Abstract
The mammalian flagellum is a specific type of motile cilium required for sperm motility and male fertility. Effective flagellar movement is dependent on axonemal function, which in turn relies on proper ion homeostasis within the flagellar compartment. This ion homeostasis is maintained by the concerted function of ion channels and transporters that initiate signal transduction pathways resulting in motility changes. Advances in electrophysiology and super-resolution microscopy have helped to identify and characterize new regulatory modalities of the mammalian flagellum. Here, we discuss what is currently known about the regulation of flagellar ion channels and transporters that maintain sodium, potassium, calcium, and proton homeostasis. Identification of new regulatory elements and their specific roles in sperm motility is imperative for improving diagnostics of male infertility.
Keywords: Sperm ion channels, CatSper, Hv1, Slo3, Slo1, Capacitation, Fertility, Motility, Progesterone, pH, Flagellum, EFCAB9
Introduction
The tail of mammalian sperm cells is represented by a single motile cilium known as the flagellum that generates its movement to propel the cell through the female reproductive tract and deliver paternal genetic material into an egg. Sperm cells rely on vigorous motility that is initiated once they are released from the seminal plasma coagulum. The motility initiation is partially driven by an increase in the intraflagellar pH caused by ion channels and transporters that move protons out of and bicarbonate into the cell. Intracellular alkalization is also an important part of a set of molecular changes known as ‘capacitation’ or final sperm maturation that sperm cells undergo in the female reproductive tract making them competent to reach and fertilize the egg [1,2]. Capacitation includes the alteration of membrane fluidity due to cholesterol removal, the change of intracellular pH, protein tyrosine phosphorylation, induction of ‘hyperactivated’ asymmetrical motility, and the acquisition of the ability to perform the acrosome reaction [3,4]. The former event, hyperactivation, is defined by a powerful asymmetrical flagellar bending mode, which allows sperm cell to penetrate the egg’s protective vestments. The latter, acrosome reaction [5], is an exocytotic event that takes place in the sperm head during fertilization. Specifically, an acrosome is a vesicle located in the anterior segments of the sperm head that contains hyaluronidase and other hydrolytic enzymes required for sperm penetration through zona pellucida, a protective layer of the egg.
All these physiological processes are possible because of the finely orchestrated signaling pathways between cytoskeletal protein complexes and plasma membrane elements, such as ion channels and transporters [6]. Dysfunction of these proteins or their regulation can cause defects in ciliary motility, which can lead to infertility [7,8]. In this review, we summarize recent advances in the function of ion channels and transporters and their corresponding signaling pathways in the mammalian sperm flagellum.
Bicarbonate signaling/ transporters
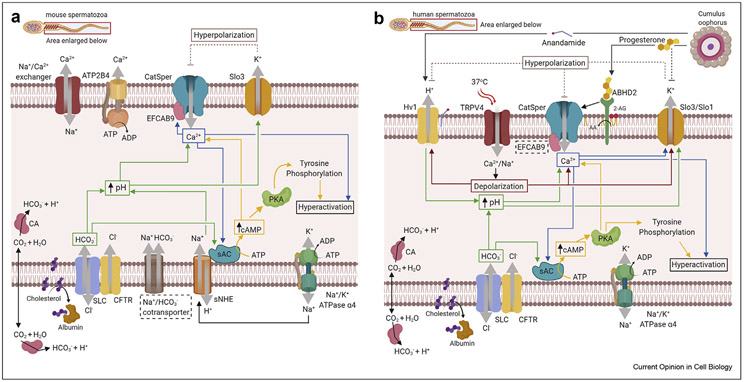
The uterus and oviduct provide an alkaline environment of pH ~7.9 and a very high concentration of bicarbonate (~50 mM, as measured in rabbit female reproductive tract) [9]. High concentration of bicarbonate is crucial for capacitation. Specifically, directly activates the atypical bicarbonate-dependent soluble adenylyl cyclase (sAC) [10] that generates cAMP required for activation of cAMP-dependent protein kinase A (PKA; Figure 1a and b). PKA signals downstream by activating tyrosine kinases [11] that phosphorylate various protein targets. Bicarbonate elevation inside sperm cells can be achieved in several ways: via CO2 diffusion through the membrane, because of its conversion into bicarbonate by carbonic anhydrases [12], and via a subset of Cl−/ exchangers, such as SLC26A3 and SLC26A6 [13]. These exchangers can directly shuttle bicarbonate between the extracellular and intracellular environments [13]. In mice and humans, the cystic fibrosis transmembrane conductance regulator channel (CFTR) works in association with these transporters, to provide a sustained regulation of Cl− transport (Figure 1a and b). As reported in Refs. [14,•15], mutation or deficiency in SLC26A3 gene is associated with impaired sperm motility in humans and mice. Interestingly, a new member of this family, SLC22A14, has also been shown to be required for male fertility in mice [16]. Additionally, the bicarbonate can be imported inside the murine sperm via the Na+/ transport mechanism [17]; however, the molecular identity of this cotransporter is yet to be revealed. Overall, the /sAC/cAMP/PKA signaling pathway is essential for the process of capacitation and male fertility, as demonstrated by sAC-deficient mice [18,19] and mice lacking catalytic subunit Cβ2 of sperm-specific PKA [20].
Figure 1.
(a) Signaling pathways in the murine sperm flagellum. pH-activated CatSper opens and carries Ca2+ into the cell. This channel is additionally regulated by intracellular Ca2+ via its EFCAB9 cytoplasmic subunit. Calcium clearance mechanisms of mouse sperm is provided mainly by ATPase (ATP2B4) and by Na+/Ca+ exchanger. Intracellular alkalization can be caused by the action of NHE exchangers. Sperm capacitation is triggered by an increase in intracellular , which enters the cell in two different ways. First, CO2 can diffuse through the membrane and then is converted by CA into . Second, extracellular can be carried into the cell by Cl−/ SLC cotransporter interacting with Cl−-permeable CFTR. Na+/K+ gradient across the plasma membrane and, hence, membrane potential are maintained by the Na+/K+-ATPase α4. This gradient could be used to power the sNHE exchanger to promote influx of Na+ and efflux of H+ and to ensure intracellular alkalization. Potassium enters the cell through the Na+/K+-ATPase α4 and can leave through the Slo3 channel, which in mouse is activated by intracellular alkalization. Efflux of K+ causes hyperpolarization, further inhibiting CatSper. and intracellular Ca2+ can trigger activation of sAC, which produces cAMP, leading to activation of PKA and tyrosine kinases, resulting in broad tyrosine phosphorylation leading to sperm capacitation and hyperactivation. Capacitation is also associated with cholesterol removal from the plasma membrane by albumin. Solid lines represent activation, and dotted lines represent inhibition. Dotted rectangles indicate proteins with yet to be conformed functional role in male fertility. (b) Signaling pathways in human sperm flagellum. P4 released from the cumulus oophorus binds to ABHD2, which cleaves 2-AG to AA and thus removes the inhibition imposed by 2-AG on the CatSper channel. CatSper opens and carries Ca2+ into the cell. Warm temperature (37 °C) in the female reproductive tract activates the TRPV4 channel, allowing cations (Na+ and Ca2+) to enter the cell and to cause membrane depolarization, which further promotes CatSper opening. Besides P4 and depolarization, CatSper also requires intracellular alkalization to produce maximal current. This can be achieved by proton efflux through Hv1, which is activated by anandamide and fatty acids released from the cumulus oophorus. As mentioned in Figure 1a, enters the cell via CO2 diffusion and via conversion by CA into . Additionally, it can be imported by SLC cotransporter interacting with Cl−-permeable CFTR. As in murine sperm, activates sAC. Flagellar alkalinity further upregulates CatSper. Potassium enters the cell through the Na+/K+-ATPase α4 and leaves through the human KSper (Slo3/Slo1) channel, which is activated by depolarization and intracellular Ca2+, and is inhibited by P4. Efflux of K+ causes hyperpolarization, thus negatively regulating CatSper and Hv1. As in murine sperm, cAMP elevation triggered by and intracellular Ca2+ leads to PKA and tyrosine kinase activation, resulting in broad tyrosine phosphorylation. The latter completes sperm capacitation and ensures hyperactivation. Additionally, cholesterol removal from the plasma membrane by albumin further facilitates capacitation. Solid lines represent activation, and dotted lines represent inhibition. Dotted rectangles indicate proteins with yet to be conformed functional role in human male fertility. Abbreviations: TRPV4, transient receptor potential cation channel subfamily member 4 vanilloid 4 channel; sAC, soluble adenylyl cyclase; PKA, protein kinase A; CatSper, cation channel of sperm; Hv1, proton voltage-gated ion channel; ABHD2, α/β hydrolase domain-containing protein 2; 2-AG, 2-arachidonoylglycerol; AA, arachidonic acid; KSper/Slo1/Slo3, potassium channel of sperm/Slowpoke homolog 1/3; SLC, solute carrier family anion exchanger; CFTR, cystic fibrosis transmembrane conductance regulator; sNHE/SLC9C1, sperm-specific Na+/H+ exchanger; EFCAB9, EF-hand calcium-binding domain-containing protein 9; ATP2B4, ATPase plasma membrane Ca2+ transporting 4; ATP, adenosine triphosphate; ADP; adenosine diphosphate; cAMP, cyclic adenosine monophosphate; P4, progesterone; CA, carbonic anhydrases. (a) and (b) figures are created with Biorender.com.
Additional hallmark of capacitation is cholesterol removal. Cholesterol is in general an essential stabilizing component of the mammalian plasma membrane. Moreover, in sperm cells its removal from the flagellar membrane is a key part of the sperm maturation process inside the female reproductive tract which is enriched in albumin and bicarbonate [9,21]. It is known that albumin sequesters cholesterol from plasma membrane (Figure 1a and b) only in the presence of bicarbonate; however, the molecular mechanism of such cholesterol sequestration is still poorly understood [22]. Cholesterol removal is associated with changes in sperm plasma membrane fluidity, the activation of a signal transduction pathway involving protein kinase A and tyrosine kinase and resulting in protein tyrosine phosphorylation and capacitation [23].
Na+/H+ exchangers
Sperm intraflagellar pH is regulated by a concerted action of ion channels and transporters. Na+/H+ (NHE) exchangers, such as SLC9B1 and SLC9B2, export protons from the cell in exchange for sodium and are required for sperm motility and male fertility in mice [24]. Another member of the same family, SLC9C1, also known as sperm-specific Na+/H+ exchanger (sNHE), has been proposed to play a role in pH regulation [25]. SLC9C1-deficient male mice are infertile, but their infertility is partially rescued by the addition of ammonium chloride, which increased the intracellular pH. Additionally, their infertility was completely rescued by the addition of cell-permeable cAMP [25]. As has been reported, SLC9C1-deficient male infertility results not from the absence of sNHE, but from inactive sAC that likely forms a complex with sNHE (Figure 1a) [26]. Interestingly, sea urchin sNHE has been shown to function as a genuine Na+/H+ exchanger gated by voltage and further modulated by cAMP [•27]. Further studies are needed to clarify sNHE’s function in mammalian male fertility, particularly in humans.
H+ channel
Proton efflux by ion channels is another efficient mechanism for intracellular pH regulation. The first proton voltage-gated ion channel, Hv1, was identified in 2006 [28,29]. Hv1 is expressed in human (and not mouse) spermatozoa [30], and a subset of these channels [•31] are positioned near the pH-dependent, Ca2+-permeable sperm-specific cation channel of sperm (CatSper) [32]. Specifically, the Hv1 channel is distributed asymmetrically to one side of the flagellar midline and according to the model [•31], it can activate only a subset of CatSper channels, resulting in asymmetrical local Ca2+ influx and perhaps flagellar rotation. A well-recognized feature of the native and recombinant Hv1 channel is its voltage-gating dependency on transmembrane pH-gradient [28,29]. Additionally, Hv1 can be further upregulated by fatty acids [33] and the endogenous cannabinoid anandamide [30], which is synthesized by the cumulus oophorus [34] (Figure 1b). In addition to the full-length Hv1, human sperm cells contain its N-terminally cleaved isoform (Hv1Sper), which has slightly different voltage-gating properties [35]; however, the precise function of Hv1 in human sperm motility will be elucidated only after a male infertile patient associated with mutation in Hvcn1 (the gene that encodes Hv1) is identified.
Ca2+ ion channel/CatSper
While ascending the female reproductive tract, the intracellular Ca2+ concentration of sperm raises through the activation of CatSper, which acts as the main flagellar calcium gate. CatSper is located in the principal piece of sperm flagellum [32] and is regulated by several endogenous stimuli, such as alkaline pH in rodents [32,36] and primates [30,37], as well as by progesterone (P4) and prostaglandins [37,38] produced by the cumulus oophorus in primates (Figure 1a and b). In human spermatozoa, CatSper activation by P4 occurs through P4 binding to the membrane receptor α/β hydrolase domain-containing protein 2 (ABHD2) [39]. ABHD2 is a lipid hydrolase that upon P4 binding hydrolyzes the endocannabinoid 2-arachidonoylglycerol (2-AG), removing it from the plasma membrane (Figure 1b). The 2-AG inhibits CatSper, and its removal leads to CatSper activation, followed by Ca2+ influx [39].
One of the downstream effects of P4-induced Ca2+ influx is the powerful asymmetrical flagellar bending mode known as hyperactivation. The precise mechanism of how intraflagellar calcium evaluation triggers hyperactivation by affecting dynein motility is not yet known; however, it has been shown recently that at high Ca2+ concentrations, Ca2+ binding protein EFCAB1 directly suppresses the velocity of microtubule sliding by outer-arm dynein and thus induces asymmetric flagellar bending in Ciona intestinalis sperm [40]. EFCAB1-deficient mice, however, are fertile, and their sperm have normal hyperactivated motility, which indicates that at least for mice, EFCAB1 is not required for hyperactivation [41].
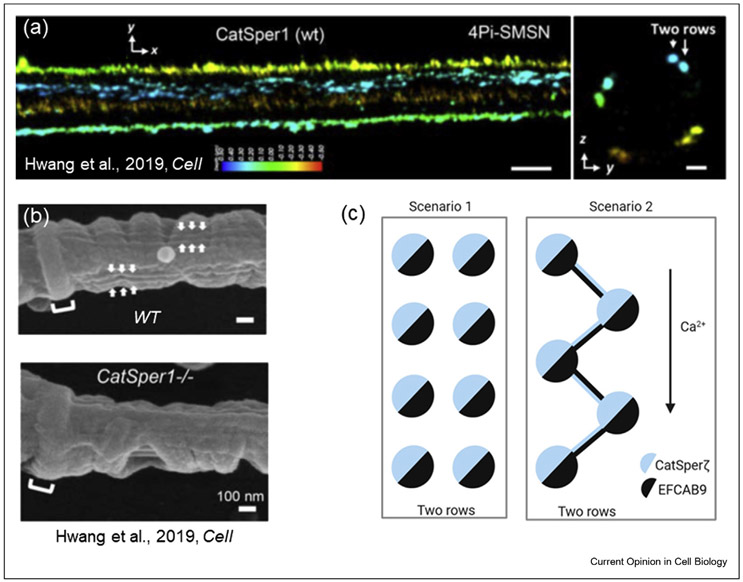
In addition to conducting Ca2+, CatSper is also positively regulated by cytoplasmic Ca2+ [••42]. The CatSper channel is a complex of at least 10 different subunits—CatSper 1 through 4 are pore-forming and CatSper beta, gamma, delta, epsilon, and zeta [••43] are auxiliary subunits. In addition, a novel member of the CatSper complex has been recently revealed: EF-hand calcium-binding domain-containing protein 9 or EFCAB9 [••42] (Figure 1a), which is a calmodulin-like protein that binds Ca2+ and acts as a dual calcium and pH sensor for CatSper. EFCAB9 exists as a complex with CatSper-zeta, and male mice with either CatSper-zeta or EFCAB9 deficiencies, or lacking both subunits, are subfertile [••42,••43]. EFCAB9—CatSper-zeta interaction is pH-dependent, and their complex breaks down at alkaline pH, thus allowing more efficient CatSper opening [••42]. The dissociation between EFCAB9 and CatSper-zeta likely increases the open probability of the channel. Interestingly, EFCAB9 also serves as an important structural element for the murine CatSper complex, as its absence results in a different organization of the CatSper nanodomains along the flagellum. Future studies will be needed to confirm functional importance of EFCAB9 for human sperm. Advances in super-resolution imaging revealed that CatSper is arranged in quadrilateral parallel lines along the sperm tail [44] (Figure 2a) by forming four lines that span the entire length of sperm principal piece (Figure 2a—b). CatSper channels have also been suggested to form a zig-zag pattern by linking two channel complexes within each line of CatSper in the flagellum [45] (Figure 2c). Indeed, it was further revealed that each of those four lines exists as two-row structures of CatSper nanodomains, and both EFCAB9 and CatSper-zeta are essential for this two-row formation. EFCAB9-deficient mice have the same quadrilateral parallel CatSper lines along the flagellum but each of these lines is made only of a single row of CatSper complexes[••42]. It was further suggested that CatSper-zeta and EFCAB9 bind to each other within a single CatSper channel complex (Figure 2); however, it is also possible that CatSper-zeta from one CatSper complex interacts with EFCAB9 from another CatSper complex located within the same two-row structure (Figure 2c). Such an arrangement could allow a coordinated opening of the channels within the line and ensure efficient and fast signal propagation along the flagellum in a domino-like effect. Such a hypothesis would require rigorous genetic proof, as well as reconstitution of an entire CatSper complex in a heterologous expression system—a task that has not yet been achieved by any research group.
Figure 2. Two possible flagellar arrangements of CatSper nanodomains.
(a) Reproduced from Ref. [••42]: 4Pi single-molecule switching nanoscopy images of murine CatSper1 in wild type (WT) flagella. x–y projection colors encode the relative distance from the focal plane along the z axis. Scale bar, 500 nm. Right panel: y–z cross sections (100 nm thick). Two-row structures are indicated with arrows. Scale bar, 200 nm. (b) SEM images of the principal piece of flagella from WT (top panel) and CatSper1−/− (bottom panel) mice. As indicated by arrows, double-row lines are observed on both sides of WT flagellum within each longitudinal column. These structures are absent in CatSper1−/− flagellum. Scale bar, 100 nm. (a) and (b) figures are from Ref. [••42]. (c) Two possible scenarios of CatSper flagellar nanodomain architecture. As shown in (a), CatSper forms two parallel rows and as suggested by Hwang et al. [••42], CatSper-zeta associates with EFCAB9 within the same CatSper complex (left panel). According to an alternative scenario (right panel), CatSper-zeta and EFCAB9 link together two neighboring channels in a zig-zag manner, in which CatSper-zeta from one CatSper complex is associated with EFCAB9 from the neighboring CatSper complex. This hypothetical arrangement would not only structurally link CatSper complexes, but also link them functionally. This linkage could permit more efficient signal propagation along the flagellar length and could be responsible for either synchronization of CatSper opening or longitudinal propagation of Ca2+ waves.
Ca2+ pump/Na+ – Ca2+ exchanger
In the human epididymis, sperm cells are kept quiescent by the acidic extracellular pH and low basal intracellular Ca2+ level kept around 100 nM, which is 15,000 times lower than the concentration measured in cauda epididymal plasma ~1.5 mM [46,47]. This gradient is maintained mainly by ATP2B4 in mouse, a Ca2+-pump, which transports Ca2+ outside powered by ATP consumption [48]. ATP2B4-deficient male mice are infertile, because of severely impaired sperm motility [49]. When the [Ca2+]i is elevated, Na+/Ca2+ exchanger is also involved in calcium clearance [48]; however, Ca2+ clearance and the role of ATP2B4 in human male fertility need to be elucidated. After the spermatozoa enter the female reproductive tract and begin to capacitate, intracellular calcium elevation plays a significant role in the initiation of hyperactivated motility and is required for the onset of acrosome reaction. In addition, cytoplasmic Ca2+ promotes tyrosine phosphorylation via activation of sAC [50], which further upregulates PKA by cAMP generation. Mice sperms also rely on the Ca2+/calmodulin-dependent phosphatase calcineurin, and calcineurin-deficient mice are infertile [51].
TRP channels and Na+–K+ ATPase
In addition to the stimuli mentioned above, CatSper is also regulated by membrane depolarization [36,37]. Interestingly, murine CatSper is less voltage-dependent than human one since the slope factor of kmouse CatSper = 30—32, while khuman CatSper = 17—21 [36,37]. Moreover, the half-maximal voltage activation V1/2 mouse CatSper = +11 mV and V1/2 human CatSper = +70 mV [36,37]. These numbers indicate that only a small fraction of human CatSpers will be opened at physiological-relevant membrane potentials. The progesterone shifts V1/2 of human CatSper to +30 mV in capacitated sperm cells [37]; however, to produce larger current, CatSper still requires both intracellular alkalization and significant membrane depolarization [37]. Therefore, membrane depolarization must occur before CatSper is activated.
Once inside the female reproductive tract, mammalian spermatozoa are exposed to increased temperatures of 37 °C, as opposed to 34.7 °C in the epididymis [52]. Additionally, human sperms also express a temperature-activated non-selective cationic channel TRPV4 [••53] that contributes to membrane depolarization by allowing positively charged ions, such as sodium and calcium, to enter the flagellum. Therefore, TRPV4 is an excellent candidate for the sperm-depolarizing channel that ensures human CatSper activation. Besides TRPV4, other members of TRP channel family (such as TRPV1 and TRPM8) were reported to be expressed in mammalian sperm flagellum; however, their functional detection by direct electrophysiology recordings did not confirm their functional presence [••53,54]. Sperm membrane potential is also controlled by K+ and Na+ equilibrium maintained by the electrogenic Na+/K+-ATPase α4 (encoded by the ATP1A4, in mouse and human) [55,56]. Na+/K+-ATPase is crucial for male fertility, since ATP1A4-deficient mice [55] is infertile. Therefore, Na+/K+-ATPase α4 is currently being considered a promising male contraceptive target [•57].
Sperm K+ channels
Potassium current in murine sperm is carried by sperm-specific Slo3 protein (encoded by the KCNU1 gene [58]), which is a strongly pH-sensitive channel activated by intracellular alkalization [59]. The Slo3 channel is important for male fertility, since mice lacking KCNU1 are infertile [60,61]. Human sperm also possess a potassium-conducting channel, KSper [62]; however, the molecular identity of human KSper is more complicated. Calcium-sensitive Slo1 channel (encoded by the KCNMA1 gene) was found to be expressed in human spermatozoa [63], in addition to Slo3 protein (Figure 1b) [64]. Surprisingly, human Slo3 channels are less pH-dependent, with currents showing activation already at pH = 6.7, a pH at which mouse Slo3 currents are not active [65]. Additionally, human KSper is regulated by intracellular calcium and inhibited by progesterone [63] and was suggested to be represented by Slo1. Calcium and progesterone sensitivities of human KSper were further confirmed by Brenker et al.; however, the group has suggested that the molecular identity of human KSper is Slo3 [64]. No human patient with either ablations or mutations in either KCNU1 or KCNMA1 genes was reported so far; however, according to Ref. [66], a male infertile patient deficient in sperm potassium current KSper, but with intact KCNU1 and KCNMA1 genes was reported. Future studies are needed to define the role of both KCNU1 and KCNMA1 genes in human sperm fertility. Specifically, the correlation between mutations in either of these genes with male infertility will be required to proof their importance for human sperm physiology.
Concluding remarks
Over the last few years, significant advances have been made in the identification of the macromolecular membrane complexes that regulate mammalian sperm motility, and a deeper understanding of their concerted action has been achieved. A combination of traditional methods, novel imaging techniques, and electrophysiology recording of ion channels from sperm cells has provided deeper insights into how these cells function; however, in order to ultimately unveil how a membrane transport system orchestrates different sperm motility patterns, computational modeling will be required. Specifically, it will be important to understand how the alteration of ion influx in response to changes in the flagellar environment modulates the beating patterns, such as basal and hyperactivated motility. This will require determination of the relative locations of the key flagellar ion transport systems and detailed characterization of their function under physiological conditions.
Acknowledgements
This work was supported by R01GM111802, Pew Biomedical Scholars Award, and Packer Wentz Endowment Will (to PVL). We thank William Skinner for reading and editing the manuscript.
Footnotes
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: PVL has a financial interest in YourChoice Therapeutics, Inc.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Austin CR: Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 1951, 4:581–596. [DOI] [PubMed] [Google Scholar]
- 2.Chang MC: Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168:697–698. [DOI] [PubMed] [Google Scholar]
- 3.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS: Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121:1139–1150. [DOI] [PubMed] [Google Scholar]
- 4.Witte TS, Schäfer-Somi S: Involvement of cholesterol, calcium and progesterone in the induction of capacitation and acrosome reaction of mammalian spermatozoa. Anim Reprod Sci 2007, 102:181–193. [DOI] [PubMed] [Google Scholar]
- 5.Dan Jean C: Studies on the acrosome. III. Effect of calcium deficiency. Biol Bull 1954, 107:335–349. [Google Scholar]
- 6.Pablo JL, DeCaen PG, Clapham DE: Progress in ciliary ion channel physiology. J Gen Physiol 2016, 149:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung J-J, Clapham DE: The control of male fertility by spermatozoan ion channels. Annu Rev Physiol 2012, 74:453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaupp UB, Strünker T: Signaling in sperm: more different than similar. Trends Cell Biol 2017, 27:101–109. [DOI] [PubMed] [Google Scholar]
- 9.Vishwakarma P: The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil Steril 1962, 13:481–485. [DOI] [PubMed] [Google Scholar]
- 10.Kleinboelting S, Diaz A, Moniot S, Van Den Heuvel J, Weyand M, Levin LR, Buck J, Steegborn C: Crystal structures of human soluble adenylyl cyclase reveal mechanisms of catalysis and of its activation through bicarbonate. Proc Natl Acad Sci U S A 2014, 111:3727–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvau A, Battistone MA, Gervasi MG, Navarrete FA, Xu X, Sánchez-Cárdenas C, de la Vega-Beltran JL, da Ros VG, Greer PA, Darszon A, et al. : The tyrosine kinase FER is responsible for the capacitation associated increase in tyrosine phosphorylation in murine sperm. Dev 2016, 143: 2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wandernoth PM, Mannowetz N, Szczyrba J, Grannemann L, Wolf A, Becker HM, Sly WS, Wennemuth G: Normal fertility requires the expression of carbonic anhydrases II and IV in sperm. J Biol Chem 2015, 290:29202–29216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardino RL, Carrageta DF, Sousa M, Alves MG, Oliveira PF: pH and male fertility: making sense on pH homeodynamics throughout the male reproductive tract. Cell Mol Life Sci 2019, 76:3783–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wedenoja S, Khamaysi A, Shimshilashvili L, Anbtawe-Jomaa S, Elomaa O, Toppari J, Höglund P, Aittomäki K, Holmberg C, Hovatta O, et al. : A missense mutation in SLC26A3 is associated with human male subfertility and impaired activation of CFTR. Sci Rep 2017, 7:14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. •.Khouri E El, Whitfield M, Stouvenel L, Kini A, Riederer B, Lores P, Romermann D, Stefano G Di, Drevet JR, Saez F, et al. : Slc26a3 deficiency is associated with epididymis dysplasia and impaired sperm fertilization potential in the mouse. Mol Reprod Dev 2018, 85:682–695.Khouri et al. showed that SLC26A3-deficient mice, previously reported to display congenital chloride-losing diarrhea (CLD) gastro-intestinal features, also have reproductive phenotype, which indicates an importance of this transporter for male fertility.
- 16.Maruyama SY, Ito M, Ikami Y, Okitsu Y, Ito C, Toshimori K, Fujii W, Yogo K: A critical role of solute carrier 22a14 in sperm motility and male fertility in mice. Sci Rep 2016, 6:36468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demarco IA, Espinosa F, Edwards J, Sosnik J, De la Vega-Beltrán JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE: Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J Biol Chem 2003, 278:7001–7009. [DOI] [PubMed] [Google Scholar]
- 18.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, et al. : The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 2005, 9:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MAM, Robben TJAA, Strik AM, Kuil C, Philipsen RLA, Van Duin M, Conti M, et al. : Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A 2004, 101:2993–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS: Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A 2004, 101:13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrenwald E, Foote RHPJ: Bovine oviductal fluid components and their potential role in sperm cholesterol efflux. Mol Reprod Dev 1990, 25:195–204. [DOI] [PubMed] [Google Scholar]
- 22.Leahy T, Gadella BM: New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J Androl 2015, 17:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS: Regulation of human sperm capacitation by a cholesterol efflux- stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod 1999, 5:1017–1026. [DOI] [PubMed] [Google Scholar]
- 24.Chen SR, Chen M, Deng SL, Hao XX, Wang XX, Liu YX: Sodium–hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis 2016, 7, e2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL: A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol 2003, 5:1117–1122. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Hu J, Alexandru Bobulescu I, Quill TA, McLeroy P, Moe OW, Garbers DL: A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc Natl Acad Sci 2007, 104:9325–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. •.Windler F, Bönigk W, Körschen HG, Grahn E, Strünker T, Seifert R, Kaupp UB: The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat Commun 2018, 9:2809.Windler et al. identified the SLC9C1 channel in sea urchin spermatozoa as a genuine Na+/H+ antiporter which is gated by voltage via a voltage-sensing domain and is directly modulated by cAMP via a cyclic nucleotide-binding domain. Thus, the group has identified Na+/H+ exchanger as a link between intracellular pH and cAMP levels.
- 28.Sasaki M, Takagi M, Okamura Y: A voltage sensor-domain protein is a voltage-gated proton channel. Science 2006, 312:589–592. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey IS, Moran MM, Chong JA, Clapham DE: A voltage-gated proton-selective channel lacking the pore domain. Nature 2006, 440:1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y: Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 2010, 140:327–337. [DOI] [PubMed] [Google Scholar]
- 31. •.Miller MR, Kenny SJ, Mannowetz N, Mansell SA, Wojcik M, Mendoza S, Zucker RS, Xu K, Lishko PV: Asymmetrically positioned flagellar control units regulate human sperm rotation. Cell Rep 2018, 24:2606–2613.Miller et al. used super-resolution microscopy, electrophysiology, and electron microscopy to show that the sperm proton channel Hv1 forms bilateral lines positioned asymmetrically down the sperm flagellum. Hv1 inhibition leads to a decrease in sperm rotation, suggesting an important role for this channel in sperm motility.
- 32.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE: A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeCoursey TE: Voltage-gated proton channels. Cell Mol Life Sci 2008, 65:2554–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Talatini MR, Taylor AH, Elson JC, Brown L, Davidson AC, Konje JC: Localisation and function of the endocannabinoid system in the human ovary. PloS One 2009, 4:e4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger TK, Fußhöller DM, Goodwin N, Bönigk W, Müller A, Dokani Khesroshahi N, Brenker C, Wachten D, Krause E, Kaupp UB, et al. : Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating. J Physiol 2017, 595:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirichok Y, Navarro B, Clapham DE: Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439:737–740. [DOI] [PubMed] [Google Scholar]
- 37.Lishko PV, Botchkina IL, Kirichok Y: Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471:387–392. [DOI] [PubMed] [Google Scholar]
- 38.Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB: The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471:382–387. [DOI] [PubMed] [Google Scholar]
- 39.Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF, Hill RZ, Bautista DM, Kirichok Y, Lishko PV: Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 2016, 352:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno K, Shiba K, Okai M, Takahashi Y, Shitaka Y, Oiwa K, Tanokura M, Inaba K: Calaxin drives sperm chemotaxis by Ca2+-mediated direct modulation of a dynein motor. Proc Natl Acad Sci U S A 2012, 109:20497–20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki K, Shiba K, Nakamura A, Kawano N, Satouh Y, Yamaguchi H, Morikawa M, Shibata D, Yanase R, Jokura K, et al. : Calaxin is required for cilia-driven determination of vertebrate laterality. Commun Biol 2019, 2:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. ••.Hwang JY, Mannowetz N, Zhang Y, Everley RA, Gygi SP, Bewersdorf J, Lishko PV, Chung JJ: Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 2019, 177:1480–1494.Hwang et al. identified EF-hand calcium-binding domain-containing protein 9 (EFCAB9) as a new CatSper auxiliary subunit that modulates channel activity and domain organization. EFCAB9 is a pH-dependent Ca2+ sensor for the CatSper channel and a direct binding partner of CatSper-zeta. Knockout mice studies demonstrate that EFCAB9, in complex with CatSper-zeta, is essential for pH-dependent and Ca2+-sensitive activation of the CatSper channel. In the absence of EFCAB9, sperm motility and fertility are compromised, and the linear arrangement of the Ca2+ signaling domains is disrupted. EFCAB9 interacts directly with CatSper-zeta in a Ca2+-dependent manner and dissociates at elevated pH. The authors suggested that EFCAB9 is a pH-dependent Ca2+ sensor that triggers changes in sperm motility.
- 43. ••.Chung J-J, Miki K, Kim D, Shim S-H, Shi HF, Hwang JY, Cai X, Iseri Y, Zhuang X, Clapham DE: CatSperζ regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. Elife 2017, 6, e23082.Chung et al. identified two genes in mice that encode new accessory proteins in the CatSper channel complex named CatSper-epsilon and CatSper-zeta. Mutant males that lack CatSper-zeta have fragmented patterns of CatSper stripes in the tails of their sperm. Moreover, fewer calcium ions were able to pass through the channels to enter the cell. Together, this made the sperm tail more rigid, which prevented it from moving efficiently within the female, resulting in reduced fertility. The authors also found that the mutant sperms were less able to penetrate the egg than normal sperms.
- 44.Chung J-J, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE: Structurally distinct Ca2+ signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 2014, 157:808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bystroff C: Intramembranal disulfide cross-linking elucidates the super-quaternary structure of mammalian CatSpers. Reprod Biol 2018, 18:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espino J, Mediero M, Lozano GM, Bejarano I, Ortiz Á, García JF, Pariente JA, Rodríguez AB: Reduced levels of intracellular calcium releasing in spermatozoa from asthenozoospermic patients. Reprod Biol Endocrinol 2009, 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morton BE, Sagadraca R, Fraser C: Sperm motility within the mammalian epididymis: species variation and correlation with free calcium levels in epididymal plasma. Fertil Steril 1978, 29:695–698. [DOI] [PubMed] [Google Scholar]
- 48.Wennemuth G, Babcock DF, Hille B: Calcium clearance mechanisms of mouse sperm. J Gen Physiol 2003, 122: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, et al. : Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J Biol Chem 2004, 279:28220–28226. [DOI] [PubMed] [Google Scholar]
- 50.Jaiswal BS, Conti M: Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci U S A 2003, 100:10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyata H, Satouh Y, Mashiko D, Muto M, Nozawa K, Shiba K, Fujihara Y, Isotani A, Inaba K, Ikawa M: Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science 2015, 350:442–445. [DOI] [PubMed] [Google Scholar]
- 52.Garolla A, Torino M, Miola P, Caretta N, Pizzol D, Menegazzo M, Bertoldo A, Foresta C: Twenty-four-hour monitoring of scrotal temperature in obese men and men with a varicocele as a mirror of spermatogenic function. Hum Reprod 2015, 30: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 53. ••.Mundt N, Spehr M, Lishko PV: TRPV4 is the temperature-sensitive ion channel of human sperm. Elife 2018, 7, e35853.Mundt et al. characterized the temperature-activated TRPV4 channel as a channel causing depolarization in human sperm. Authors suggested that TRPV4 activation triggers initial membrane depolarization, facilitating both CatSper and Hv1 gating and, consequently, sperm hyperactivation.
- 54.Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krähling M, Müller A, Benjamin Kaupp U, Strünker T: The CatSper channel: a polymodal chemosensor in human sperm. EMBO J 2012, 31:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jimenez T, McDermott JP, Sánchez G, Blanco G, Na: K-ATPase α4 isoform is essential for sperm fertility. Proc Natl Acad Sci U S A 2011, 108:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez G, Nguyen ANT, Timmerberg B, Tash JS, Blanco G: The Na,K-ATPase α4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol Hum Reprod 2006, 12:565–576. [DOI] [PubMed] [Google Scholar]
- 57. •.Syeda SS, Sánchez G, Hong KH, Hawkinson JE, Georg GI, Blanco G: Design, synthesis, and in vitro and in vivo evaluation of ouabain analogues as potent and selective Na, K-ATPase ±4 isoform inhibitors for male contraception. J Med Chem 2018, 61:1800–1820.Syeda et al. showed that ouabain’s analogs are attractive compounds that can specifically target mammalian Na,K-ATPase α4. Since ouabain itself is nonspecific and exerts toxic effects on the heart, new compounds with greater isoform specificity toward α4 are needed for the development of safe male contraceptives. They described a new ouabagenin triazole analog, which is an effective and selective inhibitor of Na,K-ATPase α4 and sperm function.
- 58.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L: Slo3, a novel pH-sensitive K+ channel from mammalian spermato-cytes. J Biol Chem 1998, 273:3509–3516. [DOI] [PubMed] [Google Scholar]
- 59.Navarro B, Kirichok Y, Clapham DE, Sper K: A pH-sensitive K current that controls sperm membrane potential. Proc Natl Acad Sci 2007, 104:7688–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L: The Slo3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 2010, 584:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM: Deletion of the Slo3 gene abolishes alkalizationactivated K+ current in mouse spermatozoa. Proc Natl Acad Sci U S A 2011, 108:5879–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith JF, Syritsyna O, Fellousc M, Serres C, Mannowetz N, Kirichok Y, Lishko PV: Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc Natl Acad Sci U S A 2013, 110:6823–6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mannowetz N, Naidoo NM, Choo S-AS, Smith JF, Lishko PV: Slo1 is the principal potassium channel of human spermatozoa. Elife 2013, 2, e01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brenker C, Zhou Y, Müller A, Echeverry FA, Trötschel C, Poetsch A, Xia XM, Bönigk W, Lingle CJ, Kaupp UB, et al. : The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife 2014, 3, e01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leonetti MD, Yuan P, Hsiung Y, MacKinnon R: Functional and structural analysis of the human SLO3 pH- and voltage-gated K+ channel. Proc Natl Acad Sci U S A 2012, 109:19274–19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown SG, Publicover SJ, Mansell SA, Lishko PV, Williams HL, Ramalingam M, Wilson SM, Barratt CLR, Sutton KA, Da Silva SM: Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum Reprod 2016, 31:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]