quality: ADC Map, and DTI Data Quality: Fiber Direction Map
eFigure 2. Anatomical MRI: Continuous Exposure Measures, with Rescaling
eFigure 3. Anatomical MRI: Dichotomous Exposure, without Rescaling
eFigure 4. Anatomical MRI: Modeling of Co-Occurring Exposures, Continuous Exposure Measures, Without Rescaling
eFigure 5. Anatomical MRI: Modeling of Co-Occurring Exposures, Continuous Exposure Measures, with Rescaling
eFigure 6. Anatomical MRI: Modeling of Co-Occurring Exposures, Dichotomous Exposure, Without Rescaling
eFigure 7. Anatomical MRI: Modeling of Co-Occurring Exposures, Dichotomous Exposure, with Rescaling
eFigure 8. Dichotomous Exposure and Dose-Response Association: Fractional Anisotropy
eFigure 9. Dichotomous Exposure and Dose-Response Association: Average Diffusion Coefficient
eFigure 10. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Fractional Anisotropy
eFigure 11. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Average Diffusion Coefficient
eFigure 12. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: NAA
eFigure 13. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Choline
eFigure 14. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Creatine
eFigure 15. Mediation Model
eFigure 16. Anatomical MRI Mediation: Vineland Adaptive Behavior Scores
eFigure 17. Anatomical MRI Mediation: Vineland Communication Scores
eFigure 18. Anatomical MRI Mediation: Bayley Gross Motor
eFigure 19. DTI Mediation: Vineland Adaptive Behavior Scores
eFigure 20. DTI Mediation: Vineland Living Skill Scores
eFigure 21. DTI Mediation: Bayley Receptive Communication Scores
eFigure 22. Scatterplots for Exposure Associations in Matched Groups: Anatomical MRI
eFigure 23. Scatterplots for Exposure Associations in Matched Groups: DTI
eFigure 24. Scatterplots for Exposure Associations in Matched Groups: Relaxometry & MRSI
eFigure 25. Scatterplots for Dose-Response Associations: Anatomical MRI
eFigure 26. Scatterplots for Inter-Modality Correlations
References
- 1.Vesga-López O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815. doi: 10.1001/archpsyc.65.7.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forray A. Substance use during pregnancy. F1000Res. 2016;5:F1000 Faculty Rev-887. Published online May 13, 2016. doi: 10.12688/f1000research.7645.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration Results from the 2015 National Survey on Drug Use and Health: detailed tables. Published September 8, 2016. Accessed April 28, 2020. https://www.samhsa.gov/data/report/results-2015-national-survey-drug-use-and-health-detailed-tables
- 4.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002-2014. JAMA. 2017;317(2):207-209. doi: 10.1001/jama.2016.17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young-Wolff KC, Tucker L-Y, Alexeeff S, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009-2016. JAMA. 2017;318(24):2490-2491. doi: 10.1001/jama.2017.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322(2):167-169. doi: 10.1001/jama.2019.7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997-1002. doi: 10.1097/AOG.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin CE, Longinaker N, Terplan M. Recent trends in treatment admissions for prescription opioid abuse during pregnancy. J Subst Abuse Treat. 2015;48(1):37-42. doi: 10.1016/j.jsat.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012 [published correction in J Perinatol. 2015;35(8):667]. J Perinatol. 2015;35(8):650-655. doi: 10.1038/jp.2015.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cain MA, Bornick P, Whiteman V. The maternal, fetal, and neonatal effects of cocaine exposure in pregnancy. Clin Obstet Gynecol. 2013;56(1):124-132. doi: 10.1097/GRF.0b013e31827ae167 [DOI] [PubMed] [Google Scholar]
- 11.Bigsby R, LaGasse LL, Lester B, et al. Prenatal cocaine exposure and motor performance at 4 months. Am J Occup Ther. 2011;65(5):e60-e68. doi: 10.5014/ajot.2011.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouin K, Murphy K, Shah PS; Knowledge Synthesis Group on Determinants of Low Birth Weight and Preterm Births . Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am J Obstet Gynecol. 2011;204(4):340.e1-340.e12. doi: 10.1016/j.ajog.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 13.Mayes LC, Cicchetti D, Acharyya S, Zhang H. Developmental trajectories of cocaine-and-other-drug-exposed and non–cocaine-exposed children. J Dev Behav Pediatr. 2003;24(5):323-335. doi: 10.1097/00004703-200310000-00003 [DOI] [PubMed] [Google Scholar]
- 14.Lester BM, Tronick EZ, LaGasse L, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110(6):1182-1192. doi: 10.1542/peds.110.6.1182 [DOI] [PubMed] [Google Scholar]
- 15.Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30(2):96-106. doi: 10.1016/j.ntt.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer LT, Arendt R, Minnes S, Farkas K, Salvator A. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicol Teratol. 2000;22(5):653-666. doi: 10.1016/S0892-0362(00)00092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tronick EZ, Messinger DS, Weinberg MK, et al. Cocaine exposure is associated with subtle compromises of infants’ and mothers’ social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Dev Psychol. 2005;41(5):711-722. doi: 10.1037/0012-1649.41.5.711 [DOI] [PubMed] [Google Scholar]
- 18.Richardson GA, Goldschmidt L, Larkby C, Day NL. Effects of prenatal cocaine exposure on child behavior and growth at 10 years of age. Neurotoxicol Teratol. 2013;40:1-8. doi: 10.1016/j.ntt.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minnes S, Robin NH, Alt AA, et al. Dysmorphic and anthropometric outcomes in 6-year-old prenatally cocaine-exposed children. Neurotoxicol Teratol. 2006;28(1):28-38. doi: 10.1016/j.ntt.2005.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covington CY, Nordstrom-Klee B, Ager J, Sokol R, Delaney-Black V. Birth to age 7 growth of children prenatally exposed to drugs: a prospective cohort study. Neurotoxicol Teratol. 2002;24(4):489-496. doi: 10.1016/S0892-0362(02)00233-7 [DOI] [PubMed] [Google Scholar]
- 21.Bandstra ES, Morrow CE, Accornero VH, Mansoor E, Xue L, Anthony JC. Estimated effects of in utero cocaine exposure on language development through early adolescence. Neurotoxicol Teratol. 2011;33(1):25-35. doi: 10.1016/j.ntt.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Lewis BA, Kirchner HL, Short EJ, et al. Prenatal cocaine and tobacco effects on children’s language trajectories. Pediatrics. 2007;120(1):e78-e85. doi: 10.1542/peds.2006-2563 [DOI] [PubMed] [Google Scholar]
- 23.Lewis BA, Minnes S, Short EJ, et al. The effects of prenatal cocaine on language development at 10 years of age. Neurotoxicol Teratol. 2011;33(1):17-24. doi: 10.1016/j.ntt.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minnes S, Min MO, Short EJ, et al. Executive function in children with prenatal cocaine exposure (12-15 years). Neurotoxicol Teratol. 2016;57:79-86. doi: 10.1016/j.ntt.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert BL, Bauer CR. Developmental and behavioral consequences of prenatal cocaine exposure: a review. J Perinatol. 2012;32(11):819-828. doi: 10.1038/jp.2012.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125(3):554-565. doi: 10.1542/peds.2009-0637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckingham-Howes S, Berger SS, Scaletti LA, Black MM. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131(6):e1917-e1936. doi: 10.1542/peds.2012-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Marroun H, Tiemeier H, Steegers EAP, et al. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1173-1181. doi: 10.1097/CHI.0b013e3181bfa8ee [DOI] [PubMed] [Google Scholar]
- 29.Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol. 1992;14(5):299-311. doi: 10.1016/0892-0362(92)90036-A [DOI] [PubMed] [Google Scholar]
- 30.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. 1999;21(2):109-118. doi: 10.1016/S0892-0362(98)00042-7 [DOI] [PubMed] [Google Scholar]
- 31.Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47(3):254-263. doi: 10.1097/CHI.0b013e318160b3f0 [DOI] [PubMed] [Google Scholar]
- 32.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22(3):325-336. doi: 10.1016/S0892-0362(00)00066-0 [DOI] [PubMed] [Google Scholar]
- 33.Fried PA, Watkinson B. Visuoperceptual functioning differs in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2000;22(1):11-20. doi: 10.1016/S0892-0362(99)00046-X [DOI] [PubMed] [Google Scholar]
- 34.Guo X, Spencer JW, Suess PE, Hickey JE, Better WE, Herning RI. Cognitive brain potential alterations in boys exposed to opiates: in utero and lifestyle comparisons. Addict Behav. 1994;19(4):429-441. doi: 10.1016/0306-4603(94)90065-5 [DOI] [PubMed] [Google Scholar]
- 35.Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84(1):29-35. doi: 10.1016/j.earlhumdev.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 36.Hickey JE, Suess PE, Newlin DB, Spurgeon L, Porges SW. Vagal tone regulation during sustained attention in boys exposed to opiates in utero. Addict Behav. 1995;20(1):43-59. doi: 10.1016/0306-4603(94)00044-Y [DOI] [PubMed] [Google Scholar]
- 37.Ornoy A, Michailevskaya V, Lukashov I, Bar-Hamburger R, Harel S. The developmental outcome of children born to heroin-dependent mothers, raised at home or adopted. Child Abuse Negl. 1996;20(5):385-396. doi: 10.1016/0145-2134(96)00014-2 [DOI] [PubMed] [Google Scholar]
- 38.Ornoy A, Segal J, Bar-Hamburger R, Greenbaum C. Developmental outcome of school-age children born to mothers with heroin dependency: importance of environmental factors. Dev Med Child Neurol. 2001;43(10):668-675. doi: 10.1017/S0012162201001219 [DOI] [PubMed] [Google Scholar]
- 39.Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology. 2015;40(1):61-87. doi: 10.1038/npp.2014.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behnke M, Smith VC; Committee on Substance Abuse; Committee on Fetus and Newborn . Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009-e1024. doi: 10.1542/peds.2012-3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLellan AT, Luborsky L, Cacciola J, et al. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173(7):412-423. doi: 10.1097/00005053-198507000-00005 [DOI] [PubMed] [Google Scholar]
- 42.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat 1992;9(3):199-213. doi: 10.1016/0740-5472(92)90062-s [DOI] [PubMed] [Google Scholar]
- 43.Zanis DA, McLellan AT, Cnaan RA, Randall M. Reliability and validity of the Addiction Severity Index with a homeless sample. J Subst Abuse Treat. 1994;11(6):541-548. doi: 10.1016/0740-5472(94)90005-1 [DOI] [PubMed] [Google Scholar]
- 44.Duyn JH, Gillen J, Sobering G, van Zijl PC, Moonen CT. Multisection proton MR spectroscopic imaging of the brain. Radiology. 1993;188(1):277-282. doi: 10.1148/radiology.188.1.8511313 [DOI] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Sisorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 46.Hollingshead AB. Four-Factor Index of Social Status. Yale University Press; 1975. [Google Scholar]
- 47.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278-296. doi: 10.1111/j.2044-8260.1967.tb00530.x [DOI] [PubMed] [Google Scholar]
- 48.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. doi: 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- 49.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed Pearson; 2003. [Google Scholar]
- 50.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed Harcourt Assessment; 2006. [Google Scholar]
- 51.Sparrow SS, Balla DA, Cicchetti DV, Doll EA. Vineland Adaptive Behavior Scales: Survey Form Manual. American Guidance Service; 1984. [Google Scholar]
- 52.Reichle CW, Smith GM, Gravenstein JS, Macris SG, Beecher HK. Comparative analgesic potency of heroin and morphine in postoperative patients. J Pharmacol Exp Ther. 1962;136:43-46. [PubMed] [Google Scholar]
- 53.Manfredonia JF. Prescribing methadone for pain management in end-of-life care. J Am Osteopath Assoc. 2005;105(3)(suppl 1):S18-S21. [PubMed] [Google Scholar]
- 54.Johnson NL. Systems of frequency curves generated by methods of translation. Biometrika. 1949;36(pt 1-2):149-176. doi: 10.1093/biomet/36.1-2.149 [DOI] [PubMed] [Google Scholar]
- 55.Burbidge JB, Magee L, Robb AL. Alternative transformations to handle extreme values of the dependent variable. J Am Stat Assoc. 1988;83(401):123-127. doi: 10.1080/01621459.1988.10478575 [DOI] [Google Scholar]
- 56.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165-1188. [Google Scholar]
- 57.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173-181. doi: 10.1023/A:1026595011371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao X, Lynch J, Chen Q. Reconsidering Baron and Kenny: myths and truths about mediation analysis. J Consum Res. 2010;37(2):197-206. doi: 10.1086/651257 [DOI] [Google Scholar]
- 59.Hermoye L, Saint-Martin C, Cosnard G, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29(2):493-504. doi: 10.1016/j.neuroimage.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 60.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48-71. doi: 10.1016/j.neuroscience.2013.12.044 [DOI] [PubMed] [Google Scholar]
- 61.Ouyang M, Dubois J, Yu Q, Mukherjee P, Huang H. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage. 2019;185:836-850. doi: 10.1016/j.neuroimage.2018.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Concha L. A macroscopic view of microstructure: using diffusion-weighted images to infer damage, repair, and plasticity of white matter. Neuroscience. 2014;276:14-28. doi: 10.1016/j.neuroscience.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 63.Ding X-Q, Kucinski T, Wittkugel O, et al. Normal brain maturation characterized with age-related T2 relaxation times: an attempt to develop a quantitative imaging measure for clinical use. Invest Radiol. 2004;39(12):740-746. doi: 10.1097/00004424-200412000-00005 [DOI] [PubMed] [Google Scholar]
- 64.Leppert IR, Almli CR, McKinstry RC, et al. ; Brain Development Cooperative Group . T(2) relaxometry of normal pediatric brain development. J Magn Reson Imaging. 2009;29(2):258-267. doi: 10.1002/jmri.21646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haynes RL, Borenstein NS, Desilva TM, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484(2):156-167. doi: 10.1002/cne.20453 [DOI] [PubMed] [Google Scholar]
- 66.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147-168. doi: 10.1038/npp.2009.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blüml S, Wisnowski JL, Nelson MD Jr, et al. Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex. 2013;23(12):2944-2955. doi: 10.1093/cercor/bhs283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomiyasu M, Aida N, Endo M, et al. Neonatal brain metabolite concentrations: an in vivo magnetic resonance spectroscopy study with a clinical MR system at 3 Tesla. PLoS One. 2013;8(11):e82746. doi: 10.1371/journal.pone.0082746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39(1):1-36. doi: 10.1007/s11064-013-1199-5 [DOI] [PubMed] [Google Scholar]
- 70.Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20(4-5):271-276. doi: 10.1159/000017321 [DOI] [PubMed] [Google Scholar]
- 71.Šlamberová R. Drugs in pregnancy: the effects on mother and her progeny. Physiol Res. 2012;61(suppl 1):S123-S135. [DOI] [PubMed] [Google Scholar]
- 72.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361(6409):258-260. doi: 10.1038/361258a0 [DOI] [PubMed] [Google Scholar]
- 73.Demerens C, Stankoff B, Logak M, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93(18):9887-9892. doi: 10.1073/pnas.93.18.9887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibson EM, Purger D, Mount CW, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Almeida RG, Lyons DA. On myelinated axon plasticity and neuronal circuit formation and function. J Neurosci. 2017;37(42):10023-10034. doi: 10.1523/JNEUROSCI.3185-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. MRI Pulse Sequences, Image Processing Methods, Post Hoc Statistical Analyses
eResults. Additional Maternal Characteristics
eDiscussion. Neurochemical Action of Exposure, Drugs Relation to Prior Studies, and Additional Limitations
eReferences.
eTable. Infant Outcomes at Postnatal Age 12 Months
eFigure 1. Examples of MPCSI Data Quality, T2 Relaxometry Image Quality, DTI Data Quality: ADC Map, and DTI Data Quality: Fiber Direction Map
eFigure 2. Anatomical MRI: Continuous Exposure Measures, with Rescaling
eFigure 3. Anatomical MRI: Dichotomous Exposure, without Rescaling
eFigure 4. Anatomical MRI: Modeling of Co-Occurring Exposures, Continuous Exposure Measures, Without Rescaling
eFigure 5. Anatomical MRI: Modeling of Co-Occurring Exposures, Continuous Exposure Measures, with Rescaling
eFigure 6. Anatomical MRI: Modeling of Co-Occurring Exposures, Dichotomous Exposure, Without Rescaling
eFigure 7. Anatomical MRI: Modeling of Co-Occurring Exposures, Dichotomous Exposure, with Rescaling
eFigure 8. Dichotomous Exposure and Dose-Response Association: Fractional Anisotropy
eFigure 9. Dichotomous Exposure and Dose-Response Association: Average Diffusion Coefficient
eFigure 10. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Fractional Anisotropy
eFigure 11. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Average Diffusion Coefficient
eFigure 12. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: NAA
eFigure 13. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Choline
eFigure 14. Modeling of Co-Occurring Exposures, Continuous Exposure Measures: Creatine
eFigure 15. Mediation Model
eFigure 16. Anatomical MRI Mediation: Vineland Adaptive Behavior Scores
eFigure 17. Anatomical MRI Mediation: Vineland Communication Scores
eFigure 18. Anatomical MRI Mediation: Bayley Gross Motor
eFigure 19. DTI Mediation: Vineland Adaptive Behavior Scores
eFigure 20. DTI Mediation: Vineland Living Skill Scores
eFigure 21. DTI Mediation: Bayley Receptive Communication Scores
eFigure 22. Scatterplots for Exposure Associations in Matched Groups: Anatomical MRI
eFigure 23. Scatterplots for Exposure Associations in Matched Groups: DTI
eFigure 24. Scatterplots for Exposure Associations in Matched Groups: Relaxometry & MRSI
eFigure 25. Scatterplots for Dose-Response Associations: Anatomical MRI
eFigure 26. Scatterplots for Inter-Modality Correlations