Abstract
Objective
We present the largest population based study of sinonasal squamous cell carcinoma (SCC) to identify risk factors for presentation with nodal metastasis.
Methods
The National Cancer Database (NCDB) was used for this study. Location codes corresponding to the nasal cavity and paranasal sinuses and histology codes representing SCC malignancy were queried. Logistic regression analysis was performed to identify factors associated with presentation with nodal metastasis.
Results
6448 cases met inclusion criteria. Nodal metastasis at presentation was seen in 13.2% of patients, with the sinus subsite (19.3%) being a significant risk factor for nodal metastasis at presentation when compared to the nasal cavity (7.9%). Logistic regression analysis showed black, uninsured and Medicaid patients were more likely than white and privately insured patients, respectively, to present with nodal metastasis.
Conclusions
In sinonasal SCC, the sinus subsite has a significantly increased risk of nodal metastasis compared to the nasal cavity. Black race, uninsured and Medicaid patients are more likely to have nodal metastasis at presentation.
Keywords: Squamous cell carcinoma, National cancer database, Nodal metastasis, Maxillary sinus, Nasal cavity
Introduction
Malignancies of the nasal cavity and paranasal sinuses are rare, representing less than 3% of all head and neck tumors.1 Squamous cell carcinoma (SCC) is the most common histology observed and accounts for 50%–60% of all malignancies in this region.1, 2, 3, 4, 5, 6 Based on the American Joint Committee on Cancer (AJCC) staging system, the sinonasal cavity is divided into two distinct subsites: tumors of the maxillary sinus and tumors of the nasal cavity and ethmoid sinus. Of these two subsites, maxillary sinus involvement is more common, comprising up to 80% of these malignancies.7
Standard treatment for primary sinonasal lesions is surgical resection with or without adjuvant radiation or chemotherapy.8 Five-year disease-specific survival ranges from 50% to 80%.4,6,9, 10, 11 Nodal involvement, advanced tumor stage, and larger tumor size have been shown to predict worse prognosis.1,12 Despite this, risk factors for nodal involvement at presentation have not been well defined. The sinonasal cavity has a heterogenous lymphatic drainage system, with known connections to the facial/buccal, submandibular, parotid, and parapharyngeal nodal regions.12
Due to the rarity of sinonasal SCC, treatment recommendations have historically been based on small retrospective series. The utility of many of these studies is further limited due to grouping together malignancies of the nasal cavity and paranasal sinuses or grouping different histological subtypes.11,12 This has made it difficult for clinicians to identify patients who are at higher risk of nodal metastasis. Based on the low incidence of sinonasal malignancies, database analysis has emerged as an important method of determining treatment outcomes for this pathology. The current study uses the National Cancer Database (NCDB) to predictors of nodal metastasis in sinonasal SCC.
Materials and methods
Information from the NCDB was obtained on March 4th, 2016 for tumors involving the head and neck diagnosed between 2004 and 2012. The NCDB is a project sponsored by both the American College of Surgeons and the American Cancer Society established in 1989. It provides a comprehensive clinical oncology database comprising data collected from more than 1500 Commission on Cancer accredited facilities and includes more than 34 million records, representing more than 70% of newly diagnosed cancer cases in the United States.9
To specifically analyze overall survival of patients with sinonasal SCC, the NCDB was queried using the ICD-O-3 (International Classification of Diseases for Oncology, Third Edition) topography codes corresponding to the nasal cavity and paranasal sinuses (C30.0, C31.0, C31.1, C31.2, C31.3, C31.8, C31.9). Histology codes included were 8070 (SCC, not otherwise specified), 8071 (SCC), and 8072 (SCC, nonkeratinizing). Only cases with behavior code of ‘3’ were included: ‘Malignant neoplasms stated or presumed to be primary’. Cases were excluded if there was evidence of metastatic disease or a record of surgery at a distant site. Patients were excluded if they did not have values for either follow-up or vital status. Cases were also excluded if they were recorded as having had surgery at a distant site to avoid confounding of different surgical procedures.
The variables investigated included age, sex, race, ethnicity, comorbidity score using the Charlson/Deyo score, insurance status, income, education level, treatment facility type, facility location, tumor histologic subtype, tumor grade, clinical T stage, clinical N stage, pathologic T stage, pathologic N stage, overall clinical stage, and treatment modality. Insurance status was categorized as ‘private’, ‘uninsured’, ‘Medicaid’, ‘Medicare’, ‘other government’, and ‘unknown’. Treatment facility type was classified as ‘Community Cancer Program’, ‘Comprehensive Cancer Program’, ‘Academic/Research Cancer Program’, ‘Integrated Network Cancer Program’, and ‘Other’. Tumor characteristics of clinical T and N stages were classified according to the 7th Edition American Joint Committee on Cancer classification. Tumor grade was classified into grade Ⅰ – well-differentiated, grade Ⅱ – moderately differentiated, grade Ⅲ – poorly differentiated, and grade Ⅳ- undifferentiated/anaplastic. The overall stage was categorized as early (stages Ⅰ and Ⅱ) and advanced (stages Ⅲ and Ⅳ). Treatment modalities included surgery, radiation, and/or chemotherapy.
Univariate analysis for categorical variables was performed using Pearson χ2 for categorical variables. Unadjusted Kaplan–Meier estimates and log-rank tests were used for univariable comparison of overall survival outcomes, and multivariable Cox proportional hazard models were generated for multivariable comparisons. Variables included in the final multivariable Cox proportional hazard model were: age, sex, race, insurance status, income, comorbidity score, facility type, facility location, education, and year of diagnosis. Logistic regression was used to examine the relationship of presentation with nodal metastasis with patient and tumor variables.
All data processing and analysis were performed with Microsoft Open R v. 3.3.2 (https://mran.microsoft.com/open/) via RStudio v. 1.1.23 (RStudio, Boston, MA, USA). The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and the hospitals participating in the CoC NCDB are the sources of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. This study was determined to be exempt by the Institutional Review Board of the Hospital of the University of Pennsylvania.
Results
A total of 6448 patients were identified with primary SCC of the nasal cavity and paranasal sinuses without metastasis in the NCDB. Demographics are shown in Table 1. There was a male predominance (64.1%) and a majority of white patients (85.4%). Tumor characteristics are shown in Table 2. Median overall survival was 58.0 months (95% CI [54.3–63.4]) with a 5-year survival of 49.6% (95% CI [48.3%–50.9%]).
Table 1.
Demographics for all subjects.
| Characteristic | n (%) |
|---|---|
| Total n | 6448 |
| Age (Mean (SD)) | 65.3 (13.2) |
| Sex | |
| Male | 4135 (64.1) |
| Female | 2313 (35.9) |
| Race | |
| White | 5506 (85.4) |
| Black | 647 (10.0) |
| Other/Unknown | 134 (2.1) |
| Asian | 161 (2.5) |
| Ethnicity | |
| Hispanic | 343 (5.3) |
| Non-hispanic | 5726 (88.8) |
| Unknown | 379 (5.9) |
| Insurance status | |
| Private | 2151 (33.4) |
| Uninsured | 281 (4.4) |
| Medicaid | 512 (7.9) |
| Medicare | 3250 (50.4) |
| Other government | 126 (2.0) |
| Unknown | 128 (2.0) |
| Comorbiditiy (Charlson/Deyo score) | |
| 0 | 5106 (79.2) |
| 1 | 1029 (16.0) |
| 2 | 228 (3.5) |
| Income | |
| Less than $38,000 | 1348 (20.9) |
| $38,000 - $47,999 | 1649 (25.6) |
| $48,000 - $62,999 | 1711 (26.5) |
| $63,000 and greater | 1653 (25.6) |
| N/A | 87 (1.3) |
| Education levela | |
| 21% or more | 1268 (19.7) |
| 13%–20.9% | 1817 (28.2) |
| 7–12.9% | 2039 (31.6) |
| Less than 7% | 1241 (19.2) |
| N/A | 262 (1.7) |
| Facility type | |
| Community Cancer Program | 453 (7.0) |
| Comprehensive Community Cancer Program | 2010 (31.2) |
| Academic/Research Cancer Program | 3107 (48.2) |
| Integrated Network Cancer Program | 714 (11.1) |
| N/A | 164 (11.1) |
SD: standard deviation; N/A: not applicable.
Education level designated as percent in area without high school diploma.
Table 2.
Tumor characteristics for all patients.
| Characteristic | n (%) |
|---|---|
| Clinical T stage at diagnosis | |
| T0 | 11 (0.2) |
| T1 | 1472 (22.8) |
| T2 | 772 (12.0) |
| T3 | 850 (13.2) |
| T4 | 113 (1.8) |
| T4a | 1367 (21.2) |
| T4b | 624 (9.7) |
| Tx | 1196 (18.5) |
| pIS | 17 (0.3) |
| N/A | 26 (0.4) |
| Clinical N stage at diagnosis | |
| N0 | 4515 (70.0) |
| N1 | 339 (5.3) |
| N2 | 93 (1.4) |
| N2a | 35 (0.5) |
| N2b | 203 (3.1) |
| N2c | 156 (2.4) |
| N3 | 28 (0.4) |
| Nx | 1074 (16.7) |
| N/A | 5 (0.1) |
| Tumor grade | |
| Grade 1: well differentiated | 983 (15.2) |
| Grade 2: moderately differentiated | 2469 (38.3) |
| Grade 3: poorly differentiated | 1702 (26.4) |
| Grade 4: anaplastic/undifferentiated | 62 (1.0) |
| Unknown | 1232 (19.1) |
N/A: not applicable; N: the extent of spread to the lymph nodes; NX: regional lymph nodes cannot be evaluated; T: tumor; Tx: primary tumor cannot be evaluated; pIS: pathologic in situ.
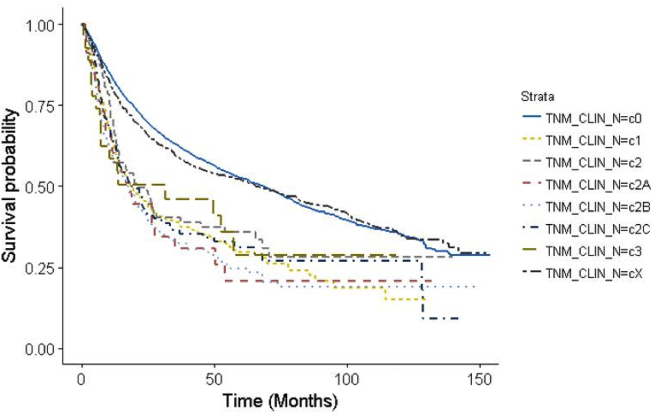
Clinical evidence of nodal metastasis was seen in 854 patients (13.2%) overall. Bilateral or contralateral neck nodal metastasis was seen in 359 patients (5.6%). For the nasal cavity subsite, nodal metastasis was seen in 271 patients (7.9%). For all sinus subsites (maxillary, ethmoid, sphenoid, frontal), nodal metastasis was seen in 583 patients (19.3%). For the maxillary sinus subsite, nodal metastasis was seen in 546 patients (21.0%). Unadjusted Kaplan–Meier overall survival based on the clinical N stage is shown in Fig. 1. Univariable analysis of overall survival by the clinical N stage is shown in Table 3.
Fig. 1.
Unadjusted Kaplan–Meier curve overall survival based on clinical nodal stage.
Table 3.
Univariable Cox proportional hazard analysis for overall survival.
| Clinical N stage | HR [95% CI] | p value |
|---|---|---|
| N0 | 1 (ref) | – |
| N1 | 1.93 [1.66–2.25] | <0.001 |
| N2 | 1.61 [1.23–2.10] | <0.001 |
| N2a | 2.04 [1.26–3.07] | <0.001 |
| N2b | 2.22 [1.84–2.66] | <0.001 |
| N2c | 1.92 [1.55–2.37] | <0.001 |
| N3 | 1.77 [1.09–2.87] | <0.001 |
| Nx | N/A | N/A |
CI: confidence interval; N: the extent of spread to the lymph nodes; NX: regional lymph nodes cannot be evaluated; HR: Hazard ratio.
Bolded values are significant with p < 0.05.
Logistic regression analysis was done on a cohort of 5369 patients who had clinical nodal staging data complete in the NCDB, patients were excluded if they were listed as N/A or cNx. On logistic regression analysis, when compared to private insurance, uninsured patients were more likely to present with nodal metastasis (OR 1.91, 95% CI [1.35–2.67], p < 0.001) after controlling for patient and tumor factors. A similar relationship was seen among Medicaid patients who were more likely to present with nodal metastasis (OR 2.00, 95% CI [1.52–2.61], p < 0.001). Black patients were more likely to present with nodal metastasis compared to white patients (OR 1.36, 95% CI [1.07–1.72], p = 0.010) on logistic regression. The highest income group of >$63,000 was less likely to present with nodal metastasis compared to the lowest income group of <$38,000 (OR 0.69, 95% CI [0.50–0.95], p = 0.022).
Discussion
Sinonasal malignancies arising from the nasal cavity and paranasal sinuses are rare neoplasms accounting for approximately 1% of all cancers.1,13 Due to the rarity of these malignancies, much of the existing literature is hindered by low patient numbers, making database analysis a more robust method of studying the behavior of these neoplasms. Nodal involvement has been shown to have a worse prognosis in sinonasal SCC.1,12 Here we present the largest population-based study examining predictors of nodal metastasis in SCC of the paranasal sinuses and nasal cavity. In agreement with prior studies, we showed that nodal metastasis at presentation was associated with worse overall survival, so we then examined patient and tumor factors associated with nodal metastasis.
To our knowledge, there exists only one other study that looks at nodal metastasis for sinonasal SCC on a database level. This study, by Ahn et al,12 uses the SEER database to evaluate the risk of lymph node metastasis in 1283 patients with sinonasal SCCs. They found that in nasal cavity SCC there were higher rates of nodal involvement for T4 tumors and primary tumor size ≥2 cm, whereas in maxillary sinus SCC, stage ≥ T2 but not size was associated with higher rates of nodal involvement.12 Among the 1283 patients included in their analysis, 182 (14.2%) had nodal involvement at presentation. For T4a nasal cavity SCC, levels Ⅰ and Ⅱ were most commonly affected; for T2 or higher maxillary sinus SCC, levels Ⅰ, Ⅱ, and/or Ⅲ were most commonly involved.12 The current study showed a similar rate of nodal metastasis for maxillary sinus 21.0% to the number cited in their study (20.7%), and this number is above the 15% threshold traditionally cited when considering elective neck treatment.12
In our study, we found that uninsured and Medicaid patients were more likely to present with nodal metastasis when compared to private insurance. A relationship between insurance status and nodal metastasis has been shown for parotid malignancy where uninsured and Medicaid patients were more likely to present with nodal metastasis compared to private insurance.14 Carey et al15 have demonstrated the impact of insurance status on overall survival for sinonasal SCC. Their study found uninsured status, Medicaid, and Medicare were associated with worse overall survival compared to private insurance.15 The relationship between insurance status and presentation with more advanced disease is relevant given the continuing healthcare reform that has led to changes in insurance status for millions of Americans. A proposed reason for the relationship between insurance status and more advanced disease at presentation is that the uninsured may be more reluctant to present to healthcare providers for their initial concerns due to financial constraints or lack of access to the healthcare system.14 Identifying at risk populations and barriers to care are important steps to improving health care.
Our findings also showed that race was a risk factor for nodal metastasis, with black patients more likely to present with nodal metastasis compared to white patients. Prior studies have revealed racial differences in survival for sinonasal malignancies.16,17 A study of all sinonasal carcinomas showed worse disease-specific survival for African American and Hispanic patients compared to white patients, and racial differences in five-year cause-specific survival between non-Hispanic whites (64%) and African American/Hispanics (52%).16 In another study of sinonasal SCC, African American patients were found to have a higher incidence and increased mortality compared to white patients.18
The NCDB is the largest clinical cancer registry in the world. It is nearly four times the size of SEER and captures 70% of newly diagnosed cancers in the United States.19 All facilities contributing to the database undergo regular auditing, ensuring a high level of data quality and completeness. Nevertheless, the NCDB is still comprised of data from various centers that may have different standards or accuracy in reporting outcomes. As in all projects with retrospective design, this study is subject to unmeasured confounding, which we aimed to control via careful multivariate analysis. In particular, staging investigations are not recorded in the NCDB, perhaps lending to confounding as prognosis may be tied to the number and type of staging evaluations performed.19 Further, although a significant amount of demographic data is included in NCDB in comparison to SEER, data points such as the cause of death, time to recurrence, and cancer-specific survival are not included, limiting our analysis.
Conclusion
This study of 6448 patients with sinonasal SCC represents the largest study on predictors of nodal metastasis. Patient factors associated with nodal metastasis at presentation included black race, uninsured and Medicaid. Tumors factors include sinus primary site. These findings are important for guiding neck management and for identifying disparities in healthcare that can lead to healthcare reform.
Edited by Zhenxiao Huang, Yi Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Turner J.H., Reh D.D. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. Cancer of the nasal cavity: survival and factors influencing prognosis. Arch Otolaryngol Head Neck Surg. 2002;128:1079–1083. doi: 10.1001/archotol.128.9.1079. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo J.F., Guemes A., Ramirez-Ortega M.C., Onate-Ocana L.F. Prognostic factors in maxillary sinus and nasal cavity carcinoma. Eur J Surg Oncol. 2005;31:1206–1212. doi: 10.1016/j.ejso.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Fornelli R.A., Fedok F.G., Wilson E.P., Rodman S.M. Squamous cell carcinoma of the anterior nasal cavity: a dual institution review. Otolaryngol Head Neck Surg. 2000;123:207–210. doi: 10.1067/mhn.2000.107450. [DOI] [PubMed] [Google Scholar]
- 5.Sanghvi S., Khan M.N., Patel N.R., Yeldandi S., Baredes S., Eloy J.A. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124:76–83. doi: 10.1002/lary.24264. [DOI] [PubMed] [Google Scholar]
- 6.Scurry W.C., Jr., Goldenberg D., Chee M.Y., Lengerich E.J., Liu Y., Fedok F.G. Regional recurrence of squamous cell carcinoma of the nasal cavity: a systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:796–800. doi: 10.1001/archotol.133.8.796. [DOI] [PubMed] [Google Scholar]
- 7.Takes R.P., Ferlito A., Silver C.E. The controversy in the management of the N0 neck for squamous cell carcinoma of the maxillary sinus. Eur Arch Otorhinolaryngol. 2014;271:899–904. doi: 10.1007/s00405-013-2591-0. [DOI] [PubMed] [Google Scholar]
- 8.Katz T.S., Mendenhall W.M., Morris C.G., Amdur R.J., Hinerman R.W., Villaret D.B. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24:821–829. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]
- 9.Allen M.W., Schwartz D.L., Rana V. Long-term radiotherapy outcomes for nasal cavity and septal cancers. Int J Radiat Oncol Biol Phys. 2008;71:401–406. doi: 10.1016/j.ijrobp.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta R., Dubal P.M., Svider P.F., Liu J.K., Baredes S., Eloy J.A. Sinonasal malignancies: a population-based analysis of site-specific incidence and survival. Laryngoscope. 2015;125:2491–2497. doi: 10.1002/lary.25465. [DOI] [PubMed] [Google Scholar]
- 11.Unsal A.A., Dubal P.M., Patel T.D. Squamous cell carcinoma of the nasal cavity: a population-based analysis. Laryngoscope. 2016;12:560–565. doi: 10.1002/lary.25531. [DOI] [PubMed] [Google Scholar]
- 12.Ahn P.H., Mitra N., Alonso-Basanta M. Risk of lymph node metastasis and recommendations for elective nodal treatment in squamous cell carcinoma of the nasal cavity and maxillary sinus: a SEER analysis. Acta Oncol. 2016;55:1107–1114. doi: 10.1080/0284186X.2016.1216656. [DOI] [PubMed] [Google Scholar]
- 13.Haerle S.K., Gullane P.J., Witterick I.J., Zweifel C., Gentili F. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24:39–49. doi: 10.1016/j.nec.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Stubbs V.C., Rajasekaran K., Chen J., Cannady S.B., Brant J.A., Newman J.G. Impact of payer status on survival in parotid malignancy. Am J Otolaryngol. 2019;40:555–559. doi: 10.1016/j.amjoto.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Carey R.M., Parasher A.K., Workman A.D. Disparities in sinonasal squamous cell carcinoma short- and long-term outcomes: analysis from the national cancer database. Laryngoscope. 2018;128:560–567. doi: 10.1002/lary.26804. [DOI] [PubMed] [Google Scholar]
- 16.Patel Z.M., Li J., Chen A.Y., Ward K.C. Determinants of racial differences in survival for sinonasal cancer. Laryngoscope. 2016;126:2022–2028. doi: 10.1002/lary.25897. [DOI] [PubMed] [Google Scholar]
- 17.Smith S.P., Russell J.L., Chen N.W., Kuo Y.F., Resto V.A. Sinonasal carcinoma: racial and ethnic disparities in survival--a review of 4714 patients. Otolaryngol Head Neck Surg. 2015;153:551–560. doi: 10.1177/0194599815593277. [DOI] [PubMed] [Google Scholar]
- 18.Ansa B., Goodman M., Ward K. Paranasal sinus squamous cell carcinoma incidence and survival based on Surveillance, Epidemiology, and End Results data, 1973 to 2009. Cancer. 2013;119:2602–2610. doi: 10.1002/cncr.28108. [DOI] [PubMed] [Google Scholar]
- 19.Boffa D.J., Rosen J.E., Mallin K. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3:1722–1728. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]