Key Points
Questions
In neurocritical care patients, what intracranial pressure values are normal and what intracranial pressure threshold is most strongly associated with Glasgow Outcome Scale score at hospital discharge?
Findings
In this observational study, intracranial pressure values of 8 to 9 mm Hg were most commonly recorded and could reflect normal values. An intracranial pressure threshold of 19 mm Hg was robustly associated with outcome in studied patients, although lower intracranial pressure values were associated with outcome in surviving patients.
Meaning
This study provides insight into what normal intracranial pressure values may be and suggests that threshold values lower than 20 mm Hg should be studied in future prospective studies.
Abstract
Importance
Intracranial pressure (ICP) elevation is a compartment syndrome that impairs blood flow to the brain. Despite the importance of ICP values in neurocritical care, normal ICP values and the precise ICP threshold at which treatment should be initiated remain uncertain.
Objective
To refine our understanding of normal ICP values and determine the ICP threshold most strongly associated with outcome.
Design, Setting, and Participants
Prospective observational study (2004-2010), with outcomes determined at hospital discharge. The study included neurocritical care patients from a single level I trauma center, San Francisco General Hospital. Three hundred eighty-three patients had a traumatic brain injury with or without craniectomy; 140 patients had another indication for ICP monitoring. Consecutive patients were studied. Data analyses were completed between March 2015 and December 2019.
Exposures
Five hundred twenty-three ICP-monitored patients.
Main Outcomes and Measures
A computer system prospectively and automatically collected 1-minute physiologic data from patients in the intensive care unit during a 6-year period. Mean ICP was calculated, as was the proportion of ICP values greater than thresholds from 1 to 80 mm Hg in 1–mm Hg increments. The association between these measures and outcome was explored for various epochs up to 30 days from the time of injury. A principal component analysis was used to explore physiologic changes at various ICP thresholds, and elastic net regression was used to identify ICP thresholds most strongly associated with Glasgow Outcome Scale score at discharge.
Results
Of the 523 studied patients, 70.7% of studied patients were men (n = 370) and 72.1% had a traumatic brain injury (n = 377). A total of 4 090 964 1-minute ICP measurements were recorded for the included patients (7.78 years of recordings). Intracranial pressure values of 8 to 9 mm Hg were most commonly recorded and could possibly reflect normal values. The principal component analysis suggested state shifts in the physiome occurred at ICPs greater than 19 mm Hg and 24 mm Hg. Elastic net regression identified an ICP threshold of 19 mm Hg as most robustly associated with outcome when considering all neurocritical care patients, patients with TBI, and patients with TBI who underwent craniectomy. Intracranial pressure values greater than 19 mm Hg were associated with mortality, while lower values were associated with outcome in surviving patients.
Conclusions and Relevance
This study provides insight into what normal ICP values could be. An ICP threshold of 19 mm Hg was robustly associated with outcome in studied patients, although lower ICP values were associated with outcome in surviving patients.
This study investigates associations between intracranial pressure values and outcomes in neurocritical care patients.
Introduction
Although outcome following traumatic brain injury (TBI) has been correlated with the magnitude and duration of intracranial pressure (ICP) elevation,1,2 the threshold at which ICP begins to exhibit detrimental effects is not known with certainty. Likewise, it remains unclear what the ideal treatment threshold for ICP is and whether a common threshold should be used for all patients and pathologies. Since their inception, the Guidelines for the Management of Severe Traumatic Brain Injury have advocated for ICP monitoring, and a treatment threshold has been recommended.3,4,5 This threshold has changed from 25 mm Hg to 20 mm Hg to 22 mm Hg in the last 2 decades, and while the precision of this recommendation is believed to be improving, the optimal value at which treatment should be initiated is not yet known with certainty.
In this study, our group has endeavored to better define the association between ICP and outcome using a large database of high-frequency physiologic data. Scientific mathematics software allowed us to explore how mean ICP and time greater than highly resolved ICP thresholds are associated with outcome, similar to our 2018 work6 exploring the association between focal brain oxygen measurements and outcome in this same population of patients with TBI.
Methods
Patient Demographics and Management
This study was approved by the University of California at San Francisco Committee on Human Research. A waiver of informed consent was granted because the study was judged to constitute minimal risk, and collected physiologic data were deidentified. All patients included in our study were admitted to San Francisco General Hospital (SFGH). The Brain Trauma Foundation’s Guidelines for the Management of Severe TBI5 are central to the care of patients with TBI at SFGH, and they are rigorously followed. Patients were selected for ICP monitoring based on the recommendations in these guidelines. The duration of ICP monitoring was based solely on medical necessity as judged by the treating neurosurgeon. An ICP treatment threshold of 20 mm Hg was used for all studied patients.4,7 Importantly, external ventricular drains used for ICP measurements were kept clamped to accurately measure ICP. When ICP values exceeded 20 mm Hg for 5 minutes, the drain was opened at 10 cm above the ear for 10 minutes.
Although physiologic data were collected prospectively, demographic data were collected retrospectively. The patients’ level of neurological disability at the time of discharge from SFGH was scored based on the Glasgow Outcome Scale (GOS).8 To facilitate a pooled analysis of all patients, all were scored with this general scale irrespective of brain pathology.
Data Collection
In conjunction with Aristein Bioinformatics LLC, our group developed a system that continuously and automatically recorded physiologic data from every bedside monitor in the SFGH neurosurgical intensive care unit (ICU) between 2004 and 2010. The time of the first recorded observation in the collection system was thus denoted as time “1,” distinct from the time of injury.
Variables displayed on the bedside monitor were collected at 1-minute intervals for the entirety of the patients’ ICU stay. In rare instances where 2 ICP monitors were used, the average was analyzed. Data on heart rate, peripheral oxygen saturation, brain oxygenation, and mean arterial pressure were also collected and analyzed.
Data Analysis
Intracranial pressure values were analyzed with the assistance of MATLAB software (MathWorks). We calculated mean ICP values and counted the number of 1-minute epochs with ICP values greater than specific thresholds between periods specified by the investigator. We analyzed ICPs greater than 79 different thresholds from 1 to 80 mm Hg in 1–mm Hg increments. The proportion of values greater than thresholds were analyzed to account for different numbers of observations between patients. Microsoft Excel, SPSS (IBM), and GraphPad were used to graph data, and figures were tiled using either PowerPoint (Microsoft) or Photoshop CC (Adobe). Error bars represent standard error of the mean (SEM).
Statistical Analysis
SPSS software, version 25, was used for statistical analyses. Analysis of variance (ANOVA) was used as a first step in the analysis of mean values from normally distributed continuous data. Post hoc tests for group differences were determined by Tukey and Bonferroni tests to adjust for multiple comparisons. Binomial logistic regression was used to analyze dichotomous categorical data. To test outcome group differences across ICP proportions, a generalized estimating equation was used; a repeated-measure model with a γ distribution. For mean values of continuous data, the “n” was considered to be the total number of observations. Where proportions greater than the threshold were analyzed, a single proportion was calculated for each patient, and the “n” in those cases was the number of patients in each group. A 2-sided P value less than .05 was set as the threshold for significance.
Principal components analyses (PCA; eMethods in the Supplement) were used to assess the association between physiologic measures (heart rate, mean arterial blood pressure, peripheral oxygen saturation, brain oxygenation [PbtO2]) and 15 binary ICP threshold categories. This physiome analysis was necessarily restricted to 229 patients who underwent both ICP and PbtO2 monitoring and was used to partition the variance in physiologic parameters across patients into orthogonal PCs. Principal component loading values reflect the unique association between each parameter and the variance explained within a particular PC. Thus, a higher loading for a particular measure indicates that measure contributes more to the variance explained by that PC.
Elastic net regression (with 10-fold cross-validation) was used to assess the relative contribution of each putative ICP threshold (between 15-30 mm Hg) in predicting GOS score at discharge. The threshold identified by this method was then included in an optimal scaling regression model along with other known factors associated with outcome to determine the strength of the threshold in predicting outcome as compared with other key factors. The elastic net optimal scaling regression workflow was repeated and performed separately for all patients, only patients with TBI, and patients with TBI who underwent a craniectomy.
Results
Patient Characteristics
Our data collection system recorded physiologic data for a total of 523 patients who underwent ICP monitoring, 383 of whom had a TBI. Of patients without TBI, 74 (52.9%) had hemorrhagic stroke, 27 (19.3%) had nontraumatic subarachnoid hemorrhage, 8 (5.7%) were being treated for a brain tumor, 6 (4.3%) had acute ischemic stroke, 6 (4.3%) had anoxic brain injury, and 19 (13.6%) were classified as other. Outcome data (GOS) were available for all but 14 patients. A total of 4 090 964 one-minute ICP measurements were recorded for the included patients (7.78 years of recordings).
Patient demographics are presented in the Table and eTable in the Supplement. Patients with poorer outcomes tended to be older (mean [SD] age at death, 57.0 [19.1] years; vegetative, 47.1 [22.3] years; severe disability, 42.5 [17.5] years; and moderate disability, 35.9 [16.7] years; P < .001; ANOVA) with longer hospital stays (mean [SD] stays for death, 15.1 [26.7] days; vegetative, 57.2 [50.8] days; severe disability, 46.3 [44.6] days; and moderate disability, 15.6 [17.0] days; P < .001; ANOVA) and a greater number of physiological measurements (death, 5178.4 [1151.4]; vegetative, 11 434.0 [1246.9]; severe disability, 9143.1 [439.6]; and moderate disability, 4187.2 [557.8]; P < .001; ANOVA), except for patients who died. Similarly, patients with poorer outcomes tended to have lower postresuscitation Glasgow Coma Scale (GCS) scores (mean [SD] score for death, 7.2 [4.1]; vegetative, 6.5 [4.3]; severe disability, 8.5 [3.9]; and moderate disability, 9.3 [4.2]; P < .001; ANOVA), except for patients who died. Patients with TBI were more likely to be younger (mean [SD] age, 43.9 [20.3] years vs 53.3 [16.0] years; P = .04; ANOVA) and men (292 patients [76.2%] vs 78 patients [55.7%]; P < .001; binomial logistic regression) and also more likely to undergo craniectomy (175 patients [45.6%] vs 37 patients [27.4%]; P = .04; binomial logistic regression).
Table. Characteristics of Included Patients in Association With Neurological Outcome at Time of Dischargea.
| Characteristic | Mean (SD) | P value | ||||
|---|---|---|---|---|---|---|
| No outcome data | Death | Vegetative | Severe disability | Moderate disability | ||
| Age | 46.9 (22.8) | 57.0 (19.1) | 47.1 (22.3) | 42.5 (17.5) | 35.9 (16.7) | <.001 |
| Sex, No. | ||||||
| Male | 11 | 103 | 22 | 192 | 42 | .57 |
| Female | 3 | 48 | 13 | 76 | 13 | |
| Craniectomy, No. | ||||||
| Yes | 6 | 68 | 16 | 139 | 33 | .32 |
| No | 7 | 83 | 19 | 131 | 21 | |
| Total hospital stay, d | 35.3 (30.8) | 15.1 (26.7) | 57.2 (50.8) | 46.3 (44.6) | 15.6 (17.0) | <.001 |
| Total measurements | 5178.4 (1151.4) | 7068.2 (506.2) | 11 434.0 (1246.9) | 9143.1 (439.6) | 4187.2 (557.8) | <.001 |
| Postresuscitation GCS | 7.5 (3.5) | 7.2 (4.1) | 6.5 (4.3) | 8.5 (3.9) | 9.3 (4.2) | <.001 |
Abbreviation: GCS, Glasgow Coma Scale score.
Continuous data were analyzed by analysis of variance, with outcome group as the independent variable. Dichotomous data were analyzed with binomial logistic regression, with outcome group as the independent variable. P less than .05 was considered statistically significant.
Among patients with TBI, Rotterdam scores are available for 345, and of these, 11 had a score of 1 (3.2%), 55 had a score of 2 (15.9%), 126 had a score of 4 (36.5%), 87 had a score of 4 (25.2%), 60 had a score of 5 (17.4%), and 6 had a score of 6 (1.7%). Traumatic subarachnoid hemorrhage was present in 239 of the patients with TBI (69.3%), and their mean postresuscitation GCS score was 8.1 (mode, 3). The mean amount of midline shift noted on cranial imaging of the patients with TBI was 9.64 mm. A total of 54.3% of patients with TBI required a craniotomy or craniectomy. Basal cistern status was assessed in 346 of these patients, and they had a normal appearance in 179 (51.7%). In 137 patients (39.6%), they were compressed, and they were absent in 30 (8.7%). In the heterogeneous patients who did not sustain a TBI, the mean age was 53.2 years, and 52 (37.4%) required a craniotomy or craniectomy.
Association Between Intracranial Pressure and Outcome
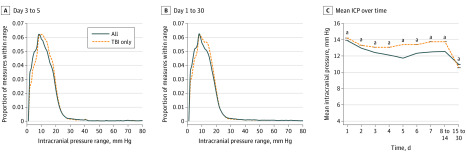
We thoroughly analyzed ICP values in different epochs from all patients and TBI-only patients (eFigure 1 in the Supplement). Salient data for days 3 to 5 and days 1 to 30 are shown in Figure 1. A discrete peak in ICP values (consistent with the mode) was consistently seen at approximately 8 to 9 mm Hg, a possible indication of normal ICP. A sharp dropoff in ICP was consistent around the treatment thresholds of 20 mm Hg, reflecting efforts to treat higher values. Nonetheless, 8.9% of all measures were greater than 20 mm Hg. Mean ICP values were generally higher in patients with TBI than those with other conditions (Figure 1C).
Figure 1. Distribution of Intracranial Pressure Measures in Studied Patients Suggests a Normal Value.
The distribution of intracranial pressure (ICP) measures is plotted for all patients (dark blue solid line) and for patients with traumatic brain injury (TBI) only (orange dotted line). A, Values obtained between 3 and 5 days following admission to the neurocritical care unit are reported while those in B represent all values obtained within 30 days of admission. The most common ICP (nearest integer) measured in all patients from day 3 to 5 was 8 mm Hg (6.19% of all measures), while in patients with TBI, it was 9 mm Hg (6.23%). The most common ICP (nearest integer) measured in all patients from day 1 to 30 was 8 (6.24% of measures), while in patients with TBI it was 9 (6.17%). Given the robust mode demonstrated by these distributions, it is possible that ICP values of 8 to 9 mm Hg may be normal. C, Mean ICP values are shown for noncumulative epochs from time of intensive care unit admission, demonstrating that all mean values are less than present and past recommended ICP treatment thresholds. Patients with TBI had higher mean values than those with other conditions. For all patients, n = 523; for patients with TBI only, n = 383.
aDenotes a statistically significant difference on analysis of variance (P < .05).
Mean ICP values were associated with outcome (Figure 2A and B). Intracranial pressure values of patients who died were much higher on day 2 and 3 after neurocritical care unit admission than surviving patients. eFigure 2 in the Supplement as well as Figure 2C-F demonstrate the assocation between time spent at greater than 79 different ICP thresholds (1-80 mm Hg in 1–mm Hg increments) and outcome during the period of maximal brain swelling (day 3-5) and for the entirety of acute care (day 1-30) in all patients and patients with TBI. For all patients between days 3 to 5, there was a significant difference in outcome across ICP thresholds (Figure 2C, generalized estimating equation [GEE], Wald χ2 = 9.29; P = .03). For patients with TBI only between days 3 to 5, there was no overall significant effect of outcome (GEE, Wald χ2 = 5.57; P = .14), but a planned comparison between death and all other outcomes revealed a significant difference (Figure 2D; GEE, Wald χ2 = 4.78; P = .03). All patients and patients with TBI only showed significant differences in outcome over ICP threshold for days 1 to 30 (Figure 2E and F; GEE, Wald χ2 >19.9; P <.001).
Figure 2. Association of Intracranial Pressure (ICP) Values With Outcome.
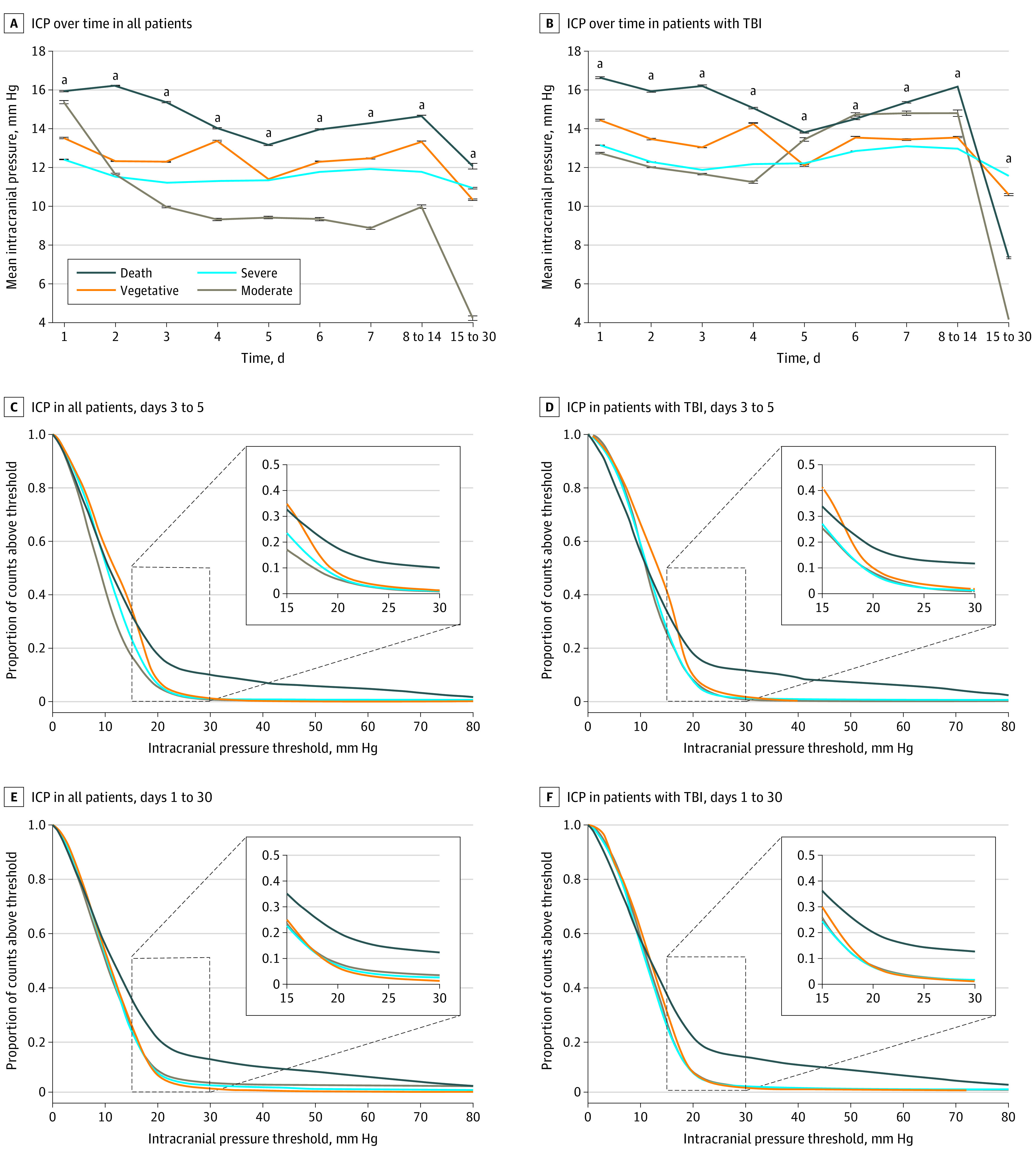
A, Data from all studied patients. B, Data only from patients with traumatic brain injury (TBI). In A and B, mean ICP values are plotted for noncumulative epochs following intensive care unit (ICU) admission in patients grouped by their level of disability (Glasgow Outcome Scale score at the time of discharge). Higher mean ICP values were associated with worse outcome. C-F, Plots of the time patients spent with ICP values at greater than 79 different ICP thresholds (1 to 80 mm Hg in 1–mm Hg increments) are shown. In the insets, the region of each graph is replotted for the range of ICP values between 15 and 30 mm Hg to improve visualization. Intracranial pressure values greater than approximately 10 mm Hg are associated with outcome, especially during the period of maximal brain swelling between days 3 and 5 following admission. A significant effect of outcome group across thresholds was seen for all patients at days 3 to 5 and 1 to 30. Patients with TBI only (D and F) showed only a significant difference between death and other groups at days 3 to 5, and main effect of outcome group for days 1 to 30. Thus, higher values seem to discriminate mortality while lower values are associated with outcome in surviving patients.
aDenotes a statistically significant difference on analysis of variance (P < .05).
In areas where different outcome lines diverge, converge, or cross, an association between ICP values and outcome is suggested. The curves look very similar whether all patients or just patients with TBI are considered, suggesting that the brain pathology does not markedly alter the association between ICP and outcome. Figure 2C and D also shows that ICP values greater than 20 mm Hg are associated with mortality, while ICP lower than 20 mm Hg is associated with outcomes in surviving patients. Indeed, ICP values higher than approximately 20 mm Hg were not robustly associated with outcome in surviving patients.
Physiologic Changes as ICP Increases
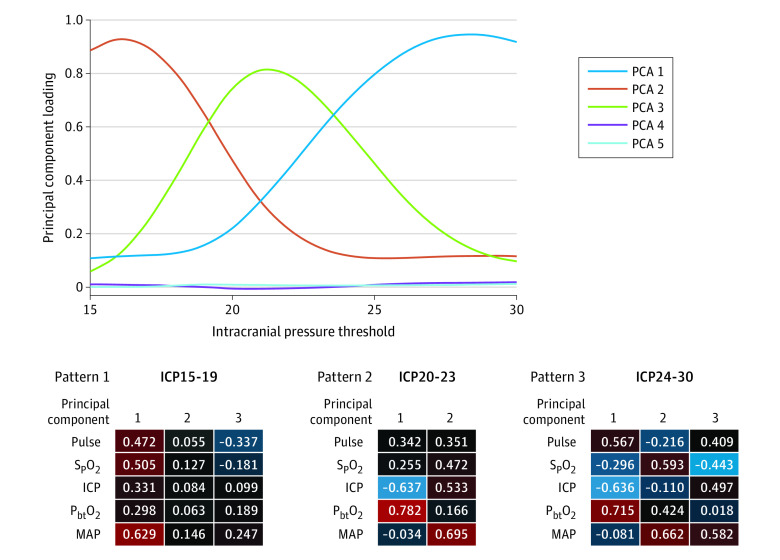
An initial linear PC analysis (PCA) was run to assess the association between numerous physiologic parameters along with each of the 15 binary ICP threshold categories (yes = 1; no = 0; eFigure 3 in the Supplement). Plotting PC loadings across ICP thresholds for 5 orthogonal principal components demonstrates 2 changes in the association between physiologic variables as ICP increases. These transitions are seen at ICP threshold of approximately 19 and 24 mm Hg (Figure 3); a plot of the PC loadings clearly shows these transitions.
Figure 3. Principal Component Analysis (PCA) Suggests Physiome Pattern Changes at Intracranial Pressure Thresholds of 19 and 24 mm Hg.
Principal component analysis was applied to the physiome (heart rate, peripheral oxygen saturation, intracranial pressure [ICP], focal brain oxygen measurements, and mean arterial blood pressure) based on a dichotomy of ICP values at 15 different ICP thresholds between 15 and 30 mm Hg. An initial PCA was run that revealed 5 unique PC patterns of loadings across thresholds. On the top, loading values for each of 5 identified principal components at each of the 15 ICP thresholds are plotted. This analysis suggests distinct physiologic associations between variables for ICP values less than 19 mm Hg, those between 19 and 24 mm Hg, and those greater than 24 mm Hg, with principal components 2, 3, and 1 dominant in each state, respectively. A second series of PCAs were then run that included each threshold (from 15-30 mm Hg) separately, along with other physiome measures. Mean loadings for 3 ranges of thresholds are shown on the bottom, depicting distinct patterns of associations between ICP threshold and other physiome measures. Blue represents negative loadings and red represents positive loadings. Principal component loadings indicate the extent to which a particular measure contributes to the variance explained within a PC, and the sign of loadings (positive or negative) indicates whether the measures correlate with the PC in the same or opposite directions.
To further assess the unique association between physiologic measures and ICP thresholds, a second set of analyses were then run that included heart rate, mean arterial pressure, peripheral oxygen saturation, and PbtO2 as before, but with a single ICP threshold (between 15-30 mm Hg) included for each PCA iteration. At the bottom of Figure 3, mean loadings for PCAs that included ICP threshold ranges 15 to 19 mm Hg, 20 to 23 mm Hg, and 24 to 30 mm Hg are shown. Heat maps for PC loadings demonstrate the distinct interrelations of physiologic variables seen, when ICPs are 15-19 mm Hg, 20-23 mm Hg, and 24-30 mm Hg. Of course, 19 mm Hg and 24 mm Hg are very close to the historic ICP thresholds recommendations of 20 mm Hg7 and 25 mm Hg.3
Identifying an Optimal ICP Threshold for Outcome Prediction
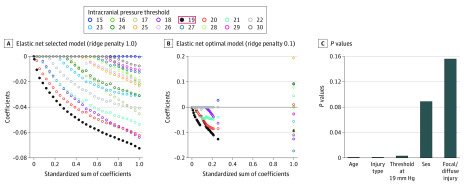
The PCA results identified 2 nodes along the spectrum of ICP threshold values at which multivariate physiologic shifts appear to occur. To further explore the association between ICP threshold and GOS outcome, we used an elastic net regression model that included all thresholds across days 1 to 30 within the threshold range identified by the PCA (15-30 mm Hg). Elastic net is a sophisticated form of regression (combing ridge and lasso regularization) that overcomes the problem of multicollinearity by shrinking the coefficients to reveal an optimal model that selects only those variables that have predictive value that can withstand the regularization penalties. The elastic net regression revealed a single ICP threshold (19 mm Hg) that remains predictive of discharge GOS (Figure 4A and B). To compare this ICP threshold with other known robust factors associated with outcome after TBI, we then ran an optimal scaling regression that incorporated sex, age, injury type, diffuse/focal injury, and the proportion of ICP counts greater than the 19 mm Hg threshold (Figure 4C). Results revealed that the 19 mm Hg threshold was a significant factor associated with outcome, with a P value comparable with age or injury type, giving further indication that the 19 mm Hg threshold is strongly associated with outcome after central nervous system insult. Moreover, the elastic net regression was repeated for patients with TBI alone as well as those patients with TBI undergoing a decompressive craniectomy, and 19 mm Hg was the ICP threshold most strongly associated with outcome in these key subpopulations as well.
Figure 4. Identifying Intracranial Pressure (ICP) Thresholds as Robust Factors Associated With Outcome.
Elastic net regression was used to determine the most robust threshold associated with outcome. Elastic net regression plots show a single ICP threshold (19 mm Hg) performed better than all other thresholds tested in an analysis of all patients, with the regression coefficient for the 19 mm Hg threshold remaining most resistant to shrinkage from ridge regression penalties of 1.0 (A, selected model) and 0.1 (B, optimal model). C, To test how this single threshold compared with other factors, an optimal scaling regression model was used and revealed that the 19 mm Hg ICP threshold performs as well as age and injury type in a predictive model of Glasgow Outcome Scale outcome at discharge. The threshold of 19 mm Hg was robust because it was also strongly associated with outcome when patients with traumatic brain injury, and those who underwent a craniectomy were analyzed with separate elastic net regressions (not shown).
Discussion
A study completed in 20159,10,11 has called the value of ICP monitoring into question, as well as the significance of ICP elevation. However, there is no question that ICP elevation is harmful when it impedes delivery of adequate nutrients to the brain. In the extreme circumstance, ICP elevation exceeds arterial pressure and prevents intracranial blood flow, as is seen with brain death. The precise thresholds of ICP and blood flow at which harm begins to occur to the brain are not known with certainty. Studies to date have tended to report ICP thresholds at which the strongest statistical association with outcome is seen6; the lowest value at which harm occurs has not been well delineated to date. Our analysis provides new insights into the association between ICP and outcome following brain injury.
Possibly Normal ICP Values
Normal ICP is difficult to define,12 and numerous normal ranges have been suggested.13,14,15,16,17,18 Because monitoring ICP in healthy patients is unethical, normal ICP is not known with certainty nor with precision. Miller argued for an ICP of less than 10 mm Hg as being normal.1 Marshall et al19 instead defined a normal ICP as being less than 15 mm Hg. Complicating matters, it has been shown that a “normal” ICP can vary with age and body position.12,20,21,22 In our study, a discrete mode of 8 to 9 mm Hg was consistently seen across distinct epochs whether all patients or just those with TBI were studied (Figure 1). It is not possible to infer normal ICP values from patients being treated for significant brain pathology. Nonetheless, these data raise the possibility that 8 to 9 mm Hg could reflect normal ICP values, at least in those undergoing monitoring in the ICU.
Literature to Date Informing the Intracranial Pressure Treatment Threshold
Although Lundberg16 first performed detailed study of ventricular fluid pressure recordings in the 1960s and associated ICP elevation with neurological decline,23 the importance of ICP elevation following TBI was not firmly established until 1977, when Miller et al1 demonstrated its correlation with outcome and its role in precipitating death. Widespread clinical application of ICP monitoring followed in the 1980s,1,19,23 and it is now viewed as important in the management of most patients with severe TBI treated in North America. Despite more than 3 decades of use and study, “the critical value of ICP… is [still] a major unanswered question.”5
Over the course of the 4 editions of the BTF Guidelines for the Management of Severe TBI, the recommended ICP treatment threshold has changed from 25 mm Hg3 to 20 to 25 mm Hg4 to 20 mm Hg7 to 22 mm Hg.22 The current guidelines recognize a total of 12 studies that inform the ICP treatment threshold.22,24 Unfortunately, strong conclusions cannot be drawn from these studies; most tended to report the ICP value most strongly associated with outcome25 and do not consider a broad range of possible thresholds. Additionally, few used unbiased and automated computer collection of high-frequency patient measurements, as we have done, which overcomes problems with threshold compliance.
It is important to consider that numerous publications have suggested an ICP treatment threshold less than 20 mm Hg may be appropriate.25,26,27,28,29 Analysis of our data suggests that an ICP threshold of 19 mm Hg is most strongly associated with patient outcome and that this threshold is robust across patient subgroups. However, our data suggest that ICP values as low as 10 mm Hg may be associated with harm.1 Our findings are also consistent with evidence for a higher ICP threshold for mortality than for good outcome.24
Are Normal ICP Values and the ICP Treatment Threshold the Same for All Patients and Conditions?
Several investigators have argued for distinct normal values or treatment thresholds with specific disease states or patient characteristics. For instance, in hydrocephalus, pressure elevations greater than 15 mm Hg are considered abnormal.12 Some have argued that a normal ICP value should be defined as less than 11 mm Hg in patients with pseudotumor cerebri,20 while others argue that the optimal treatment threshold in patients with TBI may vary with computed tomographic head findings,25 age, or sex.24 A key finding of our study is that the results of our analyses are consistent across all neurocritical care unit patients (Figure 3).
Limitations
Our study has a number of important limitations. The results of this study only pertain to patients in whom ICP monitoring was judged appropriate. Our study does not provide insight into causation. Our work describes patients from a single institution and does not capture physiologic events prior to the initiation of ICU monitoring nor the time from injury to initiation of monitoring. We used the GOS as the outcome measure for all patients, as has been permissible in other studies.30 The use of discharge neurological status was suboptimal but necessary because of a high rate of loss to follow-up. The thresholds that we identified may be confounded by ICP-directed treatments used in an effort to maintain ICP less than 20 mm Hg as well as toxicities of these treatments. However, we note that ICP-directed treatment at SFGH is not initiated until the 20 mm Hg threshold has been exceeded for 5 minutes. On this basis, we believe that such a confounding effect, if present, would likely occur at greater than the 19 mm Hg treatment threshold we identified.
Conclusions
We have performed a detailed exploration of the association between ICP and outcome using a large database of high-frequency physiologic measurements. Our study suggests that ICPs of 8 to 9 mm Hg could possibly constitute normal values and that 19 mm Hg is the ICP threshold most strongly associated with outcome in all patient groups. Intracranial pressure values at greater than this threshold are associated with mortality, while lower values are associated with outcome in surviving patients. The association between ICP and outcome was remarkably consistent among the heterogeneous pathologies studied. Our findings are consistent with those of Miller et al1 in suggesting that ICP values lower than 20 mm Hg may be harmful and that a lower ICP threshold may ultimately be judged optimal.
eMethods. Primer on Principal Component Analysis
eTable. Characteristics of Included Patients In Relation to Neurological Insult
eFigure 1. ICP Distributions for All Examined Epochs
eFigure 2. Association Between Time Spent Above ICP Thresholds and Outcome
eFigure 3. Principal Component Analyses Performed for Each Examined ICP Threshold
References
- 1.Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47(4):503-516. doi: 10.3171/jns.1977.47.4.0503 [DOI] [PubMed] [Google Scholar]
- 2.Vik A, Nag T, Fredriksli OA, et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. 2008;109(4):678-684. doi: 10.3171/JNS/2008/109/10/0678 [DOI] [PubMed] [Google Scholar]
- 3.Bullock R, Chesnut RM, Clifton G, et al. ; Brain Trauma Foundation . Guidelines for the management of severe head injury. Eur J Emerg Med. 1996;3(2):109-127. doi: 10.1097/00063110-199606000-00010 [DOI] [PubMed] [Google Scholar]
- 4.The Brain Trauma Foundation The American Association of Neurological Surgeons: the joint section on neurotrauma and critical care: intracranial pressure treatment threshold. J Neurotrauma. 2000;17(6-7):493-495. [DOI] [PubMed] [Google Scholar]
- 5.Carney NA, Ghajar J; Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS . Guidelines for the management of severe traumatic brain injury: introduction. J Neurotrauma. 2007;24(suppl 1):S1-S2. doi: 10.1089/neu.2007.9997 [DOI] [PubMed] [Google Scholar]
- 6.Hirschi R, Hawryluk GWJ, Nielson JL, et al. Analysis of high-frequency PbtO2 measures in traumatic brain injury: insights into the treatment threshold. J Neurosurg. 2018:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bratton SL, Chestnut RM, Ghajar J, et al. ; Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS . Guidelines for the management of severe traumatic brain injury, VIII: intracranial pressure thresholds. J Neurotrauma. 2007;24(suppl 1):S55-S58. doi: 10.1089/neu.2007.9988 [DOI] [PubMed] [Google Scholar]
- 8.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480-484. doi: 10.1016/S0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 9.Chesnut RM, Temkin N, Carney N, et al. ; Global Neurotrauma Research Group . A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471-2481. doi: 10.1056/NEJMoa1207363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesnut R, Bleck T, Citerio G, et al. A Consensus-based interpretation of the BEST TRIP ICP Trial. J Neurotrauma. 2015. [DOI] [PubMed] [Google Scholar]
- 11.Chesnut RM. What is wrong with the tenets underpinning current management of severe traumatic brain injury? Ann N Y Acad Sci. 2015;1345(1):74-82. doi: 10.1111/nyas.12482 [DOI] [PubMed] [Google Scholar]
- 12.Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75(6):813-821. doi: 10.1136/jnnp.2003.033126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischer AS, Payne NS, Tindall GT. Continuous monitoring of intracranial pressure in severe closed head injury without mass lesions. Surg Neurol. 1976;6(1):31-34. [PubMed] [Google Scholar]
- 14.Johnston IH, Jennett B. The place of continuous intracranial pressure monitoring in neurosurgical practice. Acta Neurochir (Wien). 1973;29(1):53-63. doi: 10.1007/BF01414616 [DOI] [PubMed] [Google Scholar]
- 15.Johnston IH, Johnston JA, Jennett B. Intracranial-pressure changes following head injury. Lancet. 1970;2(7670):433-436. doi: 10.1016/S0140-6736(70)90054-1 [DOI] [PubMed] [Google Scholar]
- 16.Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36(149):1-193. [PubMed] [Google Scholar]
- 17.Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury: a preliminary report. J Neurosurg. 1965;22(6):581-590. doi: 10.3171/jns.1965.22.6.0581 [DOI] [PubMed] [Google Scholar]
- 18.Troupp J. Intraventricular pressure in patients with severe brain injuries. J Trauma. 1965;5:373-378. doi: 10.1097/00005373-196505000-00007 [DOI] [PubMed] [Google Scholar]
- 19.Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries: part I: the significance of intracranial pressure monitoring. J Neurosurg. 1979;50(1):20-25. doi: 10.3171/jns.1979.50.1.0020 [DOI] [PubMed] [Google Scholar]
- 20.Albeck MJ, Børgesen SE, Gjerris F, Schmidt JF, Sørensen PS. Intracranial pressure and cerebrospinal fluid outflow conductance in healthy subjects. J Neurosurg. 1991;74(4):597-600. doi: 10.3171/jns.1991.74.4.0597 [DOI] [PubMed] [Google Scholar]
- 21.Chapman PH, Cosman ER, Arnold MA. The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts: a telemetric study. Neurosurgery. 1990;26(2):181-189. doi: 10.1227/00006123-199002000-00001 [DOI] [PubMed] [Google Scholar]
- 22.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Neurosurgery. 2017;80(1):6-15. doi: 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan VM, O’Neill BR, Jho D, Whiting DM, Oh MY. The history of external ventricular drainage. J Neurosurg. 2014;120(1):228-236. doi: 10.3171/2013.6.JNS121577 [DOI] [PubMed] [Google Scholar]
- 24.Sorrentino E, Diedler J, Kasprowicz M, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16(2):258-266. doi: 10.1007/s12028-011-9630-8 [DOI] [PubMed] [Google Scholar]
- 25.Chambers IR, Treadwell L, Mendelow AD. Determination of threshold levels of cerebral perfusion pressure and intracranial pressure in severe head injury by using receiver-operating characteristic curves: an observational study in 291 patients. J Neurosurg. 2001;94(3):412-416. doi: 10.3171/jns.2001.94.3.0412 [DOI] [PubMed] [Google Scholar]
- 26.Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg. 1982;56(4):498-503. doi: 10.3171/jns.1982.56.4.0498 [DOI] [PubMed] [Google Scholar]
- 27.Schreiber MA, Aoki N, Scott BG, Beck JR. Determinants of mortality in patients with severe blunt head injury. Arch Surg. 2002;137(3):285-290. doi: 10.1001/archsurg.137.3.285 [DOI] [PubMed] [Google Scholar]
- 28.Kolecki R, Dammavalam V, Bin Zahid A, et al. Elevated intracranial pressure and reversible eye-tracking changes detected while viewing a film clip. J Neurosurg. 2018;128(3):811-818. doi: 10.3171/2016.12.JNS161265 [DOI] [PubMed] [Google Scholar]
- 29.Prossinger HHH, Acimovic A, Berger R, Mostafa K, Grieb A, Steltzer H. The intervention threshold for intracranial pressure of traumatic brain injury patients can be determined by clustering algorithms and is observed to be 13 mm Hg. Clin Med Res. 2019;8(1):6-15. doi: 10.11648/j.cmr.20190801.12 [DOI] [Google Scholar]
- 30.Hawryluk GW, Austin JW, Furlan JC, Lee JB, O’Kelly C, Fehlings MG. Management of anticoagulation following central nervous system hemorrhage in patients with high thromboembolic risk. J Thromb Haemost. 2010;8(7):1500-1508. doi: 10.1111/j.1538-7836.2010.03882.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Primer on Principal Component Analysis
eTable. Characteristics of Included Patients In Relation to Neurological Insult
eFigure 1. ICP Distributions for All Examined Epochs
eFigure 2. Association Between Time Spent Above ICP Thresholds and Outcome
eFigure 3. Principal Component Analyses Performed for Each Examined ICP Threshold