Abstract
Acute intermittent hypoxia (AIH) elicits distinct mechanisms of phrenic motor plasticity initiated by brainstem neural network activation versus local (spinal) tissue hypoxia. With moderate AIH (mAIH), hypoxemia activates the carotid body chemoreceptors and (subsequently) brainstem neural networks associated with the peripheral chemoreflex, including medullary raphe serotonergic neurons. Serotonin release and receptor activation in the phrenic motor nucleus then elicits phrenic long-term facilitation (pLTF). This mechanism is independent of tissue hypoxia, since electrical carotid sinus nerve stimulation elicits similar serotonin-dependent pLTF. In striking contrast, severe AIH (sAIH) evokes a spinal adenosine-dependent, serotonin-independent mechanism of pLTF. Spinal tissue hypoxia per se is the likely cause of sAIH-induced pLTF, since local tissue hypoxia elicits extracellular adenosine accumulation. Thus, any physiological condition exacerbating spinal tissue hypoxia is expected to shift the balance towards adenosinergic pLTF. However, since these mechanisms compete for dominance due to mutual cross-talk inhibition, the transition from serotonin to adenosine dominant pLTF is rather abrupt. Any factor that compromises spinal cord circulation will limit oxygen availability in spinal cord tissue, favoring a shift in the balance towards adenosinergic mechanisms. Such shifts may arise experimentally from treatments such as carotid denervation, or spontaneous hypotension or anemia. Many neurological disorders, such as spinal cord injury or stroke compromise local circulatory control, potentially modulating tissue oxygen, adenosine levels and, thus, phrenic motor plasticity. In this brief review, we discuss the concept that local (spinal) circulatory control and/or oxygen delivery regulates the relative contributions of distinct pathways to phrenic motor plasticity.
Keywords: Respiratory motor plasticit, Acute intermittent hypoxia, Circulatory control, Serotonin, Adenosine
1. Introduction
The cardiovascular and respiratory systems are highly inter-connected, and act in concert to ensure efficient tissue oxygen delivery. Both respiratory motor and sympathetic motor systems exhibit neuroplasticity, including phrenic (pLTF) and sympathetic long-term facilitation following brief exposure to intermittent hypoxia (Mitchell et al., 2001; Dick et al., 2007; Xing and Pilowsky, 2010; Devinney et al., 2013). Although considerable advances have been made towards an understanding of neural network and cellular mechanisms giving rise to phrenic motor plasticity (Devinney et al., 2015; Fields and Mitchell, 2015), far less is known concerning mechanisms of sympathetic LTF. Another area of research that has received inadequate attention is the potential for circulatory control to indirectly impact phrenic motor plasticity. In this brief review, we propose the idea that cardiorespiratory function has considerable potential to regulate the expression of phrenic motor plasticity, acting indirectly via local (spinal) circulatory control and oxygen delivery.
Acute intermittent hypoxia (AIH) elicits distinct forms of phrenic motor plasticity, acting through brainstem neural networks versus spinal tissue hypoxia, respectively. With moderate AIH (mAIH), hypoxemia activates the carotid body chemoreceptors, stimulating carotid chemo-afferent neurons and synaptically activating brainstem respiratory neural networks, including raphe serotonergic neurons (Morris et al., 1996; Mitchell et al., 2001; Morris et al., 2001). Subsequent release of serotonin in or near the phrenic motor nucleus is both necessary and sufficient to elicit long-lasting phrenic motor facilitation (i.e. pLTF; Baker-Herman and Mitchell, 2002; MacFarlane and Mitchell, 2009; MacFarlane et al., 2011). This mechanism of serotonin-dependent pLTF is independent of tissue hypoxia, since electrical carotid sinus nerve stimulation elicits a similar serotonin-dependent response. In striking contrast, severe AIH (sAIH) evokes spinal adenosine-dependent, serotonin-independent pLTF. Spinal tissue hypoxia per se is the likely cause of sAIH-induced pLTF since local tissue hypoxia elicits extracellular ATP release and adenosine accumulation (Takahashi et al., 2010; Yamashiro and Morita, 2017). Thus, any physiological condition exacerbating spinal tissue hypoxia is expected to shift the balance from serotonergic towards adenosinergic mechanisms to pLTF.
Cardiovascular changes that compromise spinal cord circulation may limit oxygen delivery to spinal cord tissue, shifting the balance from serotonergic towards adenosinergic mechanisms of AIH-induced pLTF. Such shifts may arise experimentally from treatments such as carotid body denervation, or from spontaneous conditions such as anemia or hypotension. Many neurological disorders, including spinal cord injury or stroke compromise circulatory control, potentially modulating local tissue oxygen, adenosine levels and, thus, phrenic motor plasticity.
The functional and clinical significance of AIH-induced respiratory (and non-respiratory) motor plasticity has been reviewed in the past few years (Dale et al., 2014; Gonzalez-Rothi et al., 2015). Biologically, AIH elicits similar motor plasticity in non-respiratory motor function; thus, studies of AIH-induced pLTF have revealed novel mechanisms of motor plasticity. Such plasticity can be harnessed for therapeutic advantage. For example, five consecutive days of AIH combined with walking practice elicit the largest increase in walking ability yet reported in people with chronic, incomplete SCI (Hayes et al., 2014). In this brief review, we introduce the concept that local (spinal) circulatory control regulates the relative contributions of distinct pathways to phrenic motor plasticity, highlighting an understudied element of phrenic motor plasticity: interactions of chemoreflex/neural activation and neuromodulator release versus local oxygen delivery and tissue oxygenation.
2. Mechanisms of acute intermittent hypoxia (AIH)-induced pLTF
2.1. Q pathway to phrenic motor facilitation
mAIH elicits persistent increases in phrenic nerve activity (i.e. pLTF); rapid carotid body chemoreceptor activation and enhanced carotid sinus nerve activity are both necessary and sufficient for this response (Millhorn et al., 1980a,b). Glutamatergic chemoafferent neuron inputs stimulate neurons in caudal nucleus of the solitary tract that project to the ventral respiratory column (Housley et al., 1987; Mizusawa et al., 1994). Carotid chemoreflex activation also stimulates activity in caudal raphe neurons (Morris et al., 1996). These putative serotonergic neurons project to the spinal ventral horn, including the phrenic motor neurons (Morrison and Gebber, 1984; Holtman et al., 1986), where they release serotonin (Kinkead et al., 2001). Electrical stimulation of caudal raphe neurons mimics respiratory motor plasticity following carotid chemoafferent neuron stimulation (Millhorn, 1986), a response abolished by serotonin receptor inhibition (Millhorn, 1986; Erickson and Millhorn, 1991, 1994; Mitchell et al., 2001).
In our working model, mAIH-induced spinal serotonin release activates serotonin type 2 (5-HT2) receptors on phrenic motor neurons, initiating the intracellular signaling cascades giving rise to pLTF (Bach and Mitchell, 1996; Baker-Herman and Mitchell, 2002; Baker-Herman et al., 2004). This intracellular signaling cascade requires spinal ERK-MAP kinase activity (Hoffman et al., 2012), new BDNF protein synthesis (Baker-Herman and Mitchell, 2002; Baker-Herman et al., 2004), activation of the high affinity BDNF receptor (TrkB; Dale et al., 2017), and downstream signaling via protein kinase C-θ within phrenic motor neurons (Devinney et al., 2015; Agosto-Marlin and Mitchell, 2017). This pathway is known as the Q pathway to phrenic motor facilitation since it is initiated by Gq protein-coupled metabotropic receptors (Dale-Nagle et al., 2010).
Consistent with this working model: 1) extracellular serotonin concentration increases during AIH (Kinkead et al., 2001); 2) spinal serotonin (MacFarlane and Mitchell, 2009) or selective serotonin 2A or 2B (5-HT2A and 5-HT2B) receptor agonist injections (MacFarlane et al., 2011; Fields and Mitchell, 2017; Perim et al., 2018a) elicit phrenic motor facilitation without hypoxia; and 3) spinal serotonin receptor inhibition abolishes mAIH-induced pLTF (Bach and Mitchell, 1996; Fuller et al., 2001). Both 5- HT2A and 5-HT2B receptors are Gq protein-coupled (Q pathway), and are expressed within phrenic motor neurons (MacFarlane et al., 2011). The signaling cascades to phrenic motor facilitation utilized by 5- HT2A and 5-HT2B receptors are not identical since only 5-HT2B receptor-induced phrenic motor facilitation requires NADPH oxidase activity (MacFarlane et al., 2011), similar to mAIH-induced pLTF (MacFarlane et al., 2009). In a recent study, we found that both spinal 5- HT2A and 5-HT2B receptor activation are necessary for mAIH-induced pLTF (Tadjalli and Mitchell, 2018).
2.2. S pathway to phrenic motor facilitation
sAIH initiates similar pLTF, but through a completely distinct cellular mechanism that requires local adenosine accumulation and phrenic motor neuron adenosine 2A (A2A) receptor activation (Nichols et al., 2012; Seven et al., 2018). Mechanisms of A2A receptor-dependent pLTF are less well known (versus the Q pathway), although 5-HT7 receptor induced phrenic motor facilitation, another Gs protein-coupled metabotropic receptor, is thought to elicit phrenic plasticity via the same cellular cascade. Spinal A2A receptor inhibition blocks sAIH-induced pLTF (Nichols et al., 2012; Agosto-Marlin et al., 2017), and cervical spinal activation of A2A receptors elicits phrenic motor facilitation by a mechanism that requires new synthesis of TrkB (not BDNF) protein (Golder et al., 2008; Seven et al., 2018). Spinal A2A receptor activation is not necessary for, and actually constrains mAIH-induced pLTF (Hoffman et al., 2010). The ability of spinal adenosine to initiate the S pathway and constrain the Q pathway gave rise to our hypothesis that spinal circulation indirectly regulates the expression of phrenic motor plasticity.
Both A2A and 5-HT7 receptors are Gs protein-coupled receptors that canonically exert their effects through adenylyl cyclase-dependent cyclic adenosine monophosphate (cAMP) signaling. Cervical spinal 5-HT7 receptor activation elicits phrenic motor facilitation by a mechanism that requires activation of exchange protein activated by cyclic AMP (EPAC), Akt and mammalian target of rapamycin (mTOR), and new TrkB protein synthesis (Hoffman and Mitchell, 2011; Fields et al., 2015; Perim et al., 2018a). The relevant A2A receptors for phrenic motor facilitation are expressed within phrenic motor neurons per se (Seven et al., 2018). The common cellular cascade elicited by A2A and 5-HT7 receptors is referred to as the S pathway to phrenic motor facilitation since both receptors are Gs protein-coupled metabotropic receptors (Dale-Nagle et al., 2010).
3. Q and S pathway interactions
Interestingly, Q and S pathway co-activation does not summate their individual effects. To the contrary, they interact via powerful cross-talk inhibition, constraining or even abolishing the expression of phrenic motor plasticity (Hoffman et al., 2010; Hoffman and Mitchell, 2013; Fields and Mitchell, 2017; Perim et al., 2018a). The balance between the Q and S pathways is a bit like a seesaw. Whereas the Q pathway dominates with modest hypoxia; the S pathway opposes its effects as it slowly builds with intensity/severity/duration of hypoxia. With increasing hypoxia severity/duration, the two pathways reach balance, functionally cancelling phrenic motor facilitation (this is the point where the mass on either side of a seesaw is equal, leaving the board balanced in air—neither wins). When hypoxemia is severe enough (or long enough) to trigger yet more adenosine accumulation in the phrenic motor nucleus, the S pathway eventually overcomes the Q pathway, once again triggering pMF. This dose-response to progressively greater hypoxic burden (and adenosine accumulation) is a consequence of mutual cross-talk inhibition of the respective pathways within phrenic motor neurons. Evidence for within phrenic motor neuron effects is provided by studies using intrapleural siRNA injections that target key molecules of the Q pathway (PKC theta; Devinney et al., 2015), S pathway (A2A receptors; Seven et al., 2018) or both (TrkB; Dale et al., 2017) within phrenic motor neurons.
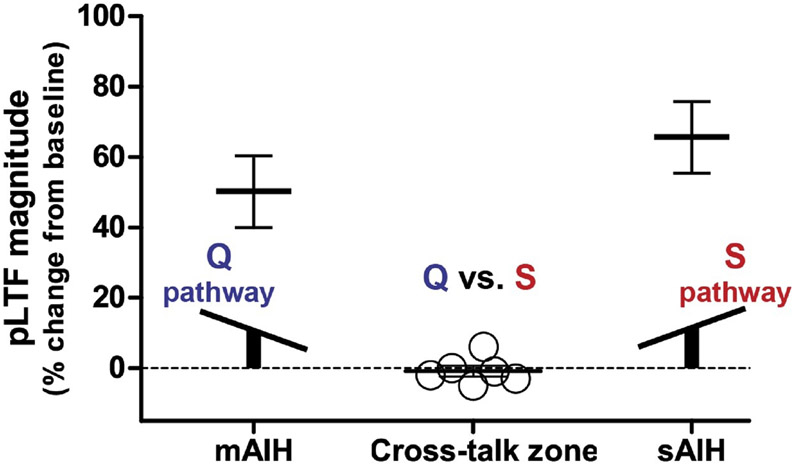
Subthreshold S pathway activation with mAIH does constrain Q pathway-dominant pLTF. However, with sAIH, adenosine accumulation is sufficient to flip the system to S pathway dominance–with Q pathway constraint (Perim et al., 2018b). Because of the reliance of this balance on tissue oxygen tension, factors regulating tissue oxygen delivery have the potential to shift the balance between Q versus S pathway contributions to pLTF through their impact on tissue adenosine accumulation. In addition, we predict a very narrow range of hypoxia where serotonin and adenosine receptor activation is equal and off-setting, thereby cancelling plasticity expression. We demonstrate that phenomenon in this review (see Fig. 2).
Fig. 2.
Similar to a seesaw (see inset), moderate AIH (mAIH: 3, 5 min episodes; PaO2 = 35–45 mmHg) and severe AIH (sAIH: 3, 5 min episodes; PaO2 = 25–30 mmHg) elicit robust phrenic long-term facilitation (pLTF; Fuller et al., 2001; Baker-Herman et al., 2004; Nichols et al., 2012; Agosto-Marlin et al., 2017; Dale et al., 2017; Perim et al., 2018b), whereas intermediate AIH (i.e. 3, 5 min episodes; PaO2 = 30–35 mmHg) fails to elicit pLTF (open circles). These data demonstrate that pLTF is cancelled with intermediate AIH (i.e. balanced cross-talk inhibition; n = 6) versus robust pLTF with less (mAIH) or more (sAIH) severe hypoxic episodes. In this “cross-talk zone,” the Q and S pathways are activated equally, canceling phrenic motor plasticity due to balanced cross-talk inhibition.
Molecules necessary for cross-talk inhibition have been identified. For example, co-activation of 5- HT2A /B and 5-HT7 serotonin receptors abolishes respiratory motor plasticity, but phrenic plasticity can be restored by inhibiting spinal PKA activity (Fields and Mitchell, 2017; Perim et al., 2018a). Thus cAMP signaling drives the S pathway via EPAC signaling, but PKA constrains the Q pathway to respiratory motor plasticity (Hoffman and Mitchell;, 2013; Fields and Mitchell, 2017). Conversely, NADPH oxidase/reactive oxygen species (Perim et al., 2018a,b) and PKCδ activity (Perim et al., 2018b) are necessary for cross-talk inhibition of the S (5-HT7) pathway from 5-HT2B and 5-HT2A receptors, respectively. Cross-talk interactions are critical regulators of phrenic motor plasticity, and likely play a key role in the ability of local circulatory control to modulate (or even dominate) the expression of phrenic motor plasticity.
4. Spinal circulatory control and phrenic motor plasticity
Low spinal oxygen levels are a potent stimulus for glia (and neurons) to release ATP, leading to extracellular adenosine accumulation (Van Wylen et al., 1986; Takahashi et al., 2010; Yamashiro and Morita, 2017). Impaired spinal oxygen delivery, whether due to central cardiovascular pathology, local ischemia and/or blood oxygen concentration (low PaO2 or low hemoglobin levels) will compromise spinal tissue oxygen levels, increasing adenosine accumulation within the phrenic motor nucleus at any given level of PaO2. If local blood flow or oxygen delivery is compromised sufficiently, adenosine accumulation may be sufficient to initiate A2A receptor-dependent phrenic motor facilitation.
Despite its potential impact on different forms of respiratory motor plasticity, there is little information available concerning the influence of cardiovascular function and changes in oxygen delivery on AIH-induced pLTF. Greater understanding of these relationships has important implications of experimental, physiological and even translational significance. Since AIH is emerging as a simple and effective means to improve function in clinical disorders that compromise movement (e.g. spinal injury, ALS), its therapeutic benefits may be undermined or exaggerated by abnormal spinal circulation. Circulation is often compromised in conditions such as spinal cord injury since they disrupt sympathetic regulation of blood flow and, therefore, oxygen delivery to the spinal cord. Greater understanding of oxygen delivery and its impact on respiratory motor plasticity is needed since it may be necessary to optimize intermittent hypoxia protocols on a case by case basis to maximize therapeutic benefits.
Tissue hypoxia is not necessary for mAIH-induced pLTF since electrical stimulation of the carotid sinus nerve evokes serotonin-dependent respiratory motor plasticity in the absence of hypoxia (Millhorn et al., 1980a, b). On the other hand, ATP release and extracellular adenosine accumulation are expected to be most prominent during severe hypoxia (Van Wylen et al., 1986; Takahashi et al., 2010; Yamashiro and Morita, 2017). Thus, adenosine-dependent pLTF will likely replace serotonin-dependent, mAIH-induced pLTF when AIH protocols consist of severe or prolonged hypoxic episodes (Nichols et al., 2012; Devinney et al., 2015). Limited spinal A2A receptor activation occurs during mAIH, but in this instance, spinal A2A receptor inhibition enhances mAIH-dependent pLTF (Hoffman et al., 2010), suggesting inhibitory S to Q pathway constraints to pLTF.
Similarly complex interactions between the Q and S pathways to phrenic motor facilitation are expressed as a bell-shaped dose-response curve in phrenic motor facilitation following episodic spinal serotonin injections (MacFarlane and Mitchell, 2009). Low serotonin doses elicit robust phrenic motor plasticity whereas high doses obscure phrenic motor facilitation due to increased 5-HT7 receptor activation (MacFarlane and Mitchell, 2009). Inhibitory cross-talk interactions have physiological implications even with mAIH-induced pLTF since spinal 5-HT7 receptor blockade enhances mAIH-induced pLTF (Hoffman and Mitchell, 2013). Inhibitory cross-talk interactions between competing pathways to phrenic motor plasticity are illustrated in Fig. 1.
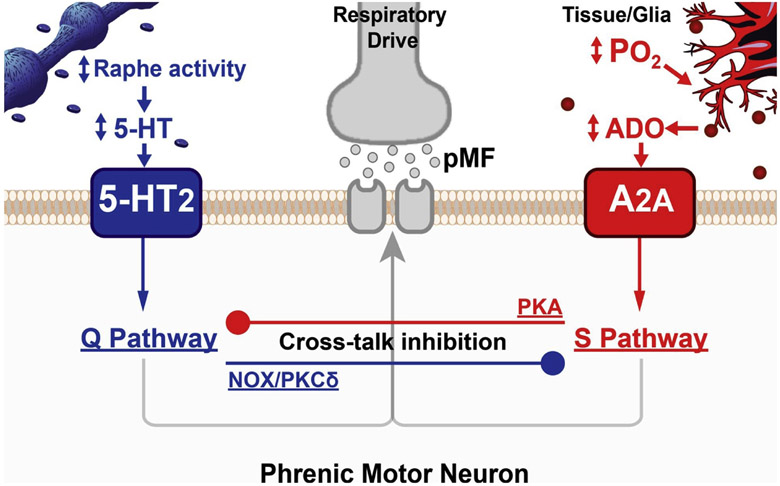
Fig. 1.
Serotonin-dependent (Q) and adenosine-dependent (S) pathways to phrenic motor facilitation (pMF) interact via powerful cross-talk inhibition. In our working model, the Q pathway is induced by 5-HT2 receptor activation on phrenic motor neurons due to AIH-induced increases in raphe neuron activity and serotonin release within the phrenic motor nucleus. However, AIH also activates 5-HT7 receptors, which constrain the dominant Q pathway (not shown). On the other hand, as local spinal tissue PO2 decreases, extracellular adenosine accumulation (ADO) contributes to the now dominant S pathway, over-riding the Q pathway via PKA-dependent cross-talk inhibition. Spinal tissue PO2 will depend on the prevailing arterial PO2 blood hemoglobin concentration (and oxygen concentration), tissue metabolic rate and perfusion of the phrenic motor nucleus. Thus, factors other than the arterial PO2 within hypoxic episodes, such as blood hemoglobin concentration and local perfusion, may shift the relative Q versus S pathway balance. “Standard” mAIH protocols could equally activate these pathways, canceling phrenic motor plasticity.
Equal activation of spinal 5-HT2 and 5-HT7 receptors (via intrathecal agonist injections) cancels respiratory motor plasticity due to cross-talk inhibition (Fields and Mitchell, 2017; Perim et al., 2018a, b). Since S to Q pathway constraints are orchestrated by cAMP-dependent PKA activity, spinal PKA inhibition: 1) restores phrenic motor plasticity with 5-HT 2A/B and 7 receptor co-activation (Perim et al., 2018a, b); and 2) enhances 5- HT2A -dependent phrenic motor plasticity (Hoffman and Mitchell, 2013; Fields and Mitchell, 2017). Conversely, while spinal NADPH oxidase inhibition restores phrenic motor plasticity with 5-HT 2B and 7 receptor co-activation (Perim et al., 2018a), spinal PKCδ inhibition restores plasticity with 5-HT 2A and 7 receptor co-activation (Perim et al., 2018b).
Available data do not conclusively demonstrate that hypoxia directly activates raphe serotonergic neurons (i.e. independent from synaptic inputs). In contrast, hypoxia directly affects many glia, inducing ATP release (Phillis et al., 1993; Wallman-Johansson and Fredholm, 1994; Gourine et al., 2005). Extracellular ATP is converted to adenosine by ecto-nucleotidases, increasing adenosine concentrations (Parkinson et al., 2005; Martin et al., 2007). When we consider the impact of impaired oxygen delivery to the cervical spinal cord, increasing adenosine levels first constrain the serotonin-dependent Q pathway to phrenic motor facilitation, and eventually dominate with the adenosine-dependent S pathway. With intermediate severities of hypoxemia during an AIH protocol, we predict intermediate adenosine levels, possibly balancing the Q and S pathways and canceling plasticity. Interestingly, only a 5 mmHg PaO2 difference seems to separate Q pathway-dependent, mAIH-induced (PaO2: 35–45 mmHg) vs. S pathway-dependent, sAIH-induced pLTF (PaO2: 25–30 mmHg).
Whereas Q pathway-dependent pLTF predominates following mAIH, and S pathway-dependent pLTF takes place following severe AIH, the small difference in arterial PO2 between these outcomes indicates that awkward moment when equivalent activation of competing pathways to pLTF is expected to cancel plasticity. This prediction is supported by preliminary experiments showing that pLTF is abolished following intermediate AIH (PaO2: 30–35 mmHg; Fig. 2). Similarly, marginal changes in local oxygen delivery due to local blood flow changes (i.e. hypotension or local vascular resistance), or blood oxygen concentration (i.e. anemia, polycythemia) may tip the balance between the Q versus S pathways to pMF.
Surprisingly, residual pLTF is still observed in carotid-denervated rats exposed to mAIH (Bavis and Mitchell, 2003; Sibigtroth and Mitchell, 2011). In this condition, unique mechanisms likely account for residual pLTF. The most likely contributor under these conditions is the profound hypotension experienced during even moderate hypoxia in carotid denervated rats. The severity of hypotension during mAIH may lower tissue PO2 into a zone where adenosine accumulates sufficiently to activate the S pathway. Thus, with carotid denervation, we predict: 1) pLTF would be blocked by preventing arterial hypotension during hypoxic episodes; and 2) residual pLTF is adenosine versus serotonin-dependent. If verified, this would be a clear experimental example of cardiovascular or circulatory modulation of phrenic motor plasticity.
Cardiovascular regulation and circulatory control have the potential to influence other forms of respiratory motor plasticity. Similar to pLTF, a prolonged increase in hypoglossal nerve activity is observed following mAIH (Neverova et al., 2007). Alpha-1 adrenergic receptor blockade with prazosin partially, but does not completely block hypoglossal LTF. Because of its actions on the peripheral vasculature, prazosin induces profound hypotension, which could account for residual hypoglossal LTF following mAIH; however, this is unlikely since equivalent hypotension from experimental blood withdrawal actually enhanced hypoglossal LTF (Neverova et al., 2007). The hypotension (i.e. from prazosin or blood withdrawal) could compromise blood flow and tissue oxygen delivery sufficiently to increase spinal adenosine accumulation, thereby activating the S pathway. This hypothesis remains to be verified.
Changes in capillary diameter also have significant implications for tissue oxygen delivery and, therefore, may shift the Q to S pathway balance. For example, spinal transection causes spinal pericyte and capillary constriction, limiting spinal tissue blood flow and decreasing spinal tissue PO2 (Li et al., 2017). Reductions in tissue PO2 will shift the balance towards S pathway activation during the same AIH protocol. Thus, with chronic cervical spinal injuries, we predict that pericyte constriction will lower tissue PO2 and increase adenosine accumulation during standard AIH protocols.
Conditions such as anemia can also affect blood oxygen concentration and, consequently, tissue oxygen delivery. Under these conditions, we predict enhanced S versus Q pathway activation during any given AIH protocol; this shift in S/Q balance may undermine phrenic motor plasticity or shift it completely towards dominant S pathway activation (Fields and Mitchell, 2017; Perim et al., 2018a). On the other hand, polycythemia should have opposite effects since spinal PO2 during AIH will be increased, minimizing adenosine-dependent inhibitory constraints arising from S pathway activation. Although preliminary data suggest cardiovascular regulation and circulatory control modulate phrenic motor plasticity, additional studies are needed to evaluate this issue.
5. Conclusion
Changes in cardiovascular function, local circulation and blood oxygen carrying capacity may modulate AIH induced pLTF expression. By reducing oxygen delivery to the phrenic motor nucleus, tissue hypoxia may be severe enough to increase adenosine concentration to levels that activate dominant S pathway phrenic motor facilitation. Given the powerful cross-talk interactions between pathways, even minor shifts could tip the balance, shifting the dominant mechanism or even cancelling all phrenic motor plasticity. Additional studies to address the importance of circulatory oxygen delivery to respiratory motor plasticity are needed. Relevant blood and cardiovascular variables (blood pressure, hematocrit, etc.) should be considered to optimize AIH as a therapeutic modality for important clinical disorders that compromise movement (Gonzalez-Rothi et al., 2015).
Acknowledgments
Sources of funding
Support provided by NIH GrantsHL69064 and the McKnight Brain Institute.
Footnotes
Conflicts of interest
The authors declare no competing financial interests.
References
- Agosto-Marlin IM, Mitchell GS, 2017. Spinal BDNF-induced phrenic motor facilitation requires PKCtheta activity. J. Neurophysiol 118, 2755–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto-Marlin IM, Nichols NL, Mitchell GS, 2017. Adenosine-dependent phrenic motor facilitation is inflammation resistant. J. Neurophysiol 117, 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS, 1996. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir. Physiol 104, 251–260. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS, 2002. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J. Neurosci 22, 6239–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci 7, 48–55. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS, 2003. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J. Appl. Physiol 94 (1985), 399–409. [DOI] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS, 2014. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Fields DP, Devinney MJ, Mitchell GS, 2017. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp. Neurol 287, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS, 2010. Multiple pathways to long-lasting phrenic motor facilitation. Adv. Exp. Med. Biol 669, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS, 2013. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann. N. Y. Acad. Sci 1279, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS, 2015. Phrenic long-term facilitation requires PKCtheta activity within phrenic motor neurons. J. Neurosci 35, 8107–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Nichols NL, Mitchell GS, 2016. Sustained hypoxia elicits competing spinal mechanisms of phrenic motor facilitation. J. Neurosci 36, 7877–7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar N, 2007. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp. Physiol 92, 87–97. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE, 1991. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res. 567, 11–24. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE, 1994. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J. Comp. Neurol 348, 161–182. [DOI] [PubMed] [Google Scholar]
- Fields DP, Mitchell GS, 2015. Spinal metaplasticity in respiratory motor control. Front. Neural Circuits 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DP, Mitchell GS, 2017. Divergent cAMP signaling differentially regulates serotonin-induced spinal motor plasticity. Neuropharmacology 113, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DP, Springborn SR, Mitchell GS, 2015. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J. Neurophysiol 114, 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS, 2001. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J. Appl. Physiol 90 (1985) 2001–20012006; discussion 2000. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS, 2008. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J. Neurosci 28, 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015. Intermittent hypoxia and neurorehabilitation. J. Appl. Physiol 119 (1985), 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM, 2005. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J. Neurosci 25, 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, 2014. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS, 2011. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J. Physiol 589, 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS, 2013. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250, 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS, 2010. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J. Physiol 588, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS, 2012. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J. Appl. Physiol. 113 (1985), 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR Jr, Dick TE, Berger AJ, 1986. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J. Neurosci. 6, 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Martin-Body RL, Dawson NJ, Sinclair JD, 1987. Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. Neuroscience 22, 237–250. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS, 2001. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp. Biochem. Physiol. A Mol. Integr. Physiol 130, 207–218. [DOI] [PubMed] [Google Scholar]
- Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ, 2017. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat. Med 23, 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS, 2009. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J. Physiol 587, 5469–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS, 2009. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J. Physiol. 587, 1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS, 2011. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V, 2007. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia 55, 36–45. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, 1986. Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J. Physiol 381, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980a. Prolonged stimulation of respiration by a new central neural mechanism. Respir. Physiol 41, 87–103. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980b. Prolonged stimulation of respiration by endogenous central serotonin. Respir. Physiol 42, 171–188. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr, 2001. Invited review: intermittent hypoxia and respiratory plasticity. J. Appl. Physiol 90 (1985), 2466–2475. [DOI] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K, 1994. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J. Physiol 478 (Pt 1), 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Arata A, Shannon R, Lindsey BG, 1996. Long-term facilitation of phrenic nerve activity in cats: responses and short time scale correlations of medullary neurones. J. Physiol 490 (Pt 2), 463–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Shannon R, Lindsey BG, 2001. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J. Physiol 532, 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Gebber GL, 1984. Raphe neurons with sympathetic-related activity: baroreceptor responses and spinal connections. Am. J. Physiol 246, R338–348. [DOI] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL, 2007. Episodic stimulation of alphal-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J. Neurosci 27, 4435–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS, 2012. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J. Appl. Physiol 112 (1985), 1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W, Zamzow CR, 2005. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol. Res 27, 153–160. [DOI] [PubMed] [Google Scholar]
- Perim RR, Fields DP, Mitchell GS, 2018a. Cross-talk inhibition between 5-HT2B and 5-HT7 receptors in phrenic motor facilitation via NADPH oxidase and PKA. Am. J. Physiol. Regul. Integr. Comp. Physiol 314, R709–R715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perim RR, Fields DP, Mitchell GS, 2018b. Protein kinase Cdelta constrains the S-pathway to phrenic motor facilitation elicited by spinal 5-HT7 receptors or severe acute intermittent hypoxia. J. Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, O’Regan MH, Perkins LM, 1993. Adenosine 5’-triphosphate release from the normoxic and hypoxic in vivo rat cerebral cortex. Neurosci. Lett 151, 94–96. [DOI] [PubMed] [Google Scholar]
- Seven YB, Perim RR, Hobson OR, Simon AK, Tadjalli A, Mitchell GS, 2018. Phrenic motor neuron adenosine 2A receptors elicit phrenic motor facilitation. J. Physiol 596, 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibigtroth CM, Mitchell GS, 2011. Carotid chemoafferent activity is not necessary for all phrenic long-term facilitation following acute intermittent hypoxia. Respir. Physiol. Neurobiol 176, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjalli A, Mitchell GS, 2018. Moderate acute hypoxia-induced phrenic long-term facilitation requires both cervical spinal 5-HT2A and 5-HT2B receptor activation Neuroscience. San Diego. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Otsuguro K, Ohta T, Ito S, 2010. Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br. J. Pharmacol 161, 1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wylen DG, Park TS, Rubio R, Berne RM, 1986. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J. Cereb. Blood Flowr Metab 6, 522–528. [DOI] [PubMed] [Google Scholar]
- Wallman-Johansson A, Fredholm BB, 1994. Release of adenosine and other purines from hippocampal slices stimulated electrically or by hypoxia/hypoglycemia. Effect of chlormethiazole. Life Sci 55, 721–728. [DOI] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM, 2010. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J. Physiol 588, 3075–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K, Morita M, 2017. Novel aspects of extracellular adenosine dynamics revealed by adenosine sensor cells. Neural Regen. Res 12, 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]